Chikungunya fever is a tropical vector-borne disease that has been spreading rapidly around the world during the last 10 years, and which has been usually misdiagnosed as dengue. Nowadays, this disease is increasing in Mexico, mainly in the southern and central zones of the country, being significantly more common in women, children and young adults (28% in < 20 years of age). The classical presentation includes fever, arthralgia, polyarthritis, back-pain, and skin rashes. Although symptoms and treatment are similar to those for dengue, there are key clinical features to differentiate these two diseases.
La enfermedad por el virus chikungunya es una enfermedad tropical transmitida por vector, que en los últimos 10 años ha tenido una gran diseminación mundial y ha sido históricamente subdiagnosticada debido a las características en común con el dengue. Actualmente la incidencia en México ha ido en aumento, sobre todo en el centro-sur del país. Es más común en mujeres, adultos jóvenes y niños (28% son menores de 20 años). El cuadro clínico suele presentarse con fiebre, artralgia, poliartritis, dolor de espalda, cefalea y erupciones cutáneas. A pesar de que el tratamiento es sintomático y similar al del dengue, existen datos clínicos clave para diferenciarlas.
The term “chikungunya” is derived from the word kungunyala, from the African kimakonde language, which means “one who slouches” in reference to the stooped posture adopted by the people because of the unbearable joint and muscular pain1.
It is an arboviral infection caused by the chikungunya virus (CHIKV), which is transmitted by the bite of infected Aedes mosquitoes. The main clinical manifestations are fever, arthralgia, polyarthritis, backache, headache, and skin rash2. The treatment is symptomatic, with the application of analgesics.
2HistoryThe first CHIKV outbreak was reported in July, 1952, in an epidemic that occurred over the plateaus of Mawia, Makonde and Rondo, now Tanzania. However, retrospective studies suggest that the epidemic of CHIKV has occurred in Africa for centuries, with outbreaks exported to Asia and America in the 18th and 19th centuries although those outbreaks were documented as dengue. Some terms such as genoux, dengue, knokkelkoorts (knuckles fever), by the inhabitants of Batavia, aburokab (fever of the knees) by the Greeks, and 3-day fever in Calcutta, have been given to this epidemic1,3.
Testimony of this is the classic report widely cited as the first description of the dengue epidemic by David Bylon, a surgeon in the city of Batavia (Jakarta), who contracted the disease and wrote the following in 1779: “It was on May 25, at 5:00 in the afternoon when I noted that while I chatted with two good friends of mine, an increased pain in my right hand and forearm joint was step by step proceeding way up toward the shoulder and then continued towards all my limbs; and by 9:00 of the same afternoon I was already in bed with high fever… There have been 3 weeks since… I was hit by the disease, and because of that I had to stay home for 5 days; but even until today, I have a continuous pain and stiffness in joints of both feet with discomfort in both ankles, so much that when I wake up in the mornings, or sit for a while and start to move again, I cannot do it very well, and going up and down stairs is very painful”4.
This report of febrile illness of sudden onset with joints involved suggests that the disease was CHIKV. That same year in Cairo and Alexandria (Egypt) another outbreak of the disease took place with a strong resemblance to CHIKV, in which attack rates reached 40–50%. The soil of these plateau is highly permeable, so local residents need to store water. This results in large populations of Aedes (Stegomyia) aegypti, generally considered to be the domesticated form of A. aegypti which efficiently transmit arboviruses, such as yellow fever and dengue.
The virus became endemic in Africa by outbreaks that occurred in Uganda, Democratic Republic of the Congo, Zimbabwe, Senegal, Nigeria, South Africa and Kenya. Particularly in Uganda, it was found that the arboreal mosquito Aedes africanus becomes infected naturally, being the first evidence of the jungle CHIKV enzootic cycle3.
After the outbreak of 1952, the virus was spread to India and countries in Southeast Asia, a leader in epidemics during the following years. The first evidence of CHIKV outside of Africa came from Bangkok, Thailand, where the virus was isolated in 1958 during the outbreak, and associated with the transmission of A. aegypti, followed by several outbreaks in Cambodia, Vietnam, Malaysia and Taiwan. On the other hand, the first epidemic reported in India was in Kolkata (formerly Calcutta), West of Bengal, in 19631,3.
In 1964, antibodies were detected in non-human primates (NHPs) captured in the current Zimbabwe, and green monkeys were shown to be hosts able to amplify the transmission of CHIKV using mosquitoes. Since then, several studies have confirmed the role of the NHPs and A. africanus, A. furcifer and other arboreal mosquitoes as enzootic vectors1.
