Childhood cancer accounts for 0.5 to 4.6% of the total number of cases in any population. According to the treatment, some side effects are present. Most adverse reactions can cause severe consequences for the survival of the patient. The more effective interventions for the management and the prevention of treatment-induced symptoms (TIS) in children with cancer are necessary to know. The objective of this review was to identify and describe available scientific evidence on the efficacy and safety of interventions used for the management and prevention of TIS in children with cancer.
MethodsWe conducted a systematic review of the literature on studies that evaluated or described the effectiveness of interventions used for the management and prevention of TIS in children with cancer in some of the major electronic databases. Results were qualitative synthesized and presented as evidence tables.
ResultsWe identified eight systematic reviews. The revisions included experimental studies. All participants, including children and adults, were patients diagnosed with some cancer about to receive or that received treatment.
ConclusionsThe results showed only a reduced number of clinical trials that have evaluated the interventions for the management of TIS in children with cancer. In addition, the available evidence was limited and of poor quality. It is necessary to conduct more clinical trials with good methodological quality and high statistical power.
El cáncer en la infancia representa del 0.5 al 4.6% del total de casos en una población. Dependiendo del tratamiento se presentan cierto tipo de efectos secundarios. La mayoría de los eventos adversos provocan consecuencias graves para la supervivencia del paciente. Es importante conocer cuáles son las intervenciones más eficaces para el manejo y la prevención de los síntomas inducidos por el tratamiento (SIT) en niños con cáncer. El objetivo de esta revisión fue identificar y describir la evidencia científica disponible sobre la eficacia y seguridad de las intervenciones utilizadas para el manejo y la prevención de los SIT en niños con cáncer.
MétodosSe realizaron búsquedas bibliográficas en las principales bases de datos electrónicas para identificar revisiones sistemáticas que evaluaran o describieran la efectividad de las intervenciones utilizadas para el manejo y la prevención de los SIT en niños con cáncer. Se realizó la síntesis cualitativa de los resultados, que se presentaron mediante tablas de evidencia.
ResultadosSe identificaron ocho revisiones sistemáticas. Las revisiones incluyeron estudios experimentales; los participantes de los estudios, incluyendo niños y adultos, fueron pacientes diagnosticados con algún tipo de cáncer a punto de recibir o que recibieron tratamiento.
ConclusionesLos resultados demuestran que un número reducido de ensayos clínicos han evaluado las intervenciones para el manejo de SIT en niños con cáncer. Además, la evidencia existente es limitada y de baja calidad. Existe la necesidad de realizar ensayos clínicos con mayor poder estadístico y adecuada calidad metodológica.
Cancer is one of the leading causes of death worldwide. So far, this disease affects populations from all countries and regions of the world. According to with global estimates, 14 million of new cases and 8 million of deaths related to cancer were registered until 2012. It is also known that at least 70% of deaths occur in low- and middle-income countries.1•3
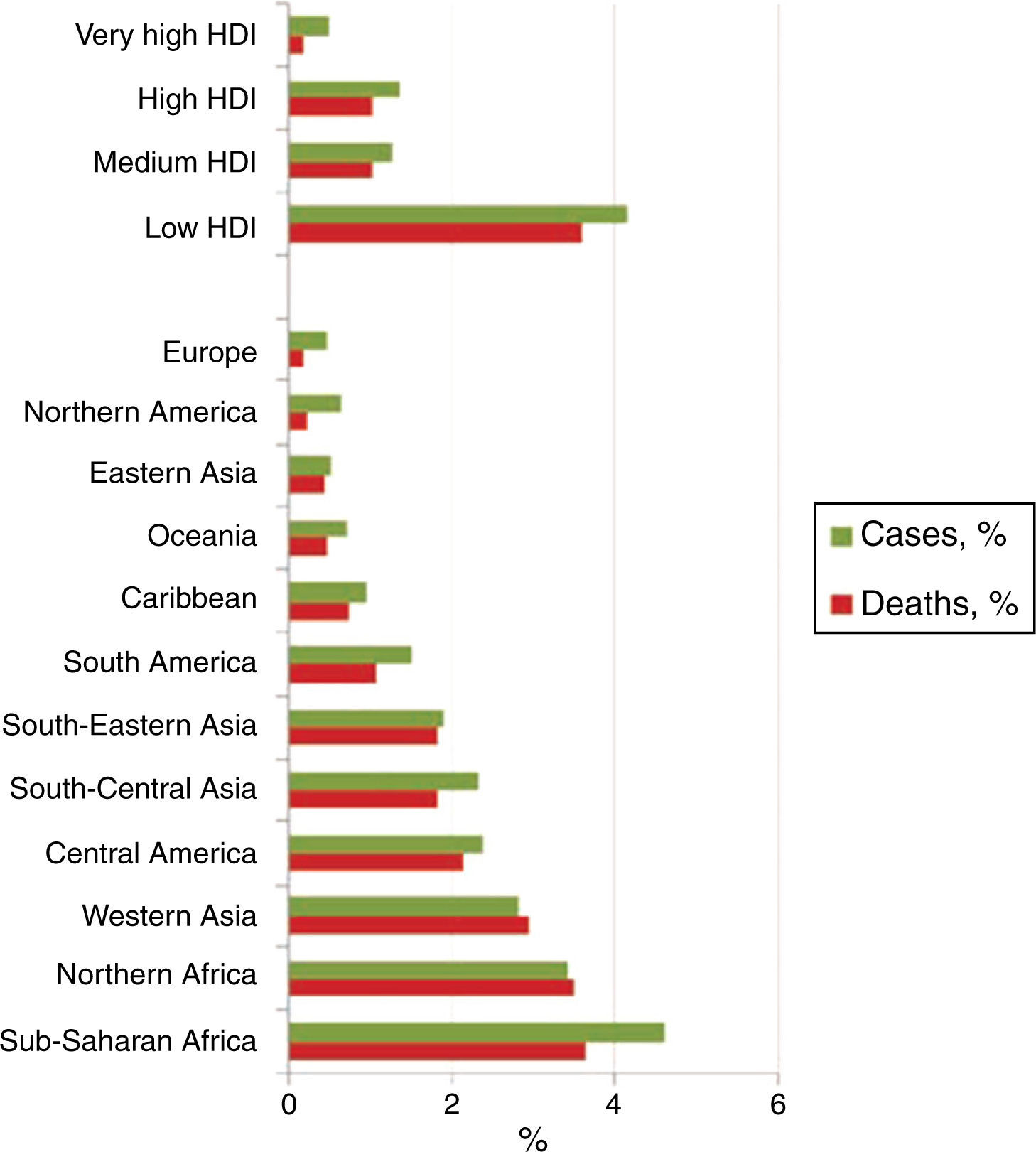
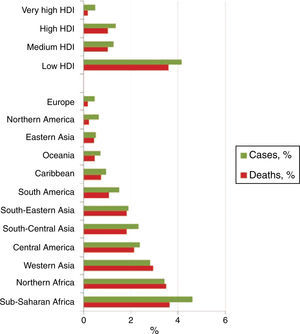
Childhood cancer accounts for 0.5 and 4.6% of the total number of cases of cancer in a population.1,2,4 Each year around 165,000 new cases of cancer are diagnosed in children between 0 and 14 years of age (about 95,000 in boys and 70,000 in girls).5 Furthermore, 80% of children diagnosed around the world are found in low-income countries. In addition, approximately 90,000 children die every year as a result of this disease (Figure 1).1,2,5,6
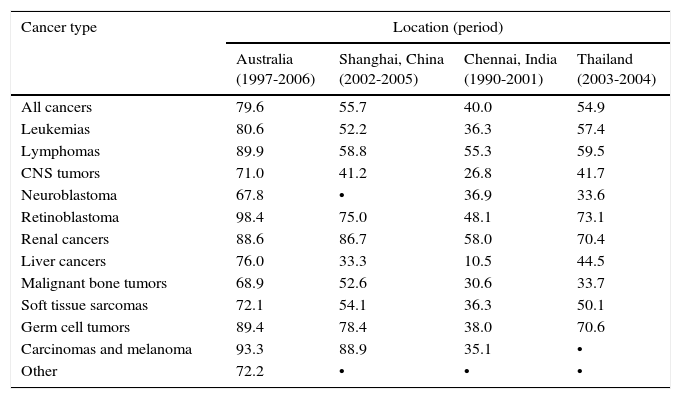
Survival rates are different and depend on the geographical region in which children with cancer live, among other factors (Table 1).1,4 That is, only 1-2/10 children with cancer receiving treatment survive in low-income countries (10-20%); in contrast, only 1-2/10 children diagnosed and treated for cancer die in high-income countries (80-90% survival).1,4•6
