Aldosterone is synthesized in the adrenal cortex and is the main regulator of sodium and potassium metabolism and the extracellular volume. Acting through the mineralocorticoid receptor, it is the final endocrine signal of the renin-angiotensin-aldosterone system with effects on the renal tubular epithelium and distal colon stimulating sodium reabsorption and potassium secretion. Water is absorbed by osmosis favoring expansion of circulating volume and increasing arterial blood pressure.
Recently there has been great interest in the non-classical actions of aldosterone on the vascular endothelium, heart and kidney. There is evidence suggesting that aldosterone participates in vascular remodeling, endothelial function and collagen deposition, contributing to heart failure progression and kidney damage. Clinical and experimental evidence supporting the use of aldosterone blocking agents in different models of kidney damage is reviewed.
La aldosterona, sintetizada en la zona glomerulosa de la corteza suprarrenal, es la principal hormona reguladora del metabolismo de sodio y potasio y del volumen extracelular. A través del receptor de mineralocorticoides, actúa como la señal endocrina final del sistema renina-angiotensina-aldosterona sobre el epitelio del túbulo renal y del colon distal, que estimula la reabsorción de sodio y la secreción de potasio. El agua se reabsorbe, vía ósmosis, favoreciendo la expansión del volumen circulante y, por ende, incrementando la presión arterial.
Recientemente, se ha centrado el interés en las acciones no clásicas de la aldosterona sobre el endotelio vascular, corazón y riñón. Existe evidencia de que la aldosterona está involucrada en la remodelación vascular, la función endotelial y la formación de colágena, y que contribuye a la progresión de la insuficiencia cardiaca, así como del daño renal. Se revisa la evidencia clínica y experimental que fundamenta el uso de bloqueadores de aldosterona para detener la progresión del daño renal en diferentes modelos.
1. Introduction
The renin-angiotensin-aldosterone system (RAAS) plays a fundamental role in the preservation of hemodynamic stability in humans. It includes the regulation of fluid and electrolyte balance, extracellular space volume and blood pressure.
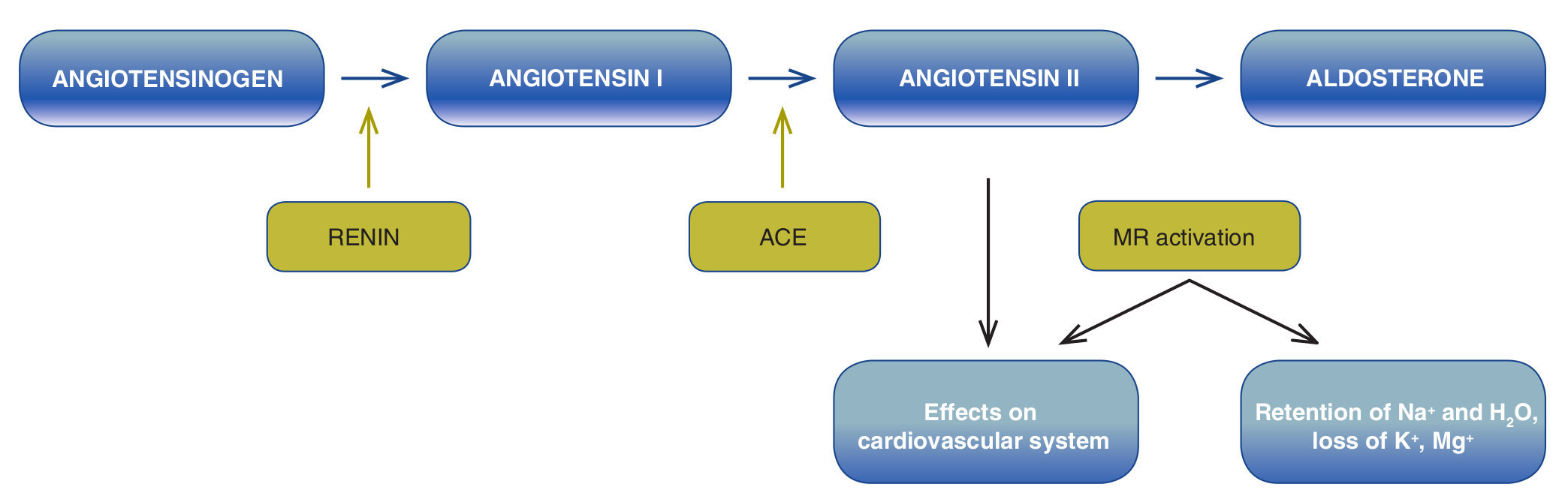
RAAS is formed by a cascade of enzymes (Fig. 1). In classic form, onset of this cascade is described by the release of renin in the kidney. This proteolytic enzyme is produced by the juxtaglomerular cells (JGC) that are found in the mid-intima of the afferent arterioles and have smooth muscle as well as endocrine characteristics.1 The number of JGC changes depending on the age of the individual and state of the extracellular volume.2 In a rat fetus model it has been observed that renin is expressed in practically all the renal vasculature including the afferent arteriole and the interlobular and arcuate arteries in the newborn. Renin expression is limited throughout the afferent arterioles and in the adult animal is confined to the JG apparatus,3 which is located in the area of contact between the afferent arteriole that reaches the glomerulus (vascular pole) and the distal convoluted tubule. Studies in humans have shown that plasma levels of renin and aldosterone change with age and are lower in the elderly population.4
Figure 1 Renin-angiotensin-aldosterone system (RAAS). ACE, angiotensin-converting enzyme; MR, mineralocorticoid receptor.
