Introducción: Se presenta la evaluación de la asociación entre la reserva de hierro (Fe) y los polimorfismos del gen de la hemocromatosis (HFE) en neonatos de alto riesgo perinatal.
Métodos: Se incluyó una serie de neonatos de alto riesgo perinatal en los que se evaluó la reserva de Fe con la medición de la ferritina sérica (FS). Se dividieron en tres grupos: sobrecarga de Fe (SoFe), con FS >1,000 μg/l; reserva normal de Fe, con FS de 154-1,000 μg/l; y reserva baja de Fe, con FS <154 μg/l. Mediante PCR en tiempo real se buscaron las mutaciones C282Y, H63D y S65C del gen HFE.
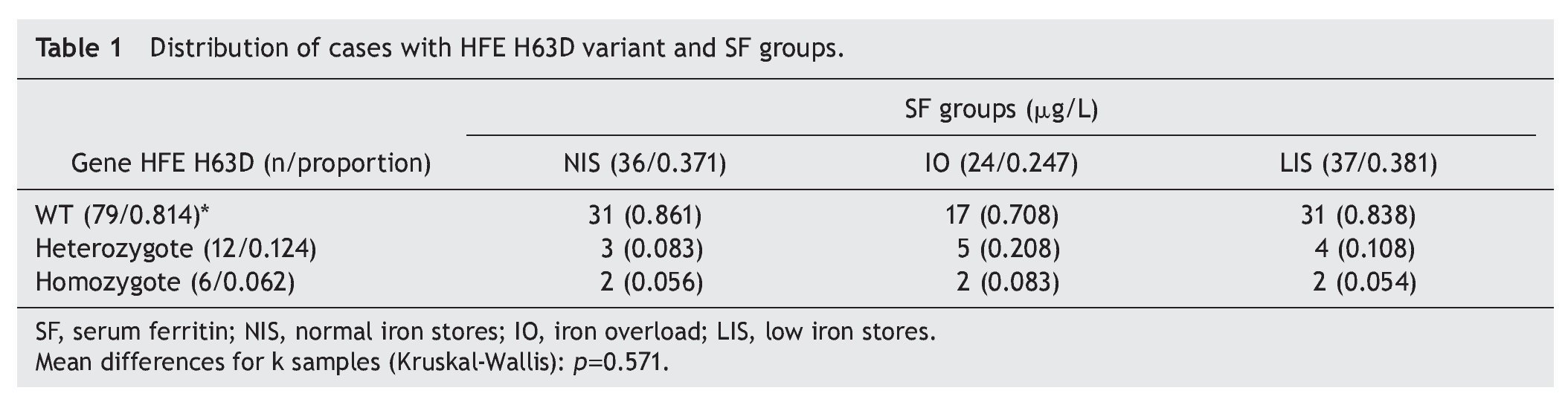
Resultados: Se estudiaron 97 neonatos. De ellos, 24 casos presentaron SoFe (proporción 0.247) y FS de 1,789 μg/l (IC 95% 1,376-2,201); 36 casos, reserva normal de FS (0.371), FS de 461 μg/l (389-533); y 37 casos, reserva baja de FS (0.381) y FS 82 μg/l (69-96). No hubo casos detectados para las mutaciones C282Y o S65C. Se identificó la variante H63D HFE en 18 neonatos (frecuencia génica de 0.185): la condición de heterocigoto (H63D/WT) en doce casos (frecuencia génica 0.124) y de homocigoto (H63D/H63D) en seis casos (frecuencia génica 0.062). La frecuencia alélica de H63D fue de 0.092. Los variante H63D HFE no mostró asociación con los neonatos de reserva normal de Fe contra reserva baja (OR 1.2; IC 95% 0.3-4.3) ni los de reserva normal contra neonatos con SoFe (OR 2.5; 0.7-9.2).
Conclusiones: Cerca del 25% de neonatos de alto riesgo tendrá sobrecarga de Fe. Aún con el posible sesgo de selección, las variantes del gen HFE no influyen sobre el estado de la reserva de Fe.
Background: The association between iron stores (Fe) and HFE gene polymorphisms on high-risk neonates is shown.