Percentage survival at 5 years after initial diagnosis in children (age 0-14 years) at four locations in different countries.
| Cancer type | Location (period) | |||
|---|---|---|---|---|
| Australia (1997-2006) | Shanghai, China (2002-2005) | Chennai, India (1990-2001) | Thailand (2003-2004) | |
| All cancers | 79.6 | 55.7 | 40.0 | 54.9 |
| Leukemias | 80.6 | 52.2 | 36.3 | 57.4 |
| Lymphomas | 89.9 | 58.8 | 55.3 | 59.5 |
| CNS tumors | 71.0 | 41.2 | 26.8 | 41.7 |
| Neuroblastoma | 67.8 | • | 36.9 | 33.6 |
| Retinoblastoma | 98.4 | 75.0 | 48.1 | 73.1 |
| Renal cancers | 88.6 | 86.7 | 58.0 | 70.4 |
| Liver cancers | 76.0 | 33.3 | 10.5 | 44.5 |
| Malignant bone tumors | 68.9 | 52.6 | 30.6 | 33.7 |
| Soft tissue sarcomas | 72.1 | 54.1 | 36.3 | 50.1 |
| Germ cell tumors | 89.4 | 78.4 | 38.0 | 70.6 |
| Carcinomas and melanoma | 93.3 | 88.9 | 35.1 | • |
| Other | 72.2 | • | • | • |
CNS, central nervous system
Source: International Agency for Research on Cancer. The First 50 Years, 1965•2015 (ref. 5).
According to several reports, the most common cancer in children ages 0-14 years are acute lymphocytic leukemia (ALL), brain tumors, neuroblastomas (which represent more than 50% of the new cases), nephroblastomas, retinoblastomas and other tumors of the central nervous system (CNS).1,4•7 The causes of most childhood cancers are not known, and for the most part, they cannot be prevented. Depending on the type of cancer, children receive different treatments such as surgery, chemotherapy, radiation, chemotherapy with stem cell transplantation, and biological or targeted therapy. Furthermore, pediatric patients present different side effects depending on their treatment.1,4 For example, chemotherapy, radiation or targeted therapy for head and neck cancer can cause toxic side effects as oral mucositis (OM).8 In the long-term, anthracyclines, which are used as chemotherapy in childhood, can cause symptomatic cardiotoxicity (severe cardiac dysfunction)•a condition associated with high morbidity and mortality.9 Febrile neutropenia, a condition potentially dangerous for life, is a common adverse event experienced by patients treated with chemotherapy.10 Similarly, nausea and vomiting are some of the most common side effects in children undergoing anticancer treatment; as they are unpleasant, they cause aversion and anguish.11 Some other symptoms frequently related to anticancer treatment are fatigue, loss of weight and muscle mass, and deterioration of the immune system.12
Currently, health professionals are overwhelmed by the amount of available information in cancer treatment, which makes it difficult to handle. Therefore, it is important to have valid and reliable scientific data that summarizes the evidence. Systematic reviews are studies that highlight available results from primary studies on specific themes to answer research questions, which are considered as the analysis and synthesis of the best evidence available to make clinical decisions, and offer information on the efficiency of health interventions and when it is possible to apply their results.13,14
Cochrane is an international organization, which focuses particularly on the development of systematic reviews of clinical trials. Cochrane reviews have a standard format produced by a pre-established methodology developed by experienced methodologists; also, it has the particularity of being constantly updated. Cochrane systematic reviews are considered as the highest quality scientific evidence.14
Therefore, it would be important to identify the scientific evidence available on which are the most effective interventions for the management and prevention of anticancer treatment-induced symptoms in children. At present, little is known about interventions that may be useful for the management of these patients. Furthermore, to our knowledge, no records of studies that describe this information exist. Therefore, the research question for this review was the following: Which are the interventions for the management and prevention of anticancer treatment-induced symptoms in children, and their effectiveness?
