Dengue continues to increase globally. Currently, the highest incidence of primo-infection occurs in the pediatric population, where severe dengue fever is potentially lethal. This study characterizes the clinical profile of pediatric patients with dengue fever in the South of Mexico.
MethodsWe undertook a case series study of 133 pediatric inpatients with a clinical diagnosis of non-severe dengue and severe dengue fever. We described univariate analysis as means or percentages using a p<0.05 as significance level and estimated the prediction of severe dengue using a GLMM (Generalized Linear Mixed Model) considering only clinical signs and symptoms.
Results58% of patients (77/133) had severe dengue. There were significant differences among the dengue groups in the following signs and symptoms: fever, abdominal pain, epistaxis and platelet count. Children older than four years of age had a higher proportion of severe dengue (p<0.05). GLMM identified a group of four clinical signs and symptoms (fever ≥39°C, myalgia, arthralgia and abdominal pain) as predictors of severe dengue.
ConclusionsThe results of this exploratory study suggest changes in the frequency of clinical signs and symptoms among pediatric inpatients. Pediatric patients with a presumptive diagnosis of dengue, showing fever of ≥ 39°C, myalgia, arthralgia and abdominal pain should be considered as potential cases of severe dengue.
El dengue sigue en incremento a nivel mundial y actualmente la mayor incidencia de primera infección ocurre en población pediátrica. El dengue grave es potencialmente letal en menores de edad. Este estudio caracteriza el perfil clínico de pacientes pediátricos con dengue atendidos en un hospital de segundo nivel de atención en Chilpancingo, Guerrero, México.
MétodosSerie de casos conformada por 133 pacientes pediátricos hospitalizados con diagnóstico de dengue no grave y dengue grave, de acuerdo a criterios clínicos. Los resultados del análisis univariado de los signos y síntomas clínicos fueron expresados como promedios o porcentajes, y se consideró nivel de significancia estadística de 0.05. Mediante GLMM (Generalised Linear Mixed Models) se estimó la predicción de dengue grave con la presencia de signos y síntomas clínicos.
ResultadosEl 58% (77/133) de los pacientes fue clasificado como dengue grave. Hubo diferencias significativas, entre los grupos de dengue, en los signos y síntomas siguientes: fiebre, dolor abdominal, epistaxis y cuenta plaquetaria. El dengue grave se presentó en mayor proporción en los pacientes mayores de cuatro años de edad (p<0.05). El GLMM identificó un conjunto de cuatro signos y síntomas clínicos (fiebre ≥39°C, mialgias, artralgias y dolor abdominal) como predictores de la gravedad del dengue.
ConclusionesLos resultados de este estudio exploratorio sugieren cambios en la frecuencia de síntomas y signos clínicos del dengue en la población pediátrica. Pacientes pediátricos con diagnóstico presuntivo de dengue que presenten fiebre ≥39°C, mialgias, artralgias y dolor abdominal deben considerarse como potenciales casos de dengue grave.
Each year, approximately 390 million cases of dengue occur worldwide, and only 25% are detected by public health surveillance systems1. Nearly 500,000 people suffer severe dengue each year, children in a significant proportion, and approximately 2.5% die2. In Mexico, dengue manifests as sporadic outbreaks which are 12-14 years apart and due to the population acquired immunity, a transition to the pediatric population and an increment in severe dengue cases occurs3.
Usually, primo-infection with any of the virus’ four serotypes happens during childhood. An increased risk of dengue virus infection in children older than 5 years old has been documented4, although there are case reports of neonatal dengue virus transmission5,6. Dengue presents as two clinical scenarios: non-severe and severe dengue. The immune response to different serotypes seems to influence disease severity7; nevertheless, García-Campos et al. have described a case of severe dengue in a breastfed infant presenting with primo-infection8. Serotype 2 dengue virus is associated with a more severe inflammatory response and the most severe cases of primo-infection have been attributed to this serotype9. The pathophysiology of severe dengue is multifactorial and it has not been completely elucidated10,11.