Methods: We included newborns with high perinatal risk. Newborns were divided into three groups for measurements of serum ferritin (SF): iron overload (IO) with SF 1000 μg/L, normal iron stores (NIS) with SF 154-1000 μg/L and low iron stores (LIS) with SF <154 μg/L. We used real-time PCR for identification of polymorphisms C282Y, H63DE, and S65C of the HFE gene.
Results: We studied 97 newborns with IO in 24 cases (ratio 0.247) and SF 1789 μg/L (95% CI 1376-2201), NIS in 36 cases (0.371), and SF of 461 μg/L (389-533) and LIS in 37 cases (0.381) and SF 82 μg/L (69-96). There were no cases detected for C282Y or S65C mutations. We identified 18 neonates with H63D HFE variant (gene frequency 0.185) with heterozygous condition (H63D/ WT) in 12 cases (gene frequency 0.124) and homozygote (H63D/H63D) in six cases (gene frequency 0.062). H63D allele frequency was 0.092. The HFE H63D variant showed no association for comparing infants with NIS vs. LIS (OR 1.2, 95% CI 0.3-4.3) and NIS vs. IO newborn infant (OR 2.5, 0.7-9.2).
Conclusions: In high-risk neonates ∼25% show IO even with the possible selection bias. HFE gene variants do not influence on the neonatal iron stores.
1. Introduction
Body iron (Fe) content in term newborns is ∼75 mg/kg; of this, 60% is accumulated during the third trimester of pregnancy;1 75-80% of iron is distributed in the form of erythrocyte hemoglobin. Approximately 10% is found in the tissues forming part of the myoglobin and cytochrome and the remaining 10-15% is stored in its soluble form as ferritin or insoluble as hemosiderin. Both are especially found in the reticular endothelial system, mainly in the liver and spleen.2
Serum ferritin (SF) is the best approximation for measuring Fe reserves in the body.3 SF values in the fetus and newborns are different from those reported for other ages.1,4 Iron in the body, estimated by SF concentrations is influenced by the different effects of the macroenvironment with varied impact.5 The state of maternal iron reserves at the beginning of the pregnancy and the status during labor is included;6-8 gestational age,3 time of umbilical cord clamping,9 collection of the umbilical cord,10 and collection of placental blood for storing.
During the neonatal stage, the speed of body growth and type of lactation provided is added.2,8 This is regardless of the presence of different neonatal complications such as preterm birth,4 iatrogenic blood loss due to repeated blood samples obtained for laboratory tests2 or red blood transfusion.11,12
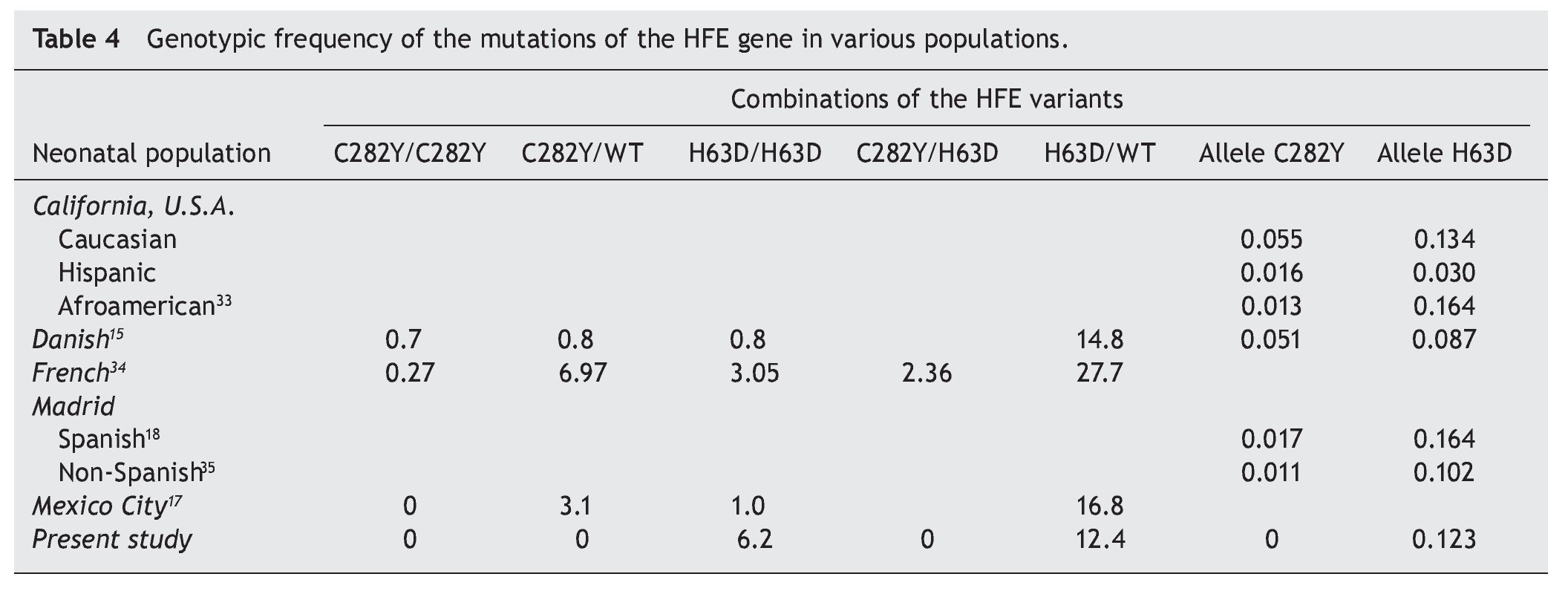