JGC contain renin granules that are secreted to the plasma in response to changes in extracellular volume, osmolality, and blood pressure. One part of this renin is filtered in the glomerulus and is resorbed in the proximal tubule. This proteolytic enzyme acts both at the level of the kidney as well as in the circulation (activity of plasma renin) and degrades the angiotensinogen produced in the liver to form angiotensin I (AngI), an inactive decapeptide which, in turn, is fragmented into angiotensin II (AngII), an octapeptide, by angiotensin-converting enzyme (ACE). ACE is found mainly in the lung but is also expressed in the heart, kidney and brain. AngII has two types of receptors, AT-1 and AT-2. When it binds to AT-1, it acts as a potent vasoconstrictor that stimulates the production of aldosterone in the glomerular zone of the adrenal gland. Aldosterone, the final signal of the RAAS, is a mineralocorticoid hormone that binds to the cytosolic mineralocorticoid receptor (MR) in the distal nephron (distal convoluted tubule, connecting tubules and principal cells in the connecting tubules) and in the colon. The aldosterone-receptor complex translocates to the nucleus where it binds to specific sequences of DNA (hormone response elements) and regulates expression of multiple proteins induced by aldosterone. It also stimulates the transepithelial transport of sodium with consequent reabsorption of sodium and water and the elimination of potassium,5 favoring the expansion of circulating volume and, therefore, increasing blood pressure.
RAAS played a key evolutionary role in enabling the transition of aquatic animals to the terra firme during the Devonian period of the Paleozoic era. Aldosterone initially appeared in the first terrestrial tetrapods. Fish do not have this hormone. RAAS is the principal regulator of renal sodium absorption so as to preserve the "sea within" and maintain life in an environment with little salt and water.6,7 It has been proposed that, when the first humans appeared, salt was scarce and very precious. Those individuals who retained more salt were those who had the greatest possibility of survival. In this modern, industrialized era, there is a large availability of salt in the diet which, added to an increase in calorie ingestion, has caused an obesity pandemia and an increase in RAAS activation. Individuals who genetically have a more active RAAS have a predisposition to diseases such as salt-sensitive hyper-tension, chronic renal disease and cardiovascular disease, due to the modern diet.5,8
2. Aldosterone antagonists
There is a great interest in the development of selective aldosterone antagonists, not only because of their diuretic effect but for their potential cardio- and nephroprotective effects.