2.1Recent outbreaksIn 2004, a new outbreak emerged, which started at the coast of Kenya (Mombasa); in the next two years it spread to certain islands in the Indian Ocean, such as The Reunion, Comoros, Mayotte, Madagascar, Mauritius, Seychelles and Maldives. It is estimated that 500,000 cases occurred since the spring of 2004 until the summer of the 20065,6.
The epidemic spread from the islands of the Indian Ocean to India, where major outbreaks occurred in 2006. Once introduced, CHIKV spread to 17 of the 28 states of India, infecting more than 1.39 million people before the end of the year. The outbreak in India continued until 2010, with the emergence of new cases in non-affected areas during the initial phase of the epidemic. Outbreaks also spread from India to the Andaman and Nicobar Islands, Sri Lanka, the Maldives, Singapore, Malaysia and Indonesia through travelers who were in the viremic phase5.
Concern over the spread of CHIKV peaked in 2007, when it was detected that the virus was spreading in an autochthonous form (human-mosquito-human) in the North of Italy, after being introduced by a viremic traveler returning from India. The Italian Ministry of Health confirmed 160 cases in Rabean of which an 83-year-old man died. A year later, the first imported case was detected in Canada5,6.
In 2010, two cases not imported were detected in the Var region, in southeast France; other cases were imported in Australia, United States and Taiwan, while in October, ten cases were reported in southern China in the city of Dongguan. At the end of that year, a case was diagnosed in La Rioja (Spain) in a person who had visited the North of India shortly before. Subsequently, in September of 2013, a case was detected at the coast of Valencia in a surfer who had spent the summer in the Senegal coast5.