The intestinal absorption of Fe at the duodenal crypts is regulated by complex molecular mechanisms,2,13 which include the hemochromatosis gene (HFE). The two best known mutations of the HFE gene, C282Y and H63D, are associated with the hereditary disease hemochromatosis, a disease of recessive autosomal transmission with symptoms related to Fe overload and damage to parenchymatous organs.14 The prevalence of the mutations of the HFE gene varies according to the ethnicity of the population. The one of greatest impact, C282Y, is common in Northern Europe, with an allelic frequency of 0.082 to 0.013,15 whereas in Mexico it is <0.002.16 For the H63D variant that represents a multiethnic distribution, the allelic frequency varies between 0.224 and 0.022, whereas in Mexico a frequency of 0.136 to 0.093 is reported.
The association between the HFE gene and the neonatal reserve of Fe has been previously evaluated. In neonates seen in Mexico City, the reported allelic frequency of the mutations of C282Y and H63D was from 0.013 and 0.102, respectively.17 This information agrees with what has been reported for newborns in the Madrid community18 with an allelic frequency for C282Y of 0.017 (95% CI 0.011-0.023) and for H63D of 0.164 (95% CI 0.148-0.180). Although information on the impact during the neonatal period is scarce, an attempt has been made to associate it with birth weight or the subsequent development of leukemia.19
Therefore, the aim of the present report was to evaluate the association between SF concentrations and the polymorphisms of the HFE in newborns, independently of their clinical conditions or history of transfusion.
2. Methods
We present a case series comprised of newborns with gestational age from 28 to 37 weeks who were hospitalized in the NICU and were subjected to an evaluation of Fe stores due to different neonatal complications. Newborns were included independent of any infectious disease or stage of ventilatory management. Also included were those with a diagnosis of cholestasis and transfusion treatment provided.
From the remaining blood sample with EDTA used for performing blood cytometry, SF determination and procurement of DNA was done. SF was determined by enzyme-linked fluorescent assay (ELFA) with a commercial reagent (VIDAS Ferritin, Biomerieux). Based on the results, three SF groups were formed: Iron overload (IO) with values >1000 μg/L; normal ion stores (NIS) with SF values between 154 and 1000 μg/L and, finally, cases with low iron stores (LIS) when neonatal SF values were <154 μg/L. These groups were adapted for term low-risk newborns7 and for those newborns with neonatal hemochromatosis.20
Genomic DNA was extracted with a commercial reagent (High Pure PCR Template Preparation Kit, Roche Applied Science). For real-time PCR, sequences specific for the HFE C282Y, H63D and S65C mutations were amplified (Lightmix In-Vitro Diagnostic Kit HFE, Tib MolBiol). With these results, cases were stratified for three variants assessed under normal conditions (WT), heterozygous (Het) or homozygous (Hom). The design of the study did not consider collecting maternal samples.
The proportions of cases identified were represented. SF values were reported as averages and 95% confidence intervals. HFE polymorphisms are shown in their allelic and gene frequencies.