The aim of this review was to identify and describe available evidence on the efficacy and safety of interventions used for the management and prevention of cancer treatment-induced symptoms in children.
2Methods2.1Study designReview of the scientific literature.
2.2Eligibility criteria2.2.1Types of studiesCochrane systematic reviews (SRs) with or without meta-analysis that had evaluated any intervention for the management and prevention of the symptoms induced by the treatment of children with cancer were considered for inclusion.
Every study that did not meet the inclusion criteria and unpublished articles or publications of protocols of systematic reviews were excluded.
2.2.2Types of participantsMale or female children and adolescents (< 18 years) diagnosed with any childhood cancer (age of diagnosis ≤ 18 years), and treatment-induced symptoms. We included both inpatients and outpatients who received care in any healthcare setting (e.g. hospital, oncology center, community, or home). There were no exclusion criteria.
2.2.3Types of interventionIntervention: any medical intervention for the management or prevention of treatment-induced symptoms in children with cancer.
Comparison: any aspect to compare with (e.g. placebo, other drugs, alternative therapies, or no intervention).
We considered all the studies that met the previously established inclusion criteria, regardless of the evaluated outcomes and measures to analyze the outcome.
2.3Search strategyWe conducted a systematic literature search in the main electronic databases without any restriction of language or publication date, from 1966 until August 2016, limiting to studies that evaluated or described the efficacy and safety of medical procedures used for the management and the prevention of symptoms induced by the treatment of cancer in children.
The main sources of information were
- •
MEDLINE
- •
The Cochrane Library
- •
TRIP Database
Search terms: interventions, medical interventions, clinical interventions, prevention, treatment, symptoms, symptom relief, induced symptoms, uncomfortable symptoms, cancer, neoplasm, oncological, children, childhood, pediatrics, infants, receiving, undergoing chemotherapy, chemotherapy-induced, radiotherapy, efficacy, safety, adverse effects, side effects, harm associated, systematic review, meta-analysis.
MEDLINE searches were conducted using MeSH terms and keywords. In addition, we included methodological filters by type of article: systematic review, meta-analysis, studies in humans (indexed and with abstract).
2.4Data collection and extractionTwo reviewers separately assessed the eligibility of the studies for inclusion. Relevant studies were retrieved, and data about the methodological aspects, participants, interventions and outcome variables were extracted. In both phases (selection of the studies and extraction of data), disagreements were resolved by consensus. In the case of a persisting disagreement, a third reviewer was consulted.
Data were registered in tables previously designed by a reviewer using Excel software and double-checked by a second reviewer. The collection of data was tested with a sample of articles.
The following information extracted from the identified studies was included:
- •
Country of origin
- •
Study design
- •
Objective
- •
Type of studies included
- •
Participants
- •
Intervention
- •
Comparison
- •
Type and duration of treatment
- •
Evaluated outcomes
- •
Type of outcome measures
- •
Follow-up time
- •
Key results
A qualitative synthesis of the results was presented as evidence tables. Results were represented as numeric values, averages, percentages and according to the outcome measures expressed in the studies. A quantitative synthesis of the results was presented accordingly to as they were analyzed and expressed in the reviews. Secondary studies were identified and included, and the studies examined different types of interventions; hence, no data analysis was conducted. Results are presented in a narrative form.