Clinically, non-severe dengue is characterized by fever, headache, retro-ocular pain, myalgia and arthralgia, nausea and vomiting, lymphangitis and exanthema. Some patients present with intense abdominal pain, persistent vomiting, tachypnea, petechiae and other hemorrhagic manifestations, as well as neurological signs and a mild altered mental status, which are considered as warning signs12,13. Besides the signs and symptoms previously described, patients with severe dengue present with signs of plasma leakage or severe bleeding and severe end-organ damage14,15. Any clinical sign or symptom, or any laboratory value that helps to distinguish a severe from a non-severe dengue may be crucial to prevent the patient from dying16,17. In the pediatric population with dengue, high fevers18, abdominal pain 19, age older than 6 years, hepatomegaly and thrombocytopenia (<50,000/mm3) have been described as strong predictors of the severity of the disease20. Measuring platelet counts may be unavailable in primary care level health units. Dengue shock syndrome is a potentially lethal complication of severe dengue virus infection, especially in children21,22; therefore, maintaining hemodynamic stability is the usual recommendation for inpatient management of dengue23–25.
Dengue is endemic in Mexico, particularly in the south-southeast regions and the coasts of the Gulf of Mexico and the Pacific Ocean26. In 2014, the pediatric population had the greatest incidence of dengue27. The state of Guerrero, in 2007, had the second largest incidence rate of hemorrhagic dengue in the country; circulation of the four serotypes was documented28. In 2014, there were 1,992 confirmed cases of dengue, with 35.7 cases of non-severe dengue per 100,000 population and an incidence of severe dengue of 20.5 cases per 100,000 population. In 2015, the seroprevalence for dengue virus recent infection was 9% among children of three to nine years old of the coastal region of the state of Guerrero29.
In 2006, a study in Tamaulipas, Mexico, found no clinically relevant characteristics of dengue virus infection in pediatric patients30. Nearly a decade after and given the changes in dengue virus dynamics, we considered necessary to revise some aspects of this disease. In this study, the clinical profile of non-severe and severe dengue, and the predictive variables of severity of dengue in pediatric patients hospitalized in a secondary care hospital dependent on the Ministry of Health in Chilpancingo, Guerrero, Mexico are reported.
2MethodsA case series of 133 patients with a dengue virus infection, hospitalized in the Pediatric Service of the Hospital General Dr. Raymundo Abarca Alarcón from January 2013 to December 2014 were studied. Two groups of patients were integrated according to the classification of non-severe and severe dengue, using the diagnostic criteria established by the World Health Organization31.
The following operational case definition of non-severe dengue was used: a patient with headache, fever, retro-ocular pain, arthralgia, myalgia, exanthema, with or without warning signs (intense abdominal pain, persistent vomit, tachypnea, hemorrhagic signs and neurological disturbances or altered mental status).
The operational case definition of severe dengue included the previous clinical picture for non-severe dengue, with one or more of the following manifestations:
- a)
Plasma leakage signs, characterized by a shock syndrome caused by dengue (differential blood pressure of < 20mmHg) or tachycardia and cutaneous manifestations of peripheral vasoconstriction; fluid accumulation with acute respiratory distress syndrome (ARDS), pleural effusion or ascites; increased hematocrit (greater than 20% of the basal average for age and gender) or a progressive increase
- b)
Severe hemorrhage, represented by evident bleeding, with anemia, hematocrit changes and hypovolemic shock
- c)
Severe end-organ compromise, with liver damage (jaundice, acute liver failure, encephalopathy) or gastrointestinal (persistent vomit, progressive or intense abdominal pain), altered mental status and profound neurologic disturbances (lethargy, restlessness, coma, seizures or encephalitis), cardiac compromise (cardiomyopathy) and kidney injury (acute kidney injury) or other organs.