Differences between SF groups and each condition of genetic frequency of HFE were compared with the test of mean differences for k samples (Kruskal-Wallis). For comparing SF values and the allelic frequency of the HFE variants, the mean difference test was used (Mann-Whitney U).
3. Results
There were 97 newborns studied with an average age of 23 days (95% CI 12-44 days). According to the values of SF concentration, the following results were identified: IO in 24 cases (proportion 0.247) and SF average 1789 μg/L (95% CI 1,376-2,201 μg/L); NIS in 36 cases (proportion 0.371) and SF average of 461 μg/L (95% CI 389-533 μg/L) and finally, LIS in 37 cases (proportion 0.381) and SF average of 82 μg/L (95% CI 69-96 μg/L). With respect to the HFE polymorphisms, there were no cases detected for mutations C282Y or S65C. There were 18 newborns identified with the HFE H63D variant (genetic frequency of 0.186). The heterozygous condition (H63D/WT) was identified in 12 cases (genetic frequency 0.124) and the homozygous condition (H63D/H63D) in six cases (genetic frequency 0.062). The frequency of the mutated allele H63D was 0.124.
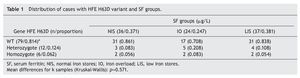
It was noted that for each category of normal, high and low Fe reserve, occurrence of the natural HFE variant was distributed in proportions of 0.861, 0.708 and 0.838, respectively, without statistically significant differences (Kruskal-Wallis test, p = 0.571). For the heterozygous variant of HFE (H63D/WT), the proportion proved to be greater for the IO category (0.208) with respect to the group of NIS and LIS (proportion 0.083 and 0.333, respectively), without statistically significant differences. Finally, for the homozygous condition HFE H63D/H63D, distribution of the cases resulted in being the same for the three SF groups (Table 1).