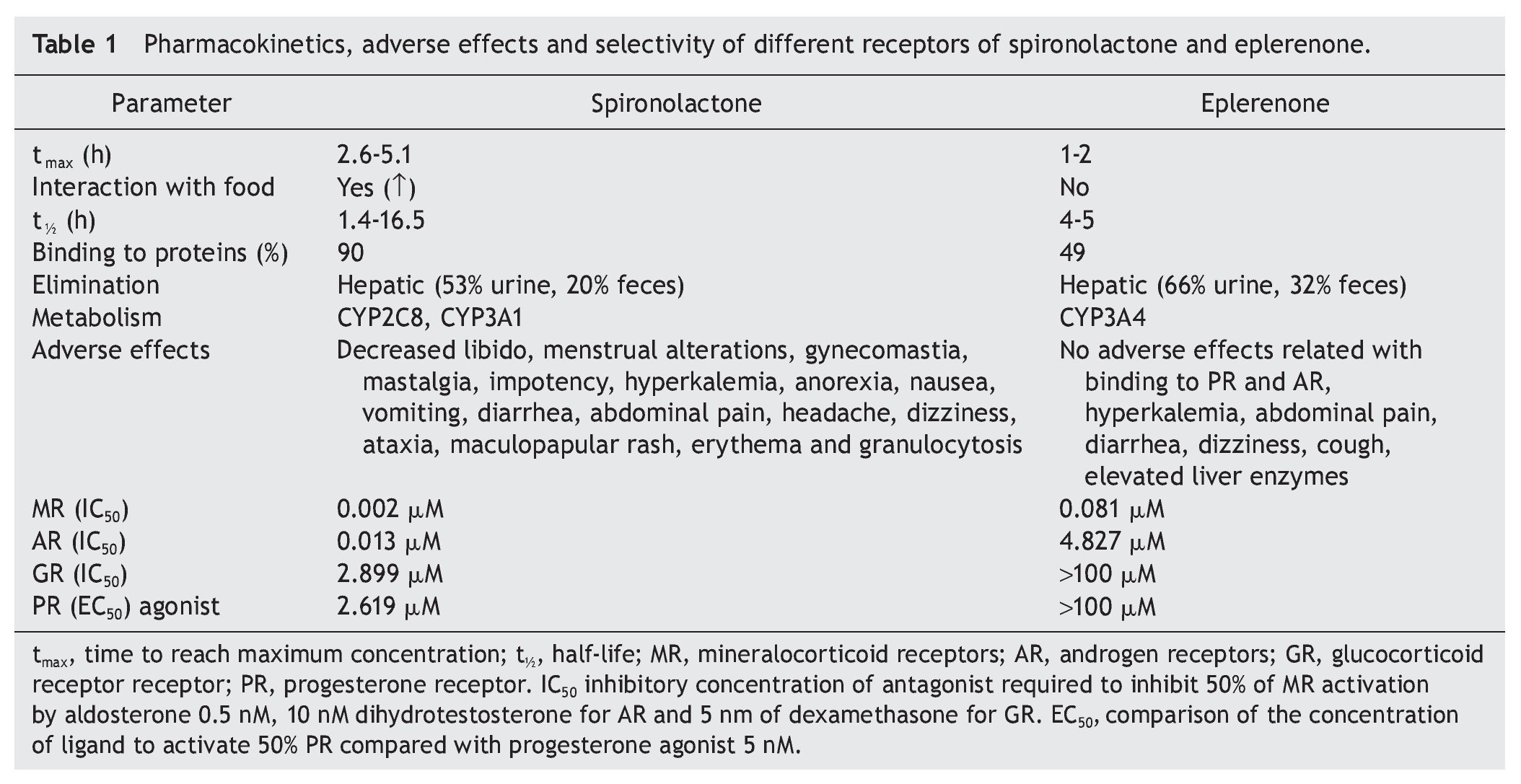
Spironolactone was first mineralocorticoid antagonist developed in 1960. It has been widely used for the treatment of primary hyperaldosteronism, peripheral edema, hypertension and hypokalemia, although in some patients it is poorly tolerated because of its side effects, mainly due to a low specificity to the MR. In addition, it binds to androgen and progesterone receptors causing menstrual disorders in women and gynecomastia in men.9
Eplerenone, a derivative of spironolactone to which an epoxy group was added, was developed in 1987.10 In contrast to spironolactone, it has a reduced affinity to estrogen and progesterone receptors; therefore producing a lower incidence of adverse sexual effects.11
Table 1 describes the pharmacokinetics of spironolactone and eplerenone as well as their selectivity on mineralocorticoid, glucocorticoid, androgen and progesterone receptors.
Like spironolactone, the most important side effect of eplerenone is hyperkalemia, therefore close monitoring during treatment with this drug is required. In case of hyperkalemia, it is recommended to decrease intake of potassium, review the concomitant treatment that can exacerbate this condition, such as the use of adrenergic beta blockers, NSAIDs, heparin, among others, and decrease the dose. Resins of cationic exchange and conventional treatments for hyperkalemia may also be given.12,13
Eplerenone interacts with other drugs that are metabolized by CYP3A4 such as clarithromycin, erythromycin, ketoconazole, fluconazole, verapamil and diltiazem, among others. It is worth mentioning that several calcium channel inhibitors available in the market, such as amlodipine and diltiazem, also have antagonistic effects on the MR.14
3. Aldosterone and organ damage
Recently there has been great interest in the non-classical actions of aldosterone on the vascular endothelium, heart and kidney endothelium. There is evidence that aldosterone is involved in vascular remodeling, endothelial function and in the formation of collagen, contributing to the progression of heart failure and kidney damage.