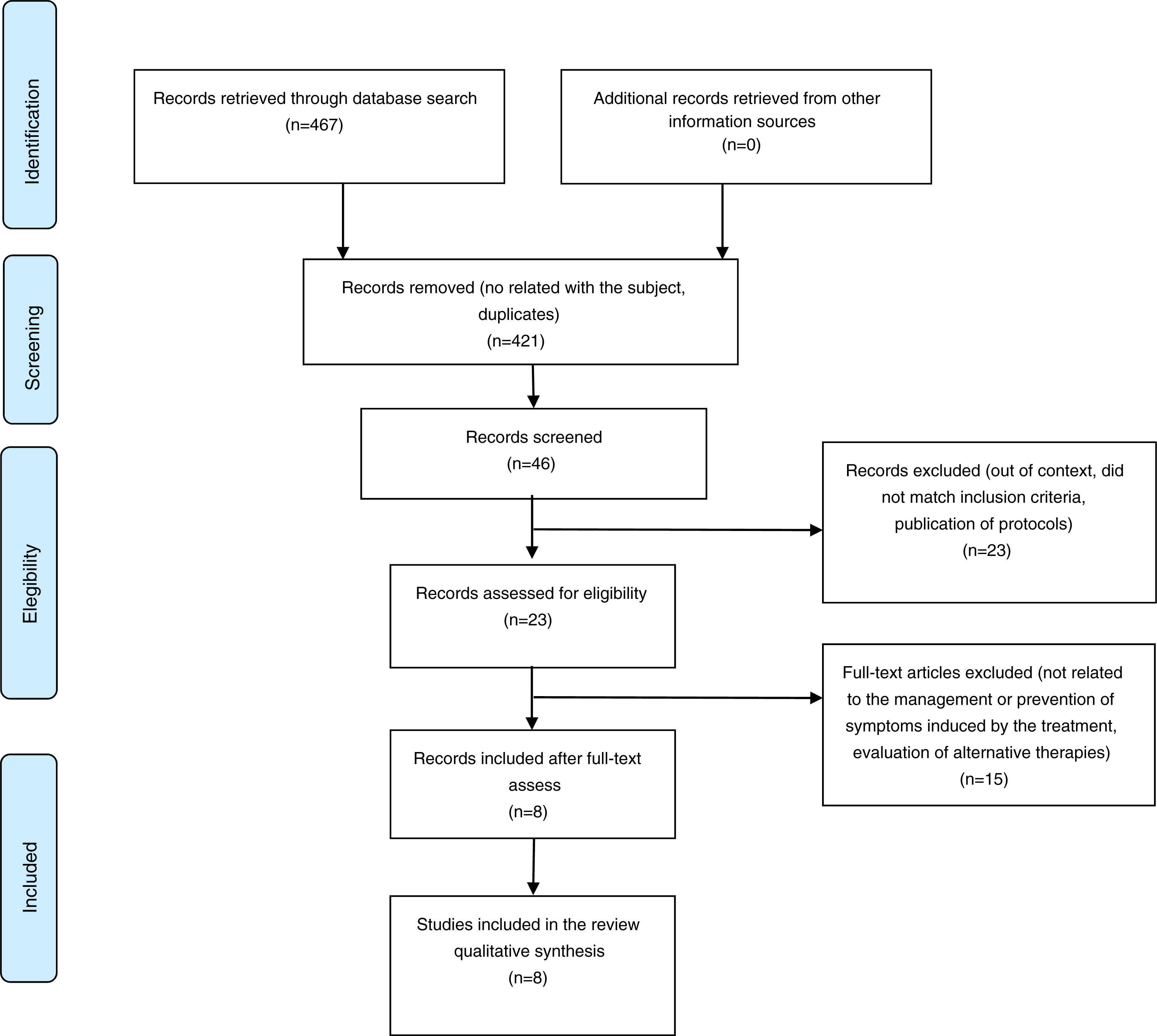
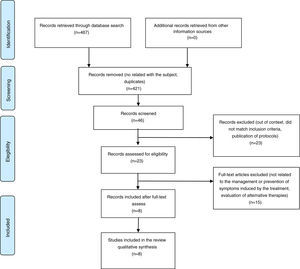
3ResultsAfter performing the search in the main electronic databases, the reviewers recovered 467 records. Titles and summaries of 46 publications were analyzed as potentially eligible for inclusion. Finally, 23 full-text articles were recovered. After analyzing the documents, eight studies that met the criteria for inclusion were selected (Figure 2).
Exclusion criteria were mainly different standards of inclusion of the population: only adults were assessed, alternative therapies were evaluated, publications of protocols, non-Cochrane systematic reviews, interventions not related to the management and prevention of symptoms induced by cancer treatment.
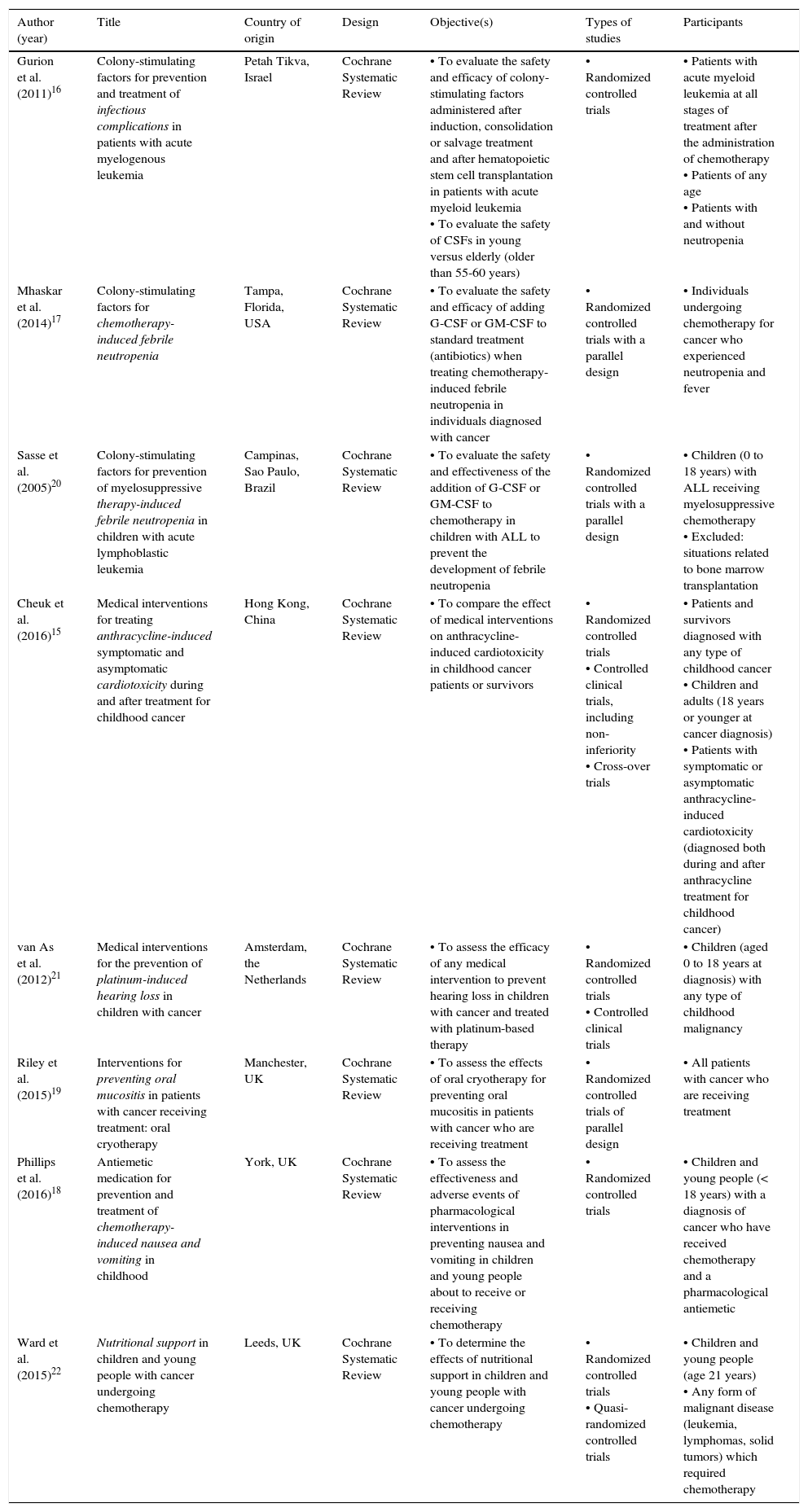
3.1Description of the studiesEight Cochrane systematic reviews that evaluated the efficacy and safety of medical procedures used for the management and prevention of the symptoms induced by the treatment of children with cancer were included.15•22
From the eight reviews included, three systematic reviews were performed in the United Kingdom; the rest were carried out in China, Brazil, Israel, the U. S. and the Netherlands. The reviews included experimental studies, which considered randomized controlled clinical trials (RCT) and controlled clinical trials (CCT) with a parallel design, cross-over