Given that none of the cases had confirmatory serology or dengue virus isolation, the diagnosis was clinical; all patients had a discharge diagnosis of dengue virus infection. Data were obtained from the official form for the “Epidemiologic study of fever and hemorrhagic fever caused by dengue virus infection”, issued by the General Direction of Epidemiologic Surveillance of Mexico's Ministry of Health32. In addition to the signs in the patient's clinical profile, this form includes information about the following variables: age, gender, municipality of residence, ethnic group, date of hospital admission, date of first contact with health services due to the probable diagnosis of dengue virus infection, travelling to other locations in the previous 15 days, and laboratory results such as hematocrit, hemoglobin, platelets, and albumin.
The study complied with the institutional proceedings and requirements in health research, and the approval of the Ethics Committee of the General Hospital Dr. Raymundo Abarca Alarcón dependent on the state's health services. Obtained information was anonym, data that could allow patient identification were not registered.
The size of the study was of 133 cases, which corresponded to the number of hospitalized patients from January 2013 to December 2014. To calculate the power of the study, we considered a detection of an effect size of 2.5, resulting in a beat-value (power) of 0.90. Analysis were performed using R statistical program, version 2.1233. The results of the analysis were expressed as averages or percentages and the level of statistical significance was set at 0.05. Given the distribution of the variables, non-parametric tests were deemed appropriate for the comparison of central tendency measures. Wilcoxon's test was used to compare continuous variables, and X2 or Fisher's exact test to compare categorical variables. A multivariate analysis with a GLMM (generalized linear mixed model) was conducted to estimate severe dengue predictive variables using clinical signs and symptoms34.
3ResultsForty-two percent of the cases (56/133) were classified as non-severe dengue; 58% (77/133) as severe dengue. Patients with non-severe dengue had their first contact with health services 4.6 days after the onset of the clinical picture, whereas patients with severe dengue at 5 days (p=0.33). There weren’t any deaths by dengue in these patients.
A smaller proportion of females (30%, 17/56) with a non-severe dengue presentation compared to males (70%, 39/56) was observed (p<0.05). The proportion of females and males with severe dengue was 49% (38/77) and 51% (39/77), respectively. Seventeen percent (10/56) of the patients with non-severe dengue were under four years old, whereas the rest (83%, 46/56) were older (p<0.05). Fever in patients with non-severe dengue presented with a mean temperature of 38.3°C, a median of 38°C, and a standard deviation (SD) of 0.48; in patients with severe dengue, these values were higher: mean of 38.6°C, median of 39°C and SD of 0.49. The difference between the median temperature between these groups was statistically significant (p<0.001). The proportion of severe dengue cases with a fever of ≥ 39°C was 51% (39/77), compared to 8% (10/56) in patients with non-severe dengue.
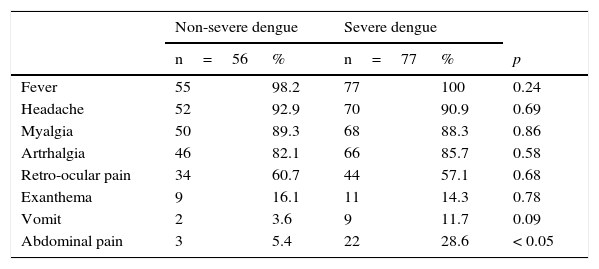
The proportion of other signs and symptoms was similar in both groups (Table 1). There were significant differences in the proportion of patients that reported abdominal pain: 5% (3/56) of patients with non-severe dengue and 29% (22/77) of patients with severe dengue reported this symptom (p<0.05).