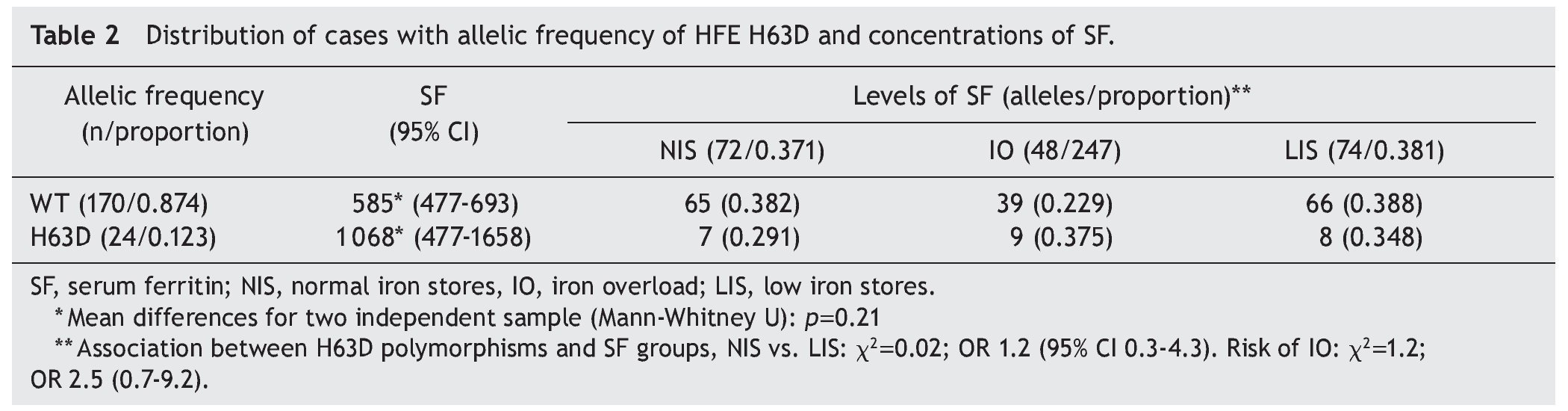
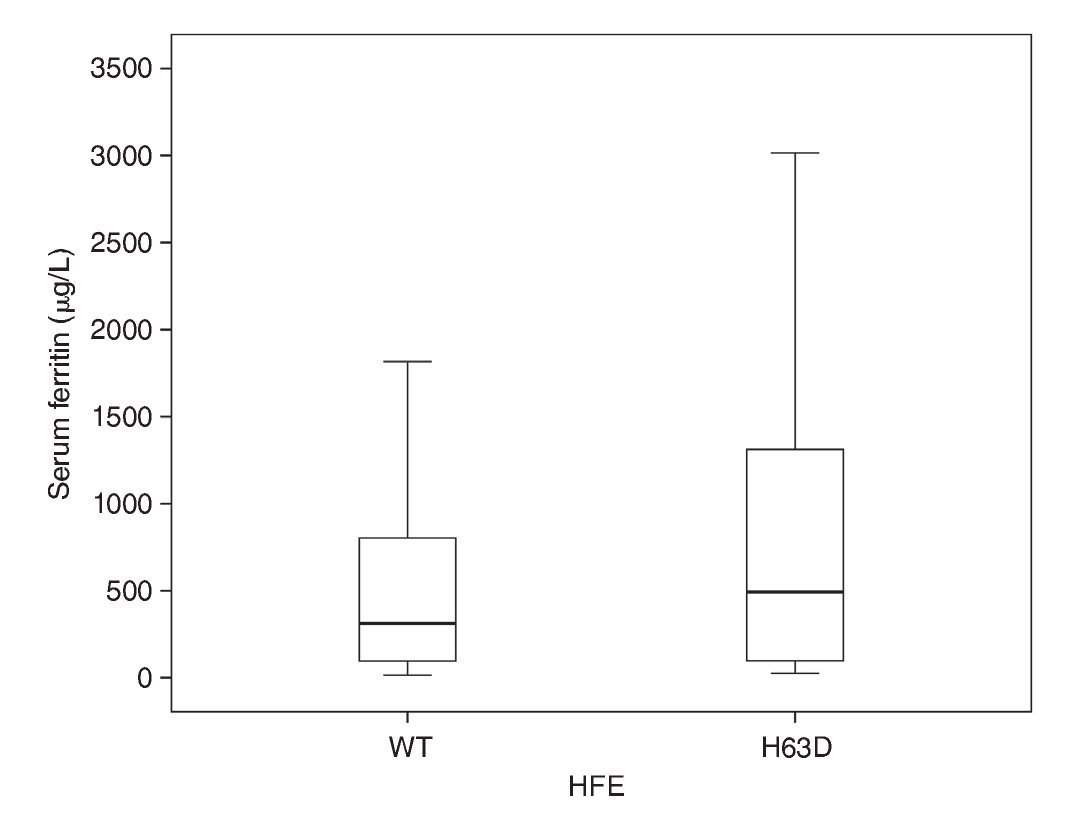
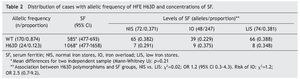
In the comparison to distribution of SF values with the natural variant of the HFE gene and of the allele HFE H63D, it was observed that, although there is a difference in the mean of the SF values between groups, the group with the allele H63D had a greater dispersion in the SF values (Fig. 1). In the comparison of the percentile frequencies (25th percentile vs. 50th and 75th percentiles), there were no statistically significant differences recorded (χ2 = 0.007, p = 0.934). Taking into consideration the allelic frequency (Table 2) and the distribution in groups of SF values, it was noted that for the natural condition of the HFE gene and the H63D allele, SF values were on average 585 μg/L (95% CI 477-693) and 1,068 μg/L (95% CI 477-1658), respectively, without statistically significant differences (Mann-Whitney U, p = 0.21).
Figure 1 Distribution of serum ferritin values according to the H63D allele of the HFE gene. WT, natural allele; H63D, mutated allele.
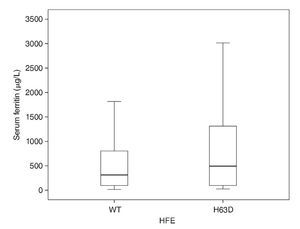
When the allelic frequency of HFE H63D is compared by group, a proportion of similar mutated alleles with respect to SF were observed (0.291, 0.375 and 0.348, respectively). It was evaluated whether the state of the H63D polymorphisms may have been a protective factor of low Fe stores or of risk for Fe overload, with results not being statistically significant. The results of the association between H63D polymorphisms when comparing newborns with normal Fe stores against those with low Fe stores were χ2 0.02; OR 1.2; 95% CI 0.3-4.3 and when comparing the cases with normal stores against newborns with IO, χ2 = 1.2; OR 2.5; 95% CI 0.7-9.2.
