Helicobacter pylori is usually acquired during childhood and remains in the gastric mucosa for years, often lifelong if untreated. It can be concluded that the gastric mucosa of children actively responds to the presence of H. pylori. Current evidences suggest that whereas H. pylori infection rarely causes peptic ulcers or gastric atrophy in children, it seems to be associated with iron deficiency and iron deficiency anemia; the evidence also suggests the infection may cause growth retardation. In contrast, H. pylori infection has been associated with a reduced risk of asthma and allergy in children and adults; also, epidemiological studies suggest that there is an inverse association between H. pylori infection and risk for esophageal adenocarcinoma. The gastric mucosa of children elicits a significant inflammatory response in the site of infection, with increased expression of toll-like receptors (TLRs) and cytokines, and increased epithelial proliferation. This response may partly be responsible for the required "immune training" needed to protect for the development of esophageal cancer, asthma, allergy or even diabetes later in life. The response may as well be associated with growth retardation, iron deficiency and increased risk for enteric infections. It then seems that our co-evolution with H. pylori has rendered benefits for human health making clear that this relationship is complex and the decision to eradicate the infection should be taken with caution.
Helicobacter pylori is usually acquired during childhood and remains in the gastric mucosa for years, often lifelong if untreated. It can be concluded that the gastric mucosa of children actively responds to the presence of H. pylori. Current evidences suggest that whereas H. pylori infection rarely causes peptic ulcers or gastric atrophy in children, it seems to be associated with iron deficiency and iron deficiency anemia; the evidence also suggests the infection may cause growth retardation. In contrast, H. pylori infection has been associated with a reduced risk of asthma and allergy in children and adults; also, epidemiological studies suggest that there is an inverse association between H. pylori infection and risk for esophageal adenocarcinoma. The gastric mucosa of children elicits a significant inflammatory response in the site of infection, with increased expression of toll-like receptors (TLRs) and cytokines, and increased epithelial proliferation. This response may partly be responsible for the required "immune training" needed to protect for the development of esophageal cancer, asthma, allergy or even diabetes later in life. The response may as well be associated with growth retardation, iron deficiency and increased risk for enteric infections. It then seems that our co-evolution with H. pylori has rendered benefits for human health making clear that this relationship is complex and the decision to eradicate the infection should be taken with caution.
Introduction
H. pylori infects the gastric mucosa of almost 50% of the world population, although major differences in prevalence occur among countries; infection is more prevalent in developing than in developed countries.1H. pylori is usually acquired during childhood and remains in the gastric mucosa for years, often lifelong if untreated. The infection invariably causes a chronic long-lasting inflammation of the gastric mucosa that in most cases causes no symptoms, although a fraction of those infected will develop gastric cancer, peptic ulcer or gastric MALT lymphoma decades after a chronic irritating inflammation.2 During the acute phase of the infection, early in childhood the infection is associated with a period of achlorydria followed by normalization of the pH and the establishment of chronic inflammation.
Adverse effects of H. pylori infection
In spite of the fact that infection starts in childhood, little is known about the clinical implications in this age group.3 There is still controversy in the role of H. pylori in chronic abdominal pain where some groups still treat the disease by giving antibiotic eradication treatment, whereas others have reported no significant effect on the evolution of the episodes.3 In our pediatric hospital it has been observed that eradication of the infection has no clinical benefit in children with recurrent abdominal pain and we currently discourage antibiotic therapy. The development of peptic ulcers usually takes decades and is very rarely reported in children, although cases have been reported in Ural Russia and Eastern Europe.4 In the Gastroenterology Service of our pediatric hospital we have observed only four cases of peptic ulcer in 500 consecutive patients attended during the period from 1994-2003; furthermore, the clinical data suggested these cases were not due to H. pylori infection. Gastric atrophy normally appears in adults although it can be seen in children living in regions with high risk for gastric cancer.5 In the same cohort of 500 cases reported above, we did not observe any case of atrophic gastritis in the studied children. These observations suggest that H. pylori infection rarely cause peptic ulcers or gastric atrophy in children of our community.