In 2013, the Panamerican Health Organization (PAHO) reported the first autochthonous cases in America, specifically on the island of St. Martin, in the Caribbean. In May 2014, it reported local circulation of the disease in several islands of the Caribbean such as Antigua and Barbuda, Haiti, Dominican Republic and St. Kitts, among others7. That same year the first case in Mexico occurred in a 39-year-old woman who traveled to the Caribbean on May 21st passing through the islands of St. Thomas, St. Martin and St. Kitts, to reach Antigua and Barbuda, where she remained until May 28th5,8.
Studies on CHIK virus in different geographical areas show the prevalence of three lineages with genotypic and antigenic distinctions. In Africa, two genotypes were attributed to epidemics and they kept the name of the region where they appeared, West Africa (WA) and the Eastern Central Region of South Africa (ECSA); and the Asian genotype was represented throughout Asia. However, the 2005–2006 epidemics included the introduction of the ECSA genotype to the Asian continent for the first time5.
3EpidemiologyCurrently, CHIKV disease has had a wide distribution in tropical and subtropical countries around the world, highlighting the Asian and African continents, the Western Pacific, the Caribbean, South America and, more recently, North America1.
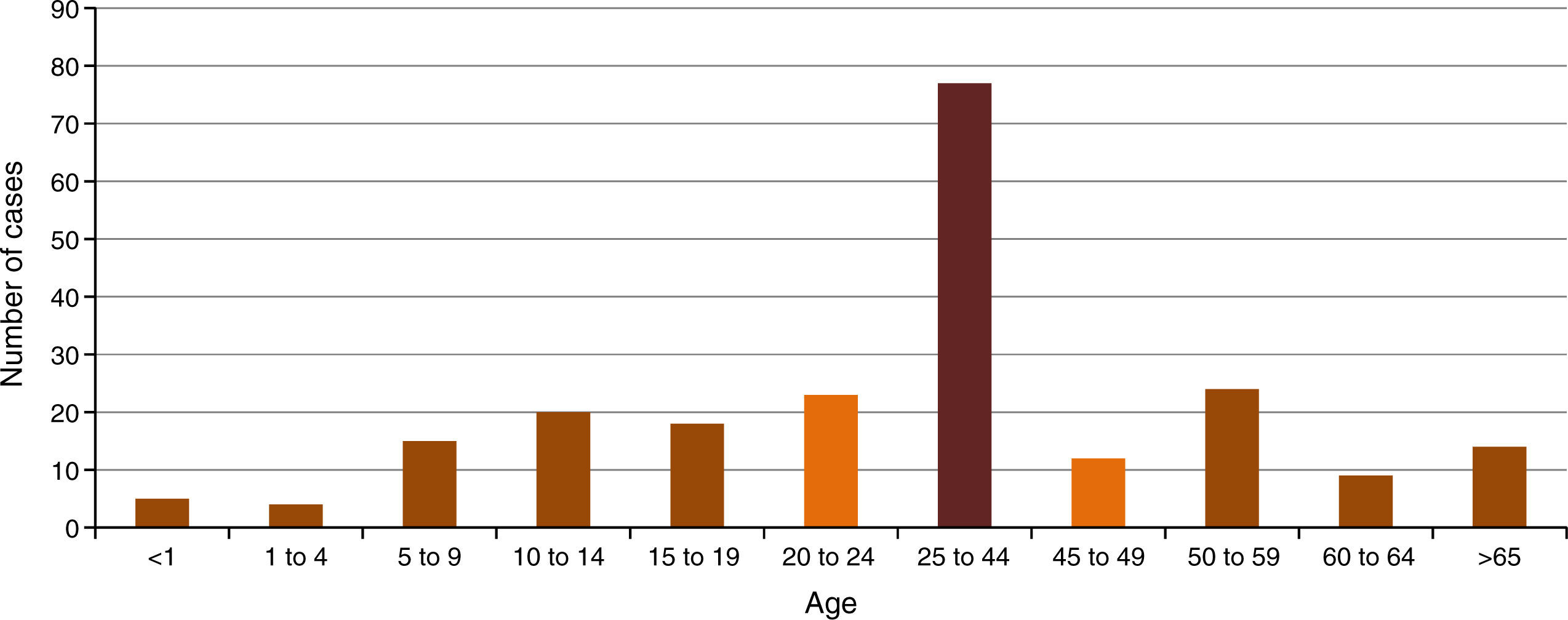
In Mexico, epidemiological surveillance started formally until 2014. However, in the data reported, a distinction between suspected and confirmed cases has been not made. In that year, data revealed that all groups of age were exposed to CHIKV. The most affected group was that of 25 to 44 years of age, with a 34.8%. Nevertheless, the population < 19 years of age represented 28% of the total population, which highlights the importance of the disease in pediatric population (Figure 1)9.
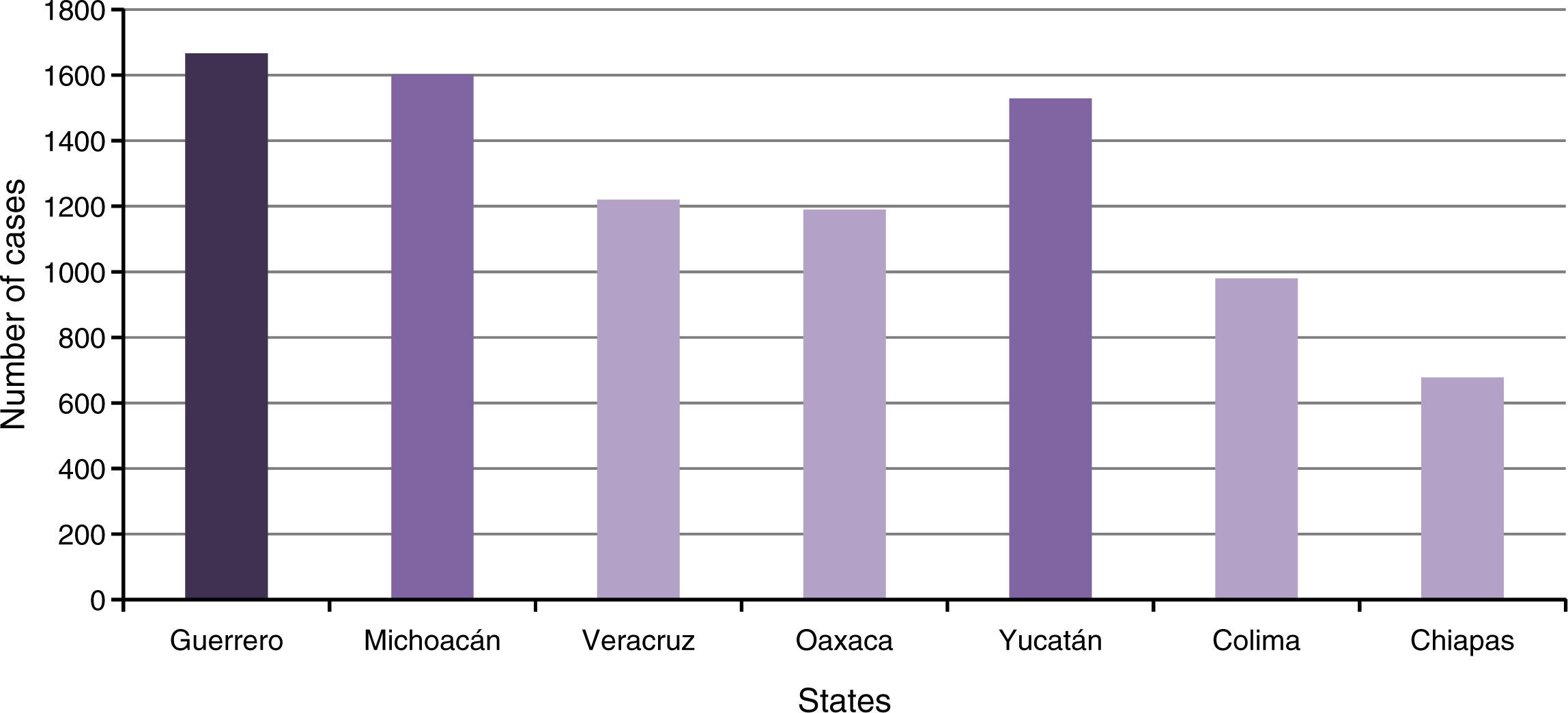
In 2015, there was a significant increase in reported cases, with a total of 11,394 cases (accumulated until December 12), compared to 222 cases in 2014. In terms of geographical location (Figure 2), mostly affected states are in the South of the country, which coincides with the trend of the vectors to settle in warm and humid zones10. Probably, low educational level in some of these states conditions a minor spreading and practice of preventive measures.