Aldosterone and the activation of the MR promote renal inflammation and fibrosis by favoring the generation of ROS.15 The addition of an aldosterone blocker such as eplere-none to the treatment of patients with heart failure reduces the risk of hospitalizations and premature death.16
It has been observed in primary hyperaldosteronism that high salt intake aggravates hypertension and organ damage. This damage can be prevented with the use of the MR blockers.17
Excessive signaling of the MR is a key mechanism in end organ damage, even with normal or low levels of aldosterone, especially in the context of high salt intake.18 The GTPasa Rac1 has been related as a pathway that modulates the MR function in models of kidney damage due to salt-sensitive hypertension as well as with cardiac damage.19-21
CRD is considered a state of relative hyperaldosteronism because, despite the expansion of the extracellular space volume, aldosterone secretion is inappropriately high, and small degrees of expansion of the extracellular space magnify the pro-hypertensive and pro-inflammatory effects of MR activation.22 Twenty-four hour urinary excretion of sodium predicts the urinary excretion of mineralocorticoids in patients with chronic kidney disease.23
In patients with diabetes, the combination of the aldosterone antagonist with spironolactone in addition to an angiotensin receptor blocker improves blood pressure and proteinuria. The advantage is that there is no deleterious effect on the glomerular filtration rate with the combination of the angiotensin receptor blocker and angiotensin-converting enzyme inhibitors, which is what is commonly used.24
Experimental evidence has arisen about the role played by aldosterone in the onset and progression of acute and chronic nephrotoxicity mediated by calcineurin inhibitors (one of the main immunosuppressive agents currently used in kidney transplantation and kidney proteinurias). This is accomplished through its participation in renal failure, tubulo-interstitial fibrosis, arterial disease and cell death by apoptosis. Alteration in the expression of mRNA at the renocortical level of different vasoactive factors such as pro-renin, endothelin, COX-2, angiotensin, AT1 and AT2 receptors has been documented as well as the TGF-β and extracellular matrix proteins. In these models these changes can be prevented or modified by blocking the MR with spironolactone or eplerenone.25-27 In addition, administration of spironolactone prevents the decrease in renal perfusion and the development of acute renal failure and tubular apoptosis during the damage due to ischemia-reperfusion in an animal model. This protection was mediated by oxidative stress reduction, by an increase in the expression of antioxidant enzymes and by the restoration of urinary excretion of nitrous oxide.28
Aldosterone may play a role in the regulation of plasminogen activator inhibitor type 1 (PAI-1)29,30 and also has a direct effect on the development of the fibrosis.31 In animal models, blocking of aldosterone with its antagonist, spironolactone, slows down the progression of fibrosis at the renal level.32 In clinical studies, aldosterone antagonists additionally decreased proteinuria when they were added to the treatment with ACE inhibitors and/or AngII antagonist.33-35
The increase of PAI-1 has been associated with an increase in the progression of fibrosis in heart and kidney disease.36 PAI-1 can be decreased by inhibiting AngII and/ or aldosterone. The latter has been associated with the prevention of fibrosis and even regression of renal damage.37 In the animal model of damage due to ischemia-reperfusion, spironolactone, whether it was previously administered at 75 min or 3 h after ischemia prevented inflammation and the activation of pre-fibrotic pathways (TGF-β), oxidative stress and proteinuria.38
Administration of spironolactone before renal trans plantation and 3 days after renal transplantation was compared vs. placebo in Mexican patients. A decrease was found in the oxidative stress assessed as urinary hydrogen peroxide. No difference was found at 72 h in renal function and in markers of tubular damage as a molecule of kidney damage KIM1, heat shock protein 72 and interleukin 8.39
In a study by our group, administration of eplerenone in Mexican children with chronic kidney disease of the graft was well tolerated, improved the glomerular filtration rate at 1, 3 and 6 months and prevented the progressive deterioration in the GFR at 12 months compared with placebo.40-42
Conflict of interest
The authors declare no conflict of interest of any nature.
Acknowledgments
Support for the study was provided by Fondo Sectorial de Investigación en Salud y Seguridad Social SS/IMSS/ ISSSTE-CONACYT Salud-2008-01-87381. A.V. received support from the program PROBEI.
Received 8 October 2013;
accepted 23 January 2014
* Corresponding author.
E-mail:medeiro.mara@gmail.com (M. Medeiros).