Colonization of the gastric mucosa by H. pylori causes a temporal reduction in acid production and this period of partial achlorydria may have other consequences. One is that the increase in pH lowers the acidic barrier. It has been suggested that children are exposed to a higher risk to acquire infections with enteropathogens, resulting in an increase of diarrheal diseases, an hypothesis suggested by some but not all groups.6-9 A prospective study followed a cohort of recently infected children and demonstrated that the increased risk for diarrheal episodes was observed only for a few weeks just after H. pylori infection was acquired.10 The increased pH may affect non-heme iron solubility, and the inflammation in the gastric mucosa may alter epithelial functions. Both may lead to reduced iron uptake, increasing the risk for iron deficiency and iron deficiency anemia.9,11 Indeed, studies have shown a higher prevalence of iron deficiency anemia in children with H. pylori infection. It has also been documented that in these children anti-H. pylori therapy plus iron supplementation improves significantly serum ferritin and hemoglobin, as contrasted by giving only iron supplementation.12,13
The effect of H. pylori on the growth of children has also been studied with controversial results. The infection has been found associated with growth retardation or short stature and with altered weight and height.14 Other studies reported only a decrease in weight for height15 or only in growth but not weight16 in H. pylori-infected children. However, a cohort study clearly demonstrated the long-term benefit of eradication of H. pylori in the recovery of the rate of growth in children followed for almost 4 years.17
We recently reported that co-infection of H. pylori with Epstein-Barr virus in children is associated with a more severe gastritis compared with single H. pylori infection, revealing a previously unknown cooperative effect of these infections to cause more serious damage during childhood.18 This finding should be pursued further as co-infection could be used as a biomarker to identify children at increased risk to develop serious disease later in life.
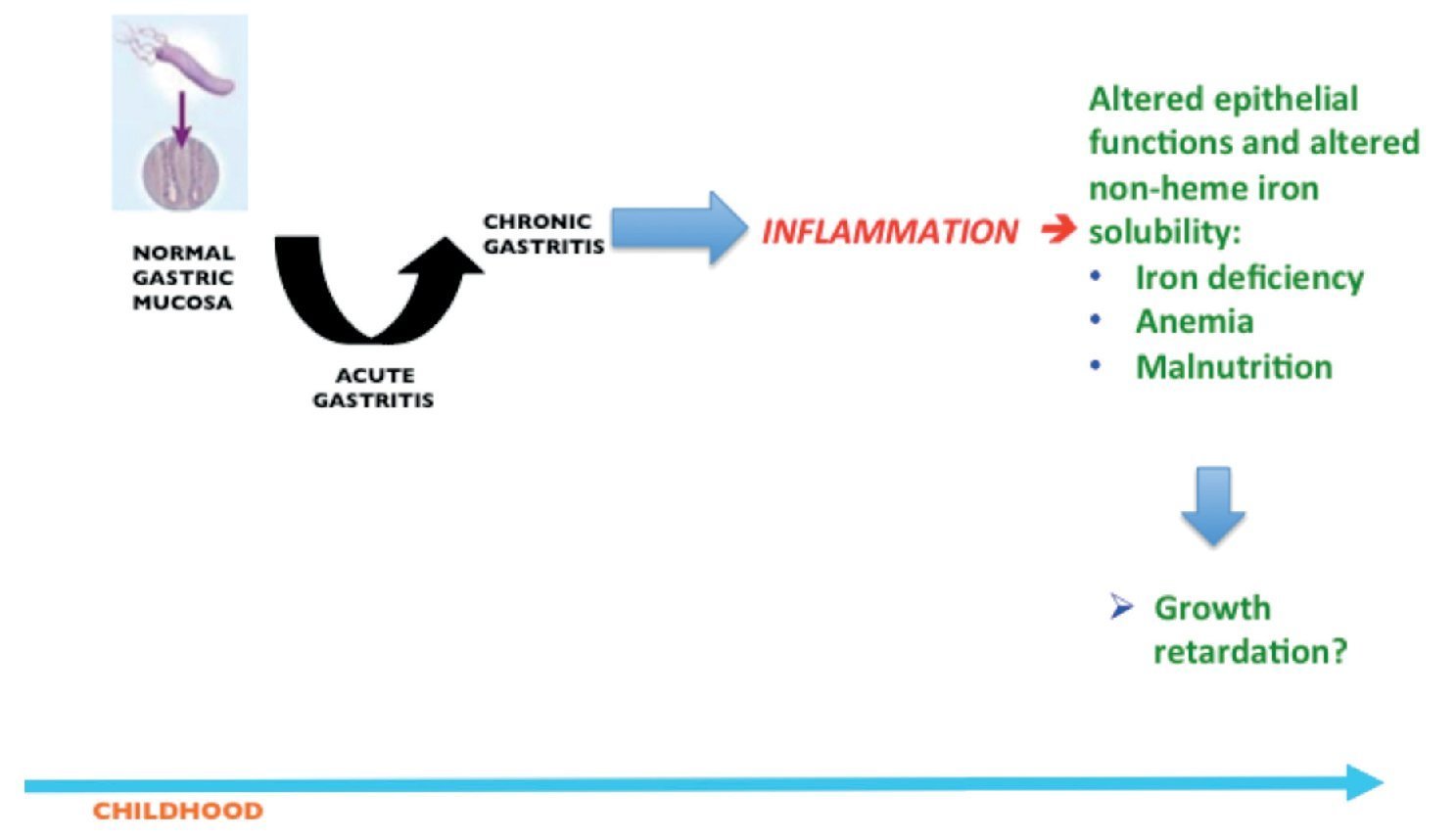
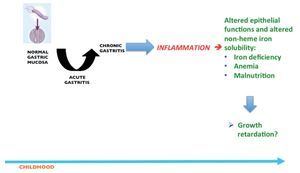
Thus, current evidences suggest that whereas H. pylori infection rarely causes peptic ulcers or gastric atrophy in children, it seems to be associated with iron deficiency and iron deficiency anemia. Evidence also suggests the infection may cause growth retardation and probably a transient increase in risk to acquire enteric infections (Fig. 1).