4. Discussion
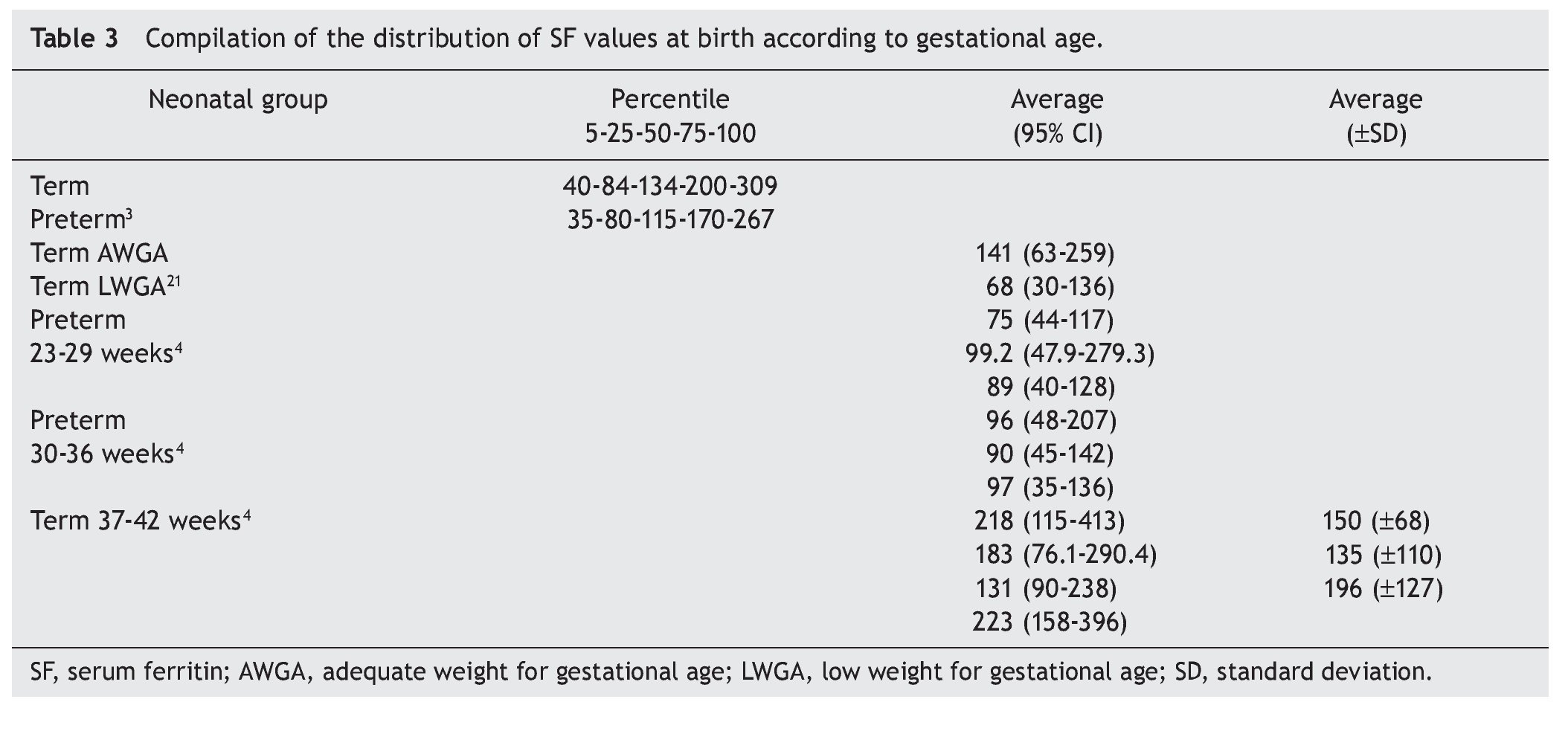
The effects of the different variables that modify Fe reserves have different weights for each condition.5 Distribution of the SF values according to the gestational age at birth has evident differences.4 Between the 23rd and 29th weeks of gestation, the SF median is 88.1 μg/L (5th-95th percentile from 44.0 to 74 μg/L). For neonates between 30 and 36 weeks of birth, the values are from 115.4 μg/L (5th-95th percentile from 55.6 to 182.1 mg/L). For those who were born between 37 and 42 weeks, SF values are from 165.8 μg/L (5th-95th percentile from 90.8 to 293.4 μg/L). These latter values are similar to those reported by our group7 with SF from 145 μg/L (24-139 μg/L). In addition, term newborns with low birth weight show lower SF values compared with eutrophic newborns.21 The compilation of results on SF values at birth points to the definitive effect of gestational age, but with a wide variability for similar ages (Table 3). More than with the difference related with the analytical method, it is related with an effect of the perinatal clinical variables.2,4
As for other ages,1 there are no established criteria for the definition of IO. In an arbitrary manner, values of SF >1,000 μg/L were chosen, whereas NIS were considered with SF values between 154 and 1000 μg/L and LIS those <154 μg/L.