Women have been notably more affected than men, which may be explained with the cultural custom of women to work at home, where the main vector (A. aegypti) of CHIKV sets, usually associated with deposits of water. In Mexico, in 2015 (up to 12/12/15), the number of cases reported by gender were 7,285 female (65%) and 3,915 male (35%)4,10,11.
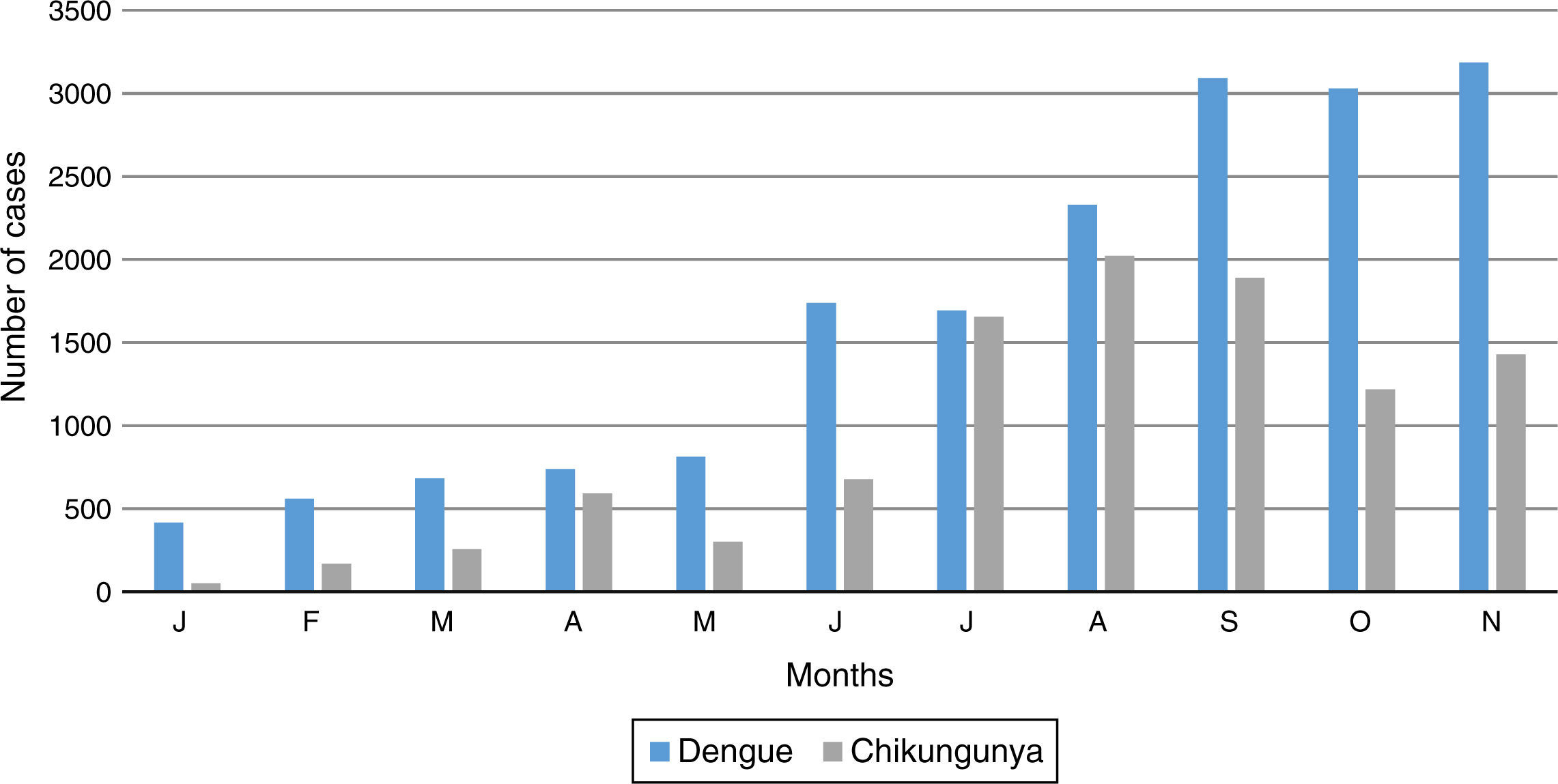
In 2015, the highest transmission of CHIKV occurred during the summer and then decreased over the course of autumn, unlike dengue which increased to its highest prevalence in November (Figure 3)10.
4EtiologyThe CHIKV belongs to the genus Alphavirus, which belongs to the family Togaviridae. It is a small size (65–70nm diameter) single-stranded RNA of positive sense virus, with a symmetrical icosahedral capsid, enveloped by 80 peaks with trimers of E1 and E2 glycoproteins in each one of them. E2 glycoprotein complexes join to unknown cell receptors and then enter the cell by endocytosis. The E1 glycoprotein includes a fusion peptide which promotes the release of nucleocapsides in the cytoplasm of host cell when it is exposed to low pH in endosomes1.
5MutationsA mutation was detected in 90% of the viruses isolated from the CHIKV outbreak on the island of Reunion, France, which began in March 2005 and ended in April 2006 registering 244,000 cases (123 severe cases, 41 cases of mother to child transmission, and 203 associated deaths). The mutation occurs at the level of residue 226 of the E1 glycoprotein of the fusion membrane (E1-A226V). It is postulated that this could facilitate the replication and transmission of the virus by reducing its dependence on cholesterol1.