Figure 1 Unwanted outcomes in children after the inflammatory response induced in the gastric mucosa as a consequence of H. pylori infection.
Is there any benefit of the infection to the host?
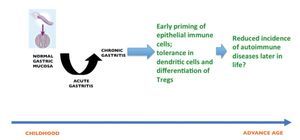
It has been argued that H. pylori has been part of the human microbiota for millennia and has evolved to successfully colonize and establish a persistent infection in the human stomach without causing disease in most infected individuals. Bacteria that are considered as normal microbiota usually interact symbiotically by performing vital functions to its human host. If we accept that H. pylori has co-evolved with humans for thousands of years, it is difficult to conceive it as a pathogen, and we are now learning which particular circumstances represent a treat for increased risk to develop a disease. The other side of the story is what benefit can H. pylori have? Epidemiological studies suggest that there is an inverse association between H. pylori infection and risk for esophageal adenocarcinoma, particularly with the more virulent cagA+ strains.19 In fact it has been suggested that the marked decline in H. pylori infection during the last decades may be partly responsible for the recent increase in esophageal adenocarcinoma incidence in Western countries.20 In addition, H. pylori infection has been associated with reduced risk of asthma and allergy in children and adults,21,22 which is consistent with the "hygiene hypothesis" that state that early exposure to microbes prevents the later development of allergic diseases. The mechanism behind this protection is unknown, although recent reports have documented that in a mouse model, H. pylori infection prevents the development of asthma most probably by induction of Tregs cells.23 This finding was further supported by the observations that dendritic cells become tolerogenic after exposure to H. pylori which, in turn, drive Treg differentiation with production of IL-18 and protection from asthma (Fig. 2).24
Figure 2 H. pylori infection during childhood elicits an early priming of the immune system, and induce tolerance in dendritic cells followed by differentiation of Tregs; these events are probably related to the observed protection against autoimmune diseases and esophageal cancer later in life.