trials, and quasi-randomised trials. Four reviews included children and young people;18,20•22 the other four included children and adults.15•17,19 All the participants were patients diagnosed with some cancer about to receive or had already received chemotherapy, radiotherapy, hematopoietic stem cell transplantation or targeted therapy. Three reviews evaluated the efficacy and safety of colony-stimulating factors (CSFs) for preventing or treating infections and febrile neutropenia induced by treatment in patients with cancer.16,17,20 Two reviews evaluated medical interventions for the prevention of cardiotoxicity induced by the treatment with anthracycline,15 and the prevention of platinum-induced hearing loss in children with cancer.21 A review assessed the effects of oral cryotherapy for the prevention of oral mucositis in patients receiving anticancer treatment.19 Another review evaluated antiemetic drugs for prevention and treatment of chemotherapy-induced nausea and vomiting in children.18 The last review determined the effects of nutritional support in children and young people with cancer undergoing chemotherapy.22
The details of the results of each study are presented in Tables 2 and 3, and key results are shown in Table 4.
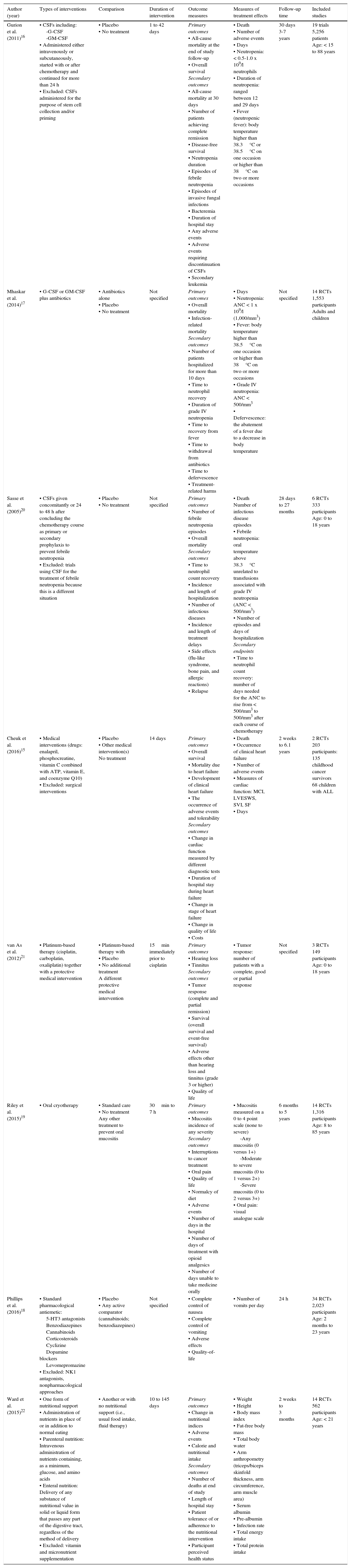
Characteristics of the studies included (I): studies, objectives, participants.