6VectorsA. aegypti and A. albopictus are invasive species that inhabit tropical and temperate regions. Their ability to breed in artificial containers facilitates their passive spread through the main routes of transport. A. aegypti has domestic and peridomestic habits. Usually, it does not occupy territories located more than 1,000m above the sea level, although it has occasionally been found up to 2,400m. In contrast, A. albopictus has jungle habits and proliferates in wild environments, and it is responsible for the transmission of the virus in rural or semi-urban areas; this is due to the adaptive mutations of the viral genome, which increase its replication in this vector. Aedes eggs resist desiccation and extreme temperatures, staying viable from 7 months to a year, which significantly hinders the eradication of the vector. In America, many regions are susceptible to invasion and spread of this virus, and the recent emergence of urban epidemics of dengue fever in South America highlights the potential danger of chikungunya fever12,13.
The main vectors of the CHIKV in Asia and Indian Ocean are also Aedes aegypti and Aedes albopictus, but other species of Aedes (A. furcifer, A. vittatus, A. fulgens, among others) can also transmit the infection12.
7TransmissionThe transmission cycle requires infection of female mosquitoes through the blood of infected people. After an adequate extrinsic incubation period (at least 48h), it is transmitted to another host through the bite of infected female mosquitoes. The hosts remain viremic approximately until fever has gone10,12.
During outbreak periods, humans are the main reservoir of the virus, which remains in circulation between mosquitoes and humans while, in interepidemic periods, various vertebrates have been implicated as reservoirs (non-humans primates, rodents, birds and small mammals). Vertical viral transmission and through breastfeeding has been documented; furthermore, when infection occurs during delivery or shortly before, the transmission rate increases to levels close to 50%. There is a possibility of transmission through transfusion of blood or blood products13.