The response of the gastric mucosa to the infection
An important issue is to understand the extent and nature of the response of the gastric mucosa of children to H. pylori infection. We engaged in a large study to address this issue in almost 600 children recruited from 1994 to 2003 in a Pediatric Hospital in Mexico City; children attended the Gastroenterology Service mainly because of recurrent abdominal pain. We included only those cases that required endoscopy and biopsies as part of their protocol for diagnosis. A sample of blood was drawn and studied for antibodies and biopsies from antrum and corpus were fixed in paraffin for immunohistochemical studies. Another fraction of biopsies was used to study expression of inflammatory mediators. We first determined production of IL-8 by the gastric epithelial cells and found that expression of IL-8 was significantly higher in the antrum and corpus of H. pylori infected children as compared with uninfected children.25 Mainly cells in the surface and crypts of the epithelium expressed IL-8.
We also analyzed the expression of toll-like receptors (TLRs) and cytokines in the gastric mucosa and found that the expression of TLRs 2, 4, 5 and 9 was significantly increased with H. pylori infection. Also, the expression of IL-8, IL-10 and TNF-α was increased in the mucosa of infected children.26
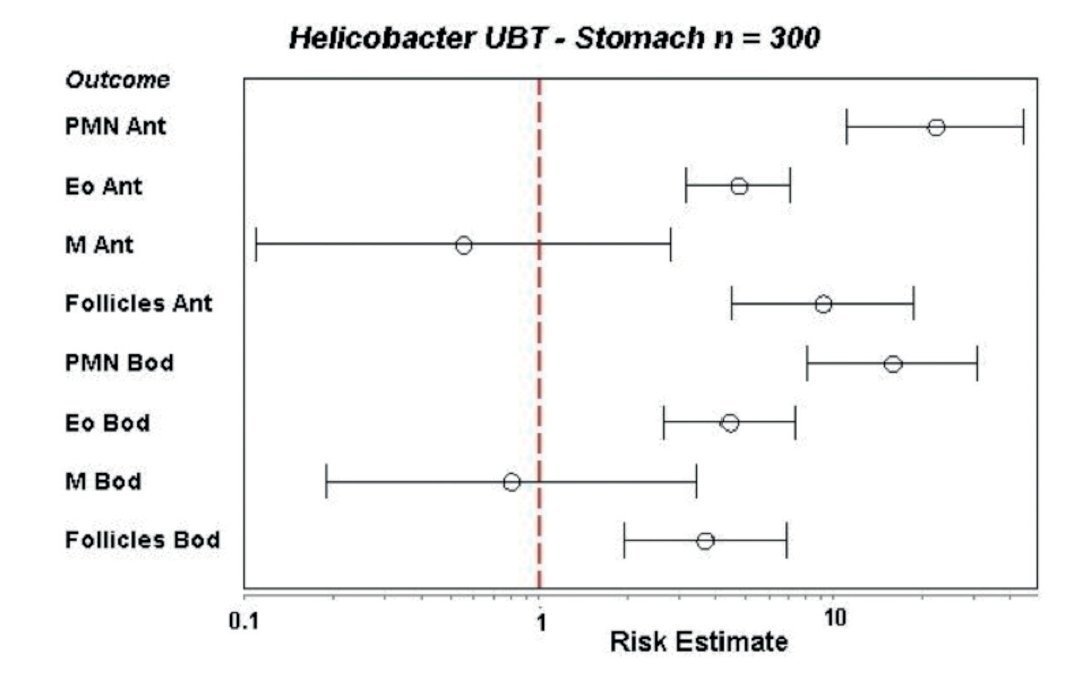
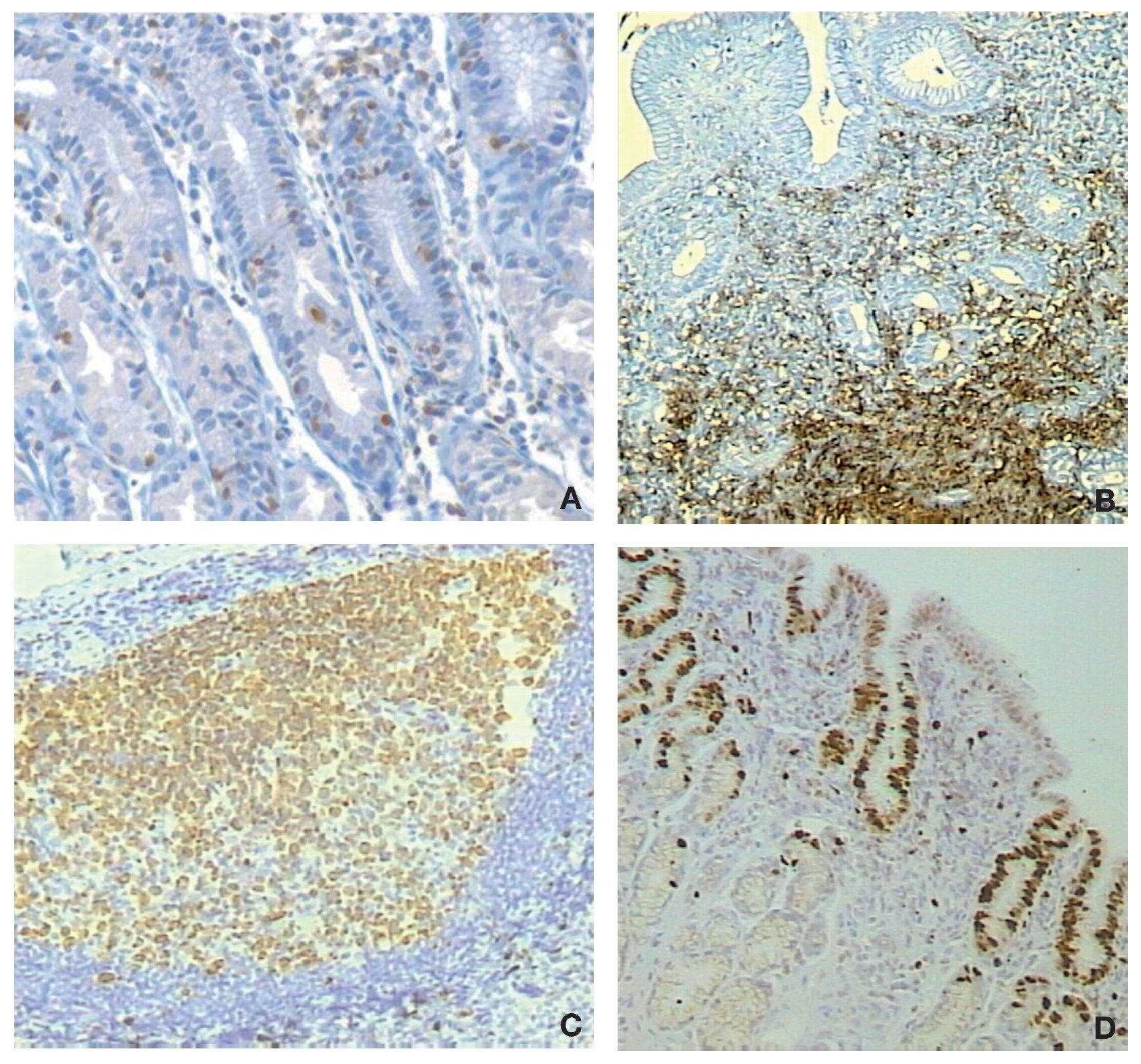
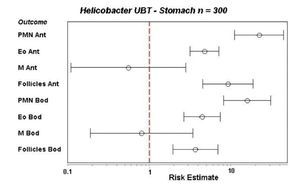
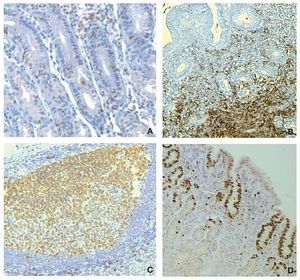
Next, we studied the recruitment of inflammatory cells to the gastric mucosa in response to the infection. For this, 300 cases were analyzed for inflammatory cells in biopsies of both regions of the stomach, the antrum and corpus using the modified Sydney system to grade the intensity of infiltration.27 Biopsies were stained with hematoxylin and eosin