Clinical characteristics of pediatric patients with non-severe and severe dengue.
| Non-severe dengue | Severe dengue | ||||
|---|---|---|---|---|---|
| n=56 | % | n=77 | % | p | |
| Fever | 55 | 98.2 | 77 | 100 | 0.24 |
| Headache | 52 | 92.9 | 70 | 90.9 | 0.69 |
| Myalgia | 50 | 89.3 | 68 | 88.3 | 0.86 |
| Artrhalgia | 46 | 82.1 | 66 | 85.7 | 0.58 |
| Retro-ocular pain | 34 | 60.7 | 44 | 57.1 | 0.68 |
| Exanthema | 9 | 16.1 | 11 | 14.3 | 0.78 |
| Vomit | 2 | 3.6 | 9 | 11.7 | 0.09 |
| Abdominal pain | 3 | 5.4 | 22 | 28.6 | < 0.05 |
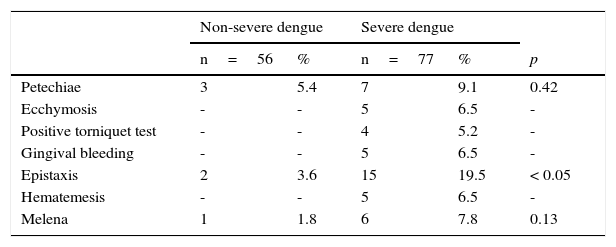
Only three patients with non-severe dengue had hemorrhagic signs. In the group of patients with severe dengue, 25 cases (32%, 35/77) presented with hemorrhagic signs (Table 2): 5% (3/56) of the patients with non-severe dengue and 19.5% (15/77) of the patients with severe dengue reported epistaxis (p<0.05).
Hemorrhagic manifestations of pediatric patients with non-severe and severe dengue.
| Non-severe dengue | Severe dengue | ||||
|---|---|---|---|---|---|
| n=56 | % | n=77 | % | p | |
| Petechiae | 3 | 5.4 | 7 | 9.1 | 0.42 |
| Ecchymosis | - | - | 5 | 6.5 | - |
| Positive torniquet test | - | - | 4 | 5.2 | - |
| Gingival bleeding | - | - | 5 | 6.5 | - |
| Epistaxis | 2 | 3.6 | 15 | 19.5 | < 0.05 |
| Hematemesis | - | - | 5 | 6.5 | - |
| Melena | 1 | 1.8 | 6 | 7.8 | 0.13 |
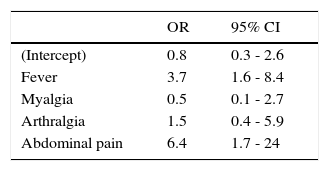
Hemoglobin, hematocrit, and platelet values for each group are presented in Table 3. There was a significant difference in the medians for platelet values in patients with non-severe and severe dengue who were older than two years-old (using Wilcoxon's sum rank test for median comparison). A 1.7% (1/56) of patients with non-severe dengue had a platelet count ≤ 50,000/mm3; compared to a 32% (25/77) in patients with severe dengue (p<0.05). The final model derived from the multivariate analysis using a GLMM identified four signs and symptoms (fever > 39°C, myalgia, arthralgia and abdominal pain), which predict severity of dengue (Table 4).
In a study regarding clinical aspects of dengue in the pediatric population, Camacho-Ramirez et al. (2006) reported that 17% of the hospitalized cases had a diagnosis of hemorrhagic dengue, considered at that time as the severe form of dengue30. In the present study, 58% of pediatric patients had a diagnosis of severe dengue. The classification of the clinical picture as classic and hemorrhagic dengue, and the classification of this study—non-severe and severe dengue—makes a comparison between these studies difficult. Nevertheless, in our study, the percentage of pediatric hospitalized patients with severe dengue is noteworthy, which indicates that nearly half of the hospitalized patients are cases of non-severe dengue and should be managed as outpatients. Inpatients with non-severe dengue are consuming resources that should be assigned to other health issues.
In this study, we found a greater proportion of pediatric patients with fever, headache, myalgia, and arthralgia compared with the study reported in 2006. Also, the proportion of patients with abdominal pain was smaller in our study. We were not able to compare the frequency of patients with exanthema and vomit because the latter symptoms were reported in a single combined group in the study by Camacho-Ramirez et al.