8PathophysiologyThe virus anchors to the cells with its E2 glycoprotein, generating its endocytosis; later, its RNA acts as mRNA to synthesize four non-structural proteins, which will influence the production of other proteins required for replication14. Prohibitine has been identified as a receptor for the virus15. CHIKV evades the immune system by means of the protein nsP2, which hydrolyzes RNA polymerase, inhibiting the transcription of some genes involved in antiviral response. Macrophages and sinusoidal endothelium of the liver have been identified as cellular reservoirs, and joints, muscle, dermis tissue and fibroblasts are targets to the virus. The presence of inflammatory infiltrate with viral replication has been documented in skeletal muscle and, in some cases, in synovial fluid12,16,17.
Inflammation of joints is mainly caused by adaptive immune response. IgM antibodies are present as early as 2 days after the onset of symptoms, and persist for weeks to months, while IgG is usually detected when the virus is eliminated, and persist for many years, which prevents the reinfection of virus13,18. The antibody response is mainly of the IgG3 isotype (neutralizing) and it is associated with the elimination of the virus13, although the persistence of anti-CHIKV IgM in serum, and of the virus in tissues of joints and in the endothelial reticulum system may explain the chronic arthropathy 12,14,16,19.
In the acute phase, the infection triggers the release of various cytokines, such as IL-6, G-CSF, GM-CSF, MCP1, TNF-¿, CXCL9, CCL2 and CXCL10, persisting only IL-17, IL-6, and GM-CSF, the latter two associated with arthralgia. There is also an increase in IL-18 induced by the Th1 response. This interleukin has a negative feedback because it induces the production of INFγ, which in turn induces the increase in the IL-18 binding protein (IL-18BP), a natural regulator of IL-1820. With respect to NK cells, these overexpress the CD94/NKG2C receptor, although the mechanism through which it interferes with the infection is still unknown5. It has been shown that the increase in IL-1¿ and IL-6 is related with a poor outcome.
The virus may spread to the CNS, showing tropism towards choroid plexuses, CSF, ependymal cells and meninges12,19. Regarding pregnancy, it has not been found in placental tissue. Increasing age is a risk factor for developing serious illness; however, in children, newborn are more susceptible12,13,16.
9Clinical featuresAfter intradermal inoculation of the virus by the mosquito, 72 to 97% of the patients develop the symptoms after an incubation period that can vary from 1 to 12 days (on average lasts from 2 to 4 days)13,16. Initially, the disease is characterized by a sudden onset of high fever with myalgia and severe arthralgia which may even interfere with sleep. Headache, photophobia, rash, weakness, fainting, confusion or deficit attention disorders can occur16,19.
Fever starts suddenly and usually exceeds 39°C, remains constant for 2–7 days until it suddenly stops13. Less than 33% of the patients have a second febrile peak21.
Joint affection is present in 70 to 100% of the patients, and it is characterized by severe arthralgia with subacute to chronic arthritis, joint effusion, stiffness and, in some cases, tenosynovitis may appear13,21. The condition tends to be symmetrical, mainly in the interphalangeal joints of hands and feet, metacarpal, metatarsophalangeal, ankles, wrists and knees12,13,16. The joint signs and symptoms are usually resolved within 1–2 weeks; however, in 12–57% of the patients, arthralgias persist in the same places for more than 3 months up to years, fluctuating in intensity and duration. People over 45 years with more severe pain initially, and especially those who have pre-existing osteoarthritis, are the most susceptible patients to chronic affection by CHIKV13,17.
Since the onset of fever until 5 days later, 50% of the patients develop vesicular or bullous dermatosis with desquamation, or maculopapular and petechial exanthema which is sometimes itchy and disappears with pressure (more often in adults); it affects the trunk, and can extend to palms, soles and face. It tends to be fleeting but it may persist over 2 days13,21,22. Hyperemia of the outer ear, which reflects chondral inflammation possibly related to infection by CHIKV, is also observed19.
Fifty percent of pediatric patients has 1 or 2 of the following symptoms: convulsions, loss of stool consistency or peripheral cyanosis21,22. Neurologic complications are frequent causes of hospitalization in these patients with CHIKV infection, and these are mainly febrile convulsions, viral encephalitis and acute encephalopathy19.
In a study conducted in pregnant women, in La Reunion, France, CHIKV infection was not associated with an increased frequency of abortions, but intrapartum vertical transmission or congenital malformations have been observed. In the first case, after the incubation period, the child has fever (79%), rash (82%), and peripheral edema (58%); regarding congenital malformations, a slight increase in the closure defects of the neural tube, chromosomal abnormalities, soft palate and cardiac defects (42%) were observed in children of women who were infected by CHIKV during pregnancy, although these data had no statistical significance to establish a causal relationship4,22. Cardiac defects include myocardial hypertrophy, ventricular dysfunction, pericarditis and dilation of the coronary arteries22.