However, other criteria have been employed: in children born at <32 weeks gestation and evaluated upon their hospital discharge, Fe deficiency (FS <76 μg/L) was present in 23% of cases, whereas Fe overload (SF >400 μg/L) affected 19%.22 In the present study, in the logistic regression analysis, the effect of prior red blood transfusion was observed (OR 1.41, 95% CI 1.2-1.6). Among the newborns who received more than three events of red blood transfusions, 50% developed IO.22 Up to 70% of newborns with alloimmune hemolytic disease had IO at birth, persisting at 50% and 18% toward the first and third months of age, respectively.11
The clinical impact of IO has been evaluated in terms of different complications such as hepatic cholestasis,12 neurodevelopmental disorders,23 diseases related with oxidative tissue damage24 and, although the evidence is still inconsistent, as a risk factor for developing childhood leukemia.19 In a similar manner, decrease in fetal or neonatal iron stores could change the neurochemical brain structure and lead to long-term cognitive dysfunction and motor changes that cannot be corrected with the Fe supplementation25 or abnormal hearing.26
Evaluation of the neonatal stores of Fe and mutations of the HFE gene in newborns with different clinical problems, which include low birth weight, has been carried out. Sufficient evidence has not demonstrated a clinical or statistically significant association27 in a manner similar to what is presented in this report. A tentative explanation for these differences may be the effect of the population frequencies of the polymorphisms of the HFE gene. The study of different populations of newborns in many geographic areas is notably similar, pointing to the multiethnic origin of the HFE H63D variant including that observed in our country (Table 4). This fact contrasts with what has been reported by the National Nutrition Survey28 with respect to the prevalence of Fe deficiency being very high, both for the adult population (18.1%) as well as for the infant stage (13-26%).29 The question arises of the expected protective effect of this mutation. Although there are indications that the effect of the HFE mutations on the mother could influence the fetal and newborn iron stores, this has only been demonstrated in animal models.30 Clinical studies have not been able to demonstrate differences between maternal hemoglobin values and fetal transferrin saturation.17
Without taking into consideration the individual clinical conditions such as the case of red blood transfusion,12 newborns with HFE mutations demonstrate a greater occurrence of cases of iron overload. Larger population studies are required to determine the occurrence of the HFE mutations in an open newborn population as well as the association between HFE and IO. The impact of IO, HFE gene and neonatal health should be evaluated and, if an association is found, the impact of preventive and corrective interventions during the preclinical stage (accumulation) and clinical (tissue damage).
In conclusion, the present report elucidates the extent of SF concentrations in high-risk newborns without taking into consideration the history of red blood transfusion or iatrogenic blood loss for laboratory studies. Only a little more than a third of these newborns will have normal Fe; ∼25% will have IO and the remaining cases will have LIS. Even with the possible bias due to the small sample size, variants of the HFE gene do not have an influence on the status of Fe stores. It should be defined if, in select groups such as newborns with neonatal cholestasis or perinatal hemolytic disease,11 evaluation of a possible association or impact of the practices of iron supplementation may have any relevance31 or the search of the HFE variants on neonatal screening.32-35
Received 13 September 2013;
accepted 3 April 2014
* Corresponding author.
Email: baptistagh@gmail.com (H.A. Baptista González).