Eighty-one percent of non-severe dengue cases and eighty-six of the severe dengue cases were over 4 years old; this result is similar to the proportion of dengue cases (80.6%) reported in children of that age at a hospital in Honduras35. It is generally accepted that an older age increases the probability of dengue virus infection36. A patient's age greater than 5 years was found to be associated with acute dengue virus infection in Tanzania4. In Thailand, patients with an age greater than 6 years were found to have the greatest risk of having severe dengue20. In our study, we found a greater proportion (86%) of children older than 4 years old diagnosed with severe dengue compared to 81% who were diagnosed with non-severe dengue; nevertheless, the difference was not statistically significant, possibly due to the sample size.
A fever >39°C, abdominal pain, epistaxis and a platelet count lower than 50,000/mm3 were associated with severe dengue. High fevers, abdominal pain and thrombocytopenia (<50,000/cm3) have also been found to be predictive factors of more severe presentations in children of other countries18,19,20; apparently, this is the first time this finding is reported in a Mexican pediatric population. Epistaxis was reported in 3% of the cases in 2006, and no significant difference comparing classic and hemorrhagic dengue was found30. In our study, the observed frequency of this finding in patients with severe dengue was 19.5% and had an association with the severity of dengue.
Hepatomegaly has been reported as a predictive sign of the severity of dengue in the pediatric population20. Our study did not report results regarding this finding because the data from this study came from the official form for the “Epidemiologic study of fever and hemorrhagic fever caused by dengue virus infection”. This form is used to register pediatric and adult patient‘s data and does not includes specific information regarding hepatomegaly. Nevertheless, our study demonstrates the usefulness of the official form to describe the clinical profile and identify clinical indicators of the severity of dengue. No patient had a confirmatory serological diagnosis of dengue. This is a limitation of this study; nevertheless, only patients with a final discharge diagnosis of dengue were selected for this study. To a reasonable extent, pediatricians had a sufficient time to confirm or reject the final diagnosis of every patient. Having results from serological or isolation studies would have allowed identification of the circulating virus serotype during the described period of study in Chilpancingo, given that dengue virus serotype 2 has been found to produce the most severe clinical presentations9.
The results of the multivariate analysis using a GLMM showed four signs and symptoms predictive of the severity of dengue virus infection. The proposal of a clinical diagnosis for the identification of pediatric patients with severe dengue must not substitute clinical criteria—perhaps the most important—of the physician, according to clinical evolution and additional signs and symptoms associated with severity. Nevertheless, in the absence of laboratory tests, these clinical markers could aid the primary care physician for the immediate referral of these patients to a hospital.
The average time for contact with health services, 4.6 days in patients with non-severe dengue and 5.0 days in severe cases, is within the range (3.8-6.3 days) reported in other studies of pediatric population37. We found a significant difference in the proportion of females (30%) and males (70%) that were referred to hospitals for non-severe dengue. This result is probably indicative of a greater preference towards male patients. An increase in the incidence of dengue with hemorrhagic manifestations in Mexico has been documented, and the pediatric population is the most affected3. This could explain why there is currently a greater incidence of some signs and symptoms in pediatric patients with severe dengue. The increase in hemorrhagic dengue could also be explained by the theory of an increased inflammatory response by immunologic processes7 when more than two dengue virus serotypes are present. Circulation of the four serotypes in Guerrero is well documented28. A greater incidence of dengue in the pediatric population could be explained by decrease of the population susceptible to infection in the elderly. Accounting for these reasons, the following dengue virus infection outbreaks should be expected to present mainly in the pediatric population, and healthcare personnel should be alert to identify clinically potential severe cases.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare no conflict of interest of any nature.
Please cite this article as: Alvarado-Castro VM, Ramírez-Hernández E, Paredes-Solís S, Legorreta-Soberanis J, Saldaña-Herrera VG, Salas-Franco LS, del Castillo-Medina JA, Andersson N. Caracterización clínica del dengue y variables predictoras de gravedad en pacientes pediátricos en un hospital de segundo nivel en Chilpancingo, Guerrero, México: serie de casos. Bol Med Hosp Infant Mex. 2016;73:237–242.