Among the rare manifestations of CHIKV infection are hemorrhaging, such as conjunctival injection and bleeding of the higher or lower digestive tract, not necessarily related to the degree of thrombocytopenia. Also, myocarditis, Guillain-Barré Syndrome, Raynaud’s disease, loss of auditory acuity (related to the chondritis), uveitis or infectious retinitis have been observed13,18.
10Confirmatory diagnosisThe gold standard for confirmation of chikungunya fever is real time polymerase chain reaction (RT-PCR) directed toward the gene of the non-structural protein of CHIKV (NSP1) or the gene of the viral envelope of CHIKV (E), either in blood or synovial fluid21.
Other methods to confirm the diagnosis are the detection of response to antibodies with ELISA, indirect immunofluorescence, or reverse transcription loop-mediated isothermal amplification (RT-LAMP)8. However, although the anti-CHIKV IgM appears after 2–3 days since onset of symptoms, titers do not reach detectable concentrations by ELISA until the first or second week, and may be present until a year later5,23.
Specific IgG for CHIKV rises 10–13 days after the onset of symptoms and persists with high titers for years, predominantly in the form of IgG31. The persistence of high titers of immunoglobulins and its correlation with chronic disease suggest an impaired antigenic depuration in these patients22.
Hematic cytometry often shows leukopenia and sometimes very mild thrombocytopenia (100,000 platelets/mm3). Unlike other viral diseases, markers of inflammation, such as the sedimentation rate of erythrocytes and C-reactive protein, tend to be high, and there is also an increase in hepatic transaminases.
Joint radiographs show no alterations except in the chronic condition after 1 year, in which there are signs of erosive arthritis and reduction of the articular space8.
Since there are often difficulties to perform the confirmatory diagnosis of this disease, the PAHO suggests the following definitions24:
Suspected case: patient with fever > 38°C (101°F) and severe arthralgia or arthritis of acute onset not explained by other medical conditions, and that endemic or epidemic areas have been affected during the two weeks prior to the onset of symptoms.
Confirmed case: suspected case with a positive specific test for CHIKV (viral isolation, RT-PCR, IgM, or a four-fold increase in titers of antibodies for chikungunya).
11Differential diagnosisDengue is the most alike and most often confused disease with chikungunya fever, partly because they share the same vector (A. aegypti) and, therefore, its same geographic space. The most important clinical data to differentiate it with dengue is the severe polyarthralgia, which is much more frequent in chikungunya fever; however, it should also be considered that it shows a more abrupt start, and a higher but of less duration fever than dengue. Rashes, conjunctival injections and arthralgias are more frequent in chikungunya fever than in dengue (Table 1)16.
Clinical comparison between fever by dengue and by chikungunya.
| Clinical features | Chikungunya fever | Dengue |
|---|---|---|
| Fever | +++ | ++ |
| Myalgia | + | ++ |
| Arthralgia | +++ | +/− |
| Rashes | ++ | + |
| Blood dyscrasia | +/− | ++ |
| Shock | − | +/− |
| Leukopenia | ++ | +++ |
| Lymphocytopenia | +++ | ++ |
| Neutropenia | + | +++ |
| Thrombocytopenia | + | +++ |
Average frequency of symptoms from studies where both diseases were compared;+ + + = 70–100% of patients; + + = 40–69%; + = 10–39%; +/− = < 10%; − = 0%.
Adapted from OPS/OMS. Preparation and response against Chikungunya virus in the Americas, 2010 (reference 15).
Other laboratory data for the differential diagnosis are normocytic normochromic anemia, hemoconcentration and thrombocytopenia and neutropenia, which are usually found in dengue and not in chikungunya fever, in which lymphopenia is more common16.
Zika virus infection is a new Aedes transmitted disease that is spreading aggressively in Africa, South and Central America. Its clinical features are similar to those of chikungunya fever and dengue; but maculopapular rash, non-purulent conjunctivitis are seen more often in Zika virus infection. Of these three entities, Zika virus infection has the higher rate of neurological and teratogenic complications, particularly Guillain-Barre syndrome and microcephalus, respectively. It is very important to make a confirmatory diagnosis by RT-PCR25–27.