| Author (year) | Title | Country of origin | Design | Objective(s) | Types of studies | Participants |
|---|---|---|---|---|---|---|
| Gurion et al. (2011)16 | Colony-stimulating factors for prevention and treatment of infectious complications in patients with acute myelogenous leukemia | Petah Tikva, Israel | Cochrane Systematic Review | • To evaluate the safety and efficacy of colony-stimulating factors administered after induction, consolidation or salvage treatment and after hematopoietic stem cell transplantation in patients with acute myeloid leukemia • To evaluate the safety of CSFs in young versus elderly (older than 55-60 years) | • Randomized controlled trials | • Patients with acute myeloid leukemia at all stages of treatment after the administration of chemotherapy • Patients of any age • Patients with and without neutropenia |
| Mhaskar et al. (2014)17 | Colony-stimulating factors for chemotherapy-induced febrile neutropenia | Tampa, Florida, USA | Cochrane Systematic Review | • To evaluate the safety and efficacy of adding G-CSF or GM-CSF to standard treatment (antibiotics) when treating chemotherapy-induced febrile neutropenia in individuals diagnosed with cancer | • Randomized controlled trials with a parallel design | • Individuals undergoing chemotherapy for cancer who experienced neutropenia and fever |
| Sasse et al. (2005)20 | Colony-stimulating factors for prevention of myelosuppressive therapy-induced febrile neutropenia in children with acute lymphoblastic leukemia | Campinas, Sao Paulo, Brazil | Cochrane Systematic Review | • To evaluate the safety and effectiveness of the addition of G-CSF or GM-CSF to chemotherapy in children with ALL to prevent the development of febrile neutropenia | • Randomized controlled trials with a parallel design | • Children (0 to 18 years) with ALL receiving myelosuppressive chemotherapy • Excluded: situations related to bone marrow transplantation |
| Cheuk et al. (2016)15 | Medical interventions for treating anthracycline-induced symptomatic and asymptomatic cardiotoxicity during and after treatment for childhood cancer | Hong Kong, China | Cochrane Systematic Review | • To compare the effect of medical interventions on anthracycline-induced cardiotoxicity in childhood cancer patients or survivors | • Randomized controlled trials • Controlled clinical trials, including non-inferiority • Cross-over trials | • Patients and survivors diagnosed with any type of childhood cancer • Children and adults (18 years or younger at cancer diagnosis) • Patients with symptomatic or asymptomatic anthracycline-induced cardiotoxicity (diagnosed both during and after anthracycline treatment for childhood cancer) |
| van As et al. (2012)21 | Medical interventions for the prevention of platinum-induced hearing loss in children with cancer | Amsterdam, the Netherlands | Cochrane Systematic Review | • To assess the efficacy of any medical intervention to prevent hearing loss in children with cancer and treated with platinum-based therapy | • Randomized controlled trials • Controlled clinical trials | • Children (aged 0 to 18 years at diagnosis) with any type of childhood malignancy |
| Riley et al. (2015)19 | Interventions for preventing oral mucositis in patients with cancer receiving treatment: oral cryotherapy | Manchester, UK | Cochrane Systematic Review | • To assess the effects of oral cryotherapy for preventing oral mucositis in patients with cancer who are receiving treatment | • Randomized controlled trials of parallel design | • All patients with cancer who are receiving treatment |
| Phillips et al. (2016)18 | Antiemetic medication for prevention and treatment of chemotherapy-induced nausea and vomiting in childhood | York, UK | Cochrane Systematic Review | • To assess the effectiveness and adverse events of pharmacological interventions in preventing nausea and vomiting in children and young people about to receive or receiving chemotherapy | • Randomized controlled trials | • Children and young people (< 18 years) with a diagnosis of cancer who have received chemotherapy and a pharmacological antiemetic |
| Ward et al. (2015)22 | Nutritional support in children and young people with cancer undergoing chemotherapy | Leeds, UK | Cochrane Systematic Review | • To determine the effects of nutritional support in children and young people with cancer undergoing chemotherapy | • Randomized controlled trials • Quasi-randomized controlled trials | • Children and young people (age 21 years) • Any form of malignant disease (leukemia, lymphomas, solid tumors) which required chemotherapy |
CSF, colony-stimulating factors; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; ALL, acute lymphoblastic leukemia.
Characteristics of the studies included (II): interventions, outcomes, measures.
| Author (year) | Types of interventions | Comparison | Duration of intervention | Outcome measures | Measures of treatment effects | Follow-up time | Included studies |
|---|---|---|---|---|---|---|---|
| Gurion et al. (2011)16 | • CSFs including: -G-CSF -GM-CSF • Administered either intravenously or subcutaneously, started with or after chemotherapy and continued for more than 24 h • Excluded: CSFs administered for the purpose of stem cell collection and/or priming | • Placebo • No treatment | 1 to 42 days | Primary outcomes • All-cause mortality at the end of study follow-up • Overall survival Secondary outcomes • All-cause mortality at 30 days • Number of patients achieving complete remission • Disease-free survival • Neutropenia duration • Episodes of febrile neutropenia • Episodes of invasive fungal infections • Bacteremia • Duration of hospital stay • Any adverse events • Adverse events requiring discontinuation of CSFs • Secondary leukemia | • Death • Number of adverse events • Days • Neutropenia: < 0.5-1.0 x 109/l neutrophils • Duration of neutropenia: ranged between 12 and 29 days • Fever (neutropenic fever): body temperature higher than 38.3°C or 38.5°C on one occasion or higher than 38°C on two or more occasions | 30 days 3-7 years | 19 trials 5,256 patients Age: < 15 to 88 years |