for polymorphonuclear (PMN), mononuclear (MN) and eosinophils cells and for the presence of lymphoid follicles. For proliferation studies we used an anti-K67 monoclonal antibody and for mast cells a monoclonal anti-tryptase antibody, whereas T and B lymphocytes were stained with a commercial integrated system.28 We found that the infiltration of PMN, eosinophils and lymphoid follicles was significantly higher in the infected children as compared with the uninfected children, both in the antrum and the corpus of the stomach. In contrast, the infiltration of MN was not different between infected and uninfected children (Fig. 3). Proliferation in the gastric epithelium was also significantly higher in H. pylori infected children, whereas infiltration of activated mast cells was lower during infection. T and B lymphocytes were significantly higher in the gastric mucosa of H. pylori infected children, observing a balance increase of CD4, CD8, and D20 lymphocytes (Fig. 4).28 From these results we may conclude that the gastric mucosa of children elicits a significant inflammatory response recruiting PMN and eosinophils to the site of infection, although H. pylori fails to induce the recruitment of MN and mast cells. The infection also induces a proliferative response in the gastric epithelium and an increases the expression of TLRs and cytokines.
Figure 3 Recruitment of inflammatory cells to the gastric mucosa of children after H. pylori infection, as referred to the recruitment in uninfected children (dotted red line, indicated as 1 on the X axis). Infiltration of polymorphonuclear (PMN), eosinophil (Eo) and lymphoid follicles (Follicles) was significantly higher in the infected group in both antrum (ant) and body (Bod) of the stomach. Mononuclear cells (M) were not increased.