Leptospirosis should be discarded in patients with a history of contact with water, mud, or rodents in an endemic area. In this disease, it is common to find serious myalgia in gastrocnemius muscles, with or without oliguria and jaundice, neutrophilia and elevated titers of creatine phosphokinase13.
To clinically rule out malaria, the febrile pattern should be observed, which tends to be periodic in this pathology. Rheumatic fever should also be considered if the disease presents an asymmetric and migratory polyarthritis that involves mainly the large joints, and complies with the Jones criteria4.
In some parts of the world there are other endemic arthritogenic alpha viroses. The Mayaro fever is typical of forest areas and has a very similar presentation than chikungunya fever, although it shows a little less frequency in joint involvement (50–90%); it also has peripheral edema (58%), retroorbital headache (40–60%), dizziness (25%), sore throat (18%), lymphadenopathy (17%), vomiting (4–14%), diarrhea (9%), and gingivorragia (5%)20. Other types of fever by alphavirus with very similar symptoms are caused by the Ross River virus and the Barmah Forest virus in Australia and some Pacific Islands, the Onyong-nyongvirus in Africa, and the Pogostavirus in Finland, among others. Therefore, it should be considered if there is a history of a recent trip to or of origin from any of these sites, although treatment does not vary much16.
12TreatmentThere is still no specific or effective antiviral treatment which reduces the therapeutic measures to physical methods, and the administration of acetaminophen (symptomatic treatment) for treating fever3,7. If acetaminophen does not work, NSAIDs or narcotics can be used28. Research is currently carried out on treatments with CHIKV antibodies, IFN-¿, chloroquine and rivabirin; the latter has been used in some severe forms but its effectiveness has not been confirmed8,12,15. Chloroquine could interfere with the entry of the virus to cells, while rivabirin is an inhibitor of viral replication28.
There are other drugs, but their effectiveness is still not evaluated in humans. The use of inhibitors of CCL2 has reduced the inflammatory infiltrate in muscle and joints in mice infected with CHIKV19. For chronic rheumatic manifestations and polyarthritis that last more than 2–3 months, the use of antirheumatics such as methrotrexate is recommended12.
13PrognosisChikungunya fever has a very low mortality rate (1 in 1,000); however, the recovery can be prolonged and arthralgia may persist for months or even years12,19. Pediatric and elderly patients are the groups with most risk of dying because CHIKV, being the main causes of death hepatitis and encephalitis in childhood, and heart failure and multiple organ failure in the eldery16,17.
14PreventionChikungunya fever prevention measures are based on avoiding mosquito stings, killing the mosquitoes and avoiding the formation of A. aegypti breeding places; just as for other diseases transmitted by these vectors5.
The evasion of mosquito bites can be done in different ways, using topic repellents such as n-diethyl-m-toluamide (DEET), which in children is recommended in concentrations below 30% and should be applied directly on the exposed skin avoiding contact with mucous membranes; or environmental repellents (insecticides, such as permethrin), so long sleeves that can be sprayed with (resists 4–5 washes); avoiding the use of perfumes that attract mosquitoes; installation of air conditioning, mosquito nets in windows and beds13.
The elimination of the vector can be done with insecticides or physical, or biological methods; however, at endemic areas, it is easier to avoid the accumulation of water and waste, periodically emptying containers that accumulate water in yards. If accumulation cannot be avoided, a light oil film can prevent the deposit of eggs, or a larvicide such as temephos can be used. There are some studies in cities of Vietnam where they promoted the proliferation of Copepods, a group of small crustaceans that eat mosquitoes, and they obtained positive results in the regulation of mosquitoes in the urban zone, and in dengue incidence29.
There have been some attempts to develop chikungunya vaccines. The US Navy has developed a promising attenuated vaccine that appeared to be safe and effective, but research has been discontinued because the military interests of this institution have been modified. Inactivated vaccines with good protective response for CHIKV, such as the detergent-inactivated vaccine and the formalin-inactivated vaccine, have also been developed but there is still not enough support of clinical research. Recently, a chikungunya virus-like particle vaccine and a recombinant measles-virus-based chikungunya one have been tried, each one in a short phase I trial (25 and 26 patients, respectively), in which both showed the induction of chikungunya virus neutralizing antibodies in 100% of the patients. Further investigation on these potential chikungunya vaccines is needed. Aside from preventing the long-lasting effects of the disease, a chikungunya virus vaccine will reduce the economic and social impact that the disease has in endemic countries due to health care costs, loss of working days and impairment in tourism, which is a very important source of income in a lot of countries17,30.
Conflict of interestThe authors declare no conflict of interest of any nature.