| Mhaskar et al. (2014)17 | • G-CSF or GM-CSF plus antibiotics | • Antibiotics alone • Placebo • No treatment | Not specified | Primary outcomes • Overall mortality • Infection-related mortality Secondary outcomes • Number of patients hospitalized for more than 10 days • Time to neutrophil recovery • Duration of grade IV neutropenia • Time to recovery from fever • Time to withdrawal from antibiotics • Time to defervescence • Treatment-related harms | • Days • Neutropenia: ANC < 1 x 109/l (1,000/mm3) • Fever: body temperature higher than 38.5°C on one occasion or higher than 38°C on two or more occasions • Grade IV neutropenia: ANC < 500/mm3 • Defervescence: the abatement of a fever due to a decrease in body temperature | Not specified | 14 RCTs 1,553 participants Adults and children |
| Sasse et al. (2005)20 | • CSFs given concomitantly or 24 to 48 h after concluding the chemotherapy course as primary or secondary prophylaxis to prevent febrile neutropenia • Excluded: trials using CSF for the treatment of febrile neutropenia because this is a different situation | • Placebo • No treatment | Not specified | Primary outcomes • Number of febrile neutropenia episodes • Overall mortality Secondary outcomes • Time to neutrophil count recovery • Incidence and length of hospitalization • Number of infectious diseases • Incidence and length of treatment delays • Side effects (flu-like syndrome, bone pain, and allergic reactions) • Relapse | • Death Number of infectious disease episodes • Febrile neutropenia: oral temperature above 38.3°C unrelated to transfusions associated with grade IV neutropenia (ANC < 500/mm3) • Number of episodes and days of hospitalization Secondary endpoints • Time to neutrophil count recovery: number of days needed for the ANC to rise from < 500/mm3 to 500/mm3 after each course of chemotherapy | 28 days to 27 months | 6 RCTs 333 participants Age: 0 to 18 years |
| Cheuk et al. (2016)15 | • Medical interventions (drugs: enalapril, phosphocreatine, vitamin C combined with ATP, vitamin E, and coenzyme Q10) • Excluded: surgical interventions | • Placebo • Other medical intervention(s) No treatment | 14 days | Primary outcomes • Overall survival • Mortality due to heart failure • Development of clinical heart failure • The occurrence of adverse events and tolerability Secondary outcomes • Change in cardiac function measured by different diagnostic tests • Duration of hospital stay during heart failure • Change in stage of heart failure • Change in quality of life • Costs | • Death • Occurrence of clinical heart failure • Number of adverse events • Measures of cardiac function: MCI, LVESWS, SVI, SF • Days | 2 weeks to 6.1 years | 2 RCTs 203 participants: 135 childhood cancer survivors 68 children with ALL |
| van As et al. (2012)21 | • Platinum-based therapy (cisplatin, carboplatin, oxaliplatin) together with a protective medical intervention | • Platinum-based therapy with • Placebo • No additional treatment A different protective medical intervention | 15min immediately prior to cisplatin | Primary outcomes • Hearing loss • Tinnitus Secondary outcomes • Tumor response (complete and partial remission) • Survival (overall survival and event-free survival) • Adverse effects other than hearing loss and tinnitus (grade 3 or higher) • Quality of life | • Tumor response: number of patients with a complete, good or partial response | Not specified | 3 RCTs 149 participants Age: 0 to 18 years |
| Riley et al. (2015)19 | • Oral cryotherapy | • Standard care • No treatment Any other treatment to prevent oral mucositis | 30min to 7 h | Primary outcomes • Mucositis incidence of any severity Secondary outcomes • Interruptions to cancer treatment • Oral pain • Quality of life • Normalcy of diet • Adverse events • Number of days in the hospital • Number of days of treatment with opioid analgesics • Number of days unable to take medicine orally | • Mucositis measured on a 0 to 4 point scale (none to severe) -Any mucositis (0 versus 1+) -Moderate to severe mucositis (0 to 1 versus 2+) -Severe mucositis (0 to 2 versus 3+) • Oral pain: visual analogue scale | 6 months to 5 years | 14 RCTs 1,316 participants Age: 8 to 85 years |
| Phillips et al. (2016)18 | • Standard pharmacological antiemetic: 5-HT3 antagonists Benzodiazepines Cannabinoids Corticosteroids Cyclizine Dopamine blockers Levomepromazine • Excluded: NK1 antagonists, nonpharmacological approaches | • Placebo • Any active comparator (cannabinoids; benzodiazepines) | Not specified | • Complete control of nausea • Complete control of vomiting • Adverse effects • Quality-of-life | • Number of vomits per day | 24 h | 34 RCTs 2,023 participants Age: 2 months to 23 years |
| Ward et al. (2015)22 | • One form of nutritional support • Administration of nutrients in place of or in addition to normal eating • Parenteral nutrition: Intravenous administration of nutrients containing, as a minimum, glucose, and amino acids • Enteral nutrition: Delivery of any substance of nutritional value in solid or liquid form that passes any part of the digestive tract, regardless of the method of delivery • Excluded: vitamin and micronutrient supplementation | • Another or with no nutritional support (i.e., usual food intake, fluid therapy) | 10 to 145 days | Primary outcomes • Change in nutritional indices • Adverse events • Calorie and nutritional intake Secondary outcomes • Number of deaths at end of study • Length of hospital stay • Patient tolerance of or adherence to the nutritional intervention • Participant perceived health status | • Weight • Height • Body mass index • Fat-free body mass • Total body water • Arm anthropometry (triceps/biceps skinfold thickness, arm circumference, arm muscle area) • Serum albumin • Pre-albumin • Infection rate • Total energy intake • Total protein intake | 2 weeks to 3 months | 14 RCTs 562 participants Age: < 21 years |
CSFs, colony-stimulating factors; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; ALL, acute lymphoblastic leukemia; RCTs, randomized control trials; ANC, absolute neutrophil count; ATP, adenosine triphosphate; MCI, maximal cardiac index; LVESWS, left ventricular end-systolic wall stress; SVI, stress-velocity index; SF, shortening fraction; 5-HT3, 5-hydroxytriptamine receptor.
Key results reported in systematic reviews.