Figure 4 Altered recruitment of cells in the gastric mucosa of children infected with H. pylori. A) Increased intra-epithelial infiltration of T-lymphocytes; B) Increased infiltration of B lymphocytes in the submucosa; C) Formation of lymphoid follicles; D) Increased proliferation of epithelial cells beyond the neck cells area.
The gastric mucosa of children actively responds to the presence of H. pylori. This response may be partly responsible for the required "immune training" needed to protect for the development of esophageal cancer, asthma or allergy later in life. The response may as well be associated with the unwanted outcomes seen during childhood-growth retardation, iron deficiency and probably increased risk for enteric infections. It then seems that our co-evolution with H. pylori has rendered benefits for human health, making clear that this relationship is not a black and white history, and the decision to eradicate the infection should be taken with caution at this point. On one side, eradication at early ages would presumably eliminate the benefit for prevention of future diseases like esophageal cancer, asthma and allergy, whereas on the other side persistent infection for decades increases the risk for peptic ulcer or gastric cancer. Perhaps a wise decision will be to find the point where we earn the most and lose the least of the relationship and eradicate H. pylori when risk for disease is higher than for protection, probably between the ages of 40 and 50 years.
Funding
The work performed in the laboratory of JT was partly financed by Fondo de Investigación, Instituto Mexicano del Seguro Social, México.
Conflict of interest
The authors declare no conflict of interest of any nature.
Acknowledgments
JT is a recipient of a scholarship from Fundación IMSS, México.
Received 8 November 2013;
accepted 12 December 2013
* Corresponding author.
E-mail address:jtorresl57@yahoo.com.mx (J. Torres).