| Name of the study | Key results |
|---|---|
| Colony-stimulating factors for prevention and treatment of infectious complications in patients with acute myelogenous leukemia16 | • CSFs should not be given routinely to AML patients post-chemotherapy since they do not improve overall survival or infectious parameters including the rate of bacteremia and invasive fungal infections • On the other hand, the results showed that they do not adversely affect hematological outcomes such as complete remission, relapse rate, and disease-free survival |
| Colony-stimulating factors for chemotherapy-induced febrile neutropenia17 | • The use of CSFs plus antibiotics in individuals with chemotherapy-induced febrile neutropenia had no effect on overall mortality but reduced the number of time participants spent in the hospital and improved their ability to achieve neutrophil recovery • It was not clear whether CSF plus antibiotics had an effect on infection-related mortality • Participants receiving CSFs had a shorter duration of neutropenia, faster recovery from fever and shorter duration of antibiotics use |
| Colony-stimulating factors for prevention of myelosuppressive therapy-induced febrile neutropenia in children with acute lymphoblastic leukemia20 | • There is evidence that the prophylactic administration of CSFs reduces the duration of hospitalization and the rate of infection during treatment • There was no evidence that CSF shortened the duration of neutropenia episodes or diminished the delay of chemotherapy courses in pediatric patients with ALL undergoing myelosuppressive chemotherapy • Although there were statistically significant fewer febrile neutropenia episodes in the CSF group, substantial heterogeneity between trials prevents it from drawing conclusions, and no useful information available on survival |
| Medical interventions for treating anthracycline-induced symptomatic and asymptomatic cardiotoxicity during and after treatment for childhood cancer15 | • Although there is some evidence that enalapril temporarily improves one parameter of the cardiac function, it is unclear whether it improves clinical outcomes • Enalapril was associated with a higher risk of dizziness or hypotension and fatigue. Clinicians should weigh the possible benefits with the known side effects of enalapril in childhood cancer survivors with asymptomatic anthracycline-induced cardiotoxicity • Only one trial evaluated the effect of phosphocreatine in childhood cancer patients with anthracycline-induced cardiotoxicity. Limited data with a high risk of bias showed no significant difference between phosphocreatine and control treatments on echocardiographic function and clinical outcomes |
| Medical interventions for the prevention of platinum-induced hearing loss in children with cancer21 | • Currently, there is no evidence from individual studies in children with osteosarcoma or hepatoblastoma treated with different platinum analogues and dosage schedules which underscores the use of amifostine as an otoprotective intervention as compared to no additional treatment • No eligible studies for other possible otoprotective medical interventions and other types of malignancies were identified; therefore, no conclusions can be made about their efficacy in preventing ototoxicity in children treated with platinum-based therapy • More high-quality research is needed |
| Interventions for preventing oral mucositis in patients with cancer receiving treatment: oral cryotherapy19 | • The oral cryotherapy leads to large reductions in the incidence of oral mucositis of all severities in adults receiving 5FU based treatment for solid cancers • There is less confidence in the ability of oral cryotherapy to reduce the incidence of oral mucositis in adults receiving high-dose melphalan-based cancer treatment prior to HSCT • Evidence suggests that oral cryotherapy does reduce oral mucositis in these adults, but we are less certain about the size of the reduction, which could be large or small |
| Antiemetic medication for prevention and treatment of chemotherapy-induced nausea and vomiting in childhood18 | • The overall picture of which antiemetics are the most effective in preventing chemotherapy-induced nausea and vomiting in childhood remains incomplete and imprecise • The 5-HT3 antagonists are effective in patients who are to receive emetogenic chemotherapy, with granisetron or palonosetron possibly better than ondansetron • Adding dexamethasone improves control of vomiting, although the risk-benefit profile of adjunctive steroid remains uncertain |
| Nutritional support in children and young people with cancer undergoing chemotherapy22 | • There is limited evidence from individual trials to suggest that PN is more effective than EN in well-nourished children and young people with cancer undergoing chemotherapy • There is limited evidence to suggest that enteral or parenteral glutamine-supplementation has no significant effect on mucositis, infection rates or length of hospital stay • Limited evidence suggests an energy dense feed increases mean daily energy intake and has a positive effect on weight gain • The evidence for other comparisons and in malnourished patients remains unclear • There is a need for more well-designed, adequately powered trials assessing the effectiveness and safety of nutritional support in children and young people with cancer |
CSFs, colony-stimulating factors; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; 5FU, 5-fluorouracil; HSCT, haematopoietic stem cell transplantation; PN, parenteral nutrition; EN, enteral nutrition.
Gurion et al. (2011) evaluated the efficacy and safety of the colony-stimulating factor (CSF) for the prevention and treatment of infectious complications in patients with acute myeloid leukemia (AML).16 Nineteen RCTs with 5,256 participants aged 0 to 88 years were included. Colony-stimulating factors used were granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage-colony-stimulating factor (GM-CSF), administered after chemotherapy induction, consolidation or salvage treatment, and after hematopoietic stem cell transplantation. Placebo or no treatment groups were compared. Most clinical trials included patients > 15 years of age (12 trials, 63%). One test was performed in children aged 0 to 18 of age. Seventeen trials included patients subjected to induction chemotherapy (89%), one trial included patients subjected to consolidation chemotherapy and one trial included patients subjected to salvage chemotherapy. Chemotherapy protocols used in the trials were heterogeneous. They consisted of different combinations of anthracyclines and cytarabine with or without etoposide. According to the results, 14 trials with 4,119 participants reported all-cause mortality. Meta-analysis of data from these studies indicated that there was no difference in all-cause mortality among chemotherapy and CSFs groups compared with chemotherapy alone, with a risk ratio (RR) of 1.01 (95% confidence interval (95% CI) 0.98 to 1.05). For overall survival, a meta-analysis of 11 trials involving 3,335 participants reported no significant difference between patients treated with chemotherapy and CSFs compared with chemotherapy alone, with a hazard ratio (HR) of 1.00 (95% CI 0.93 to 1.08). The use of CSFs did not decrease the occurrence of febrile neutropenia episodes (RR 0.98; 95% CI 0.94 to 1.03), bacteremia (RR 0.96; 95% CI 0.82 to 1.12), or the incidence of invasive fungal infections (RR 1.40; 95% CI 0.90 to 2.19). The results also reported that CSFs significantly reduced the duration of neutropenia and hospital stay. Only two trials reported adverse event data: a study reported 11 cases of events in the CSFs group, and the other study reported 25 cases in the CSFs group compared with nine cases in the control group. The review concluded that CSFs should not be given routinely to patients with AML after chemotherapy since they do not improve survival or the prevalence of infections such as bacteremia and invasive mycosis. However, they do not negatively affect hematological outcomes, complete remission, relapse rates or disease-free survival.
Mhaskar et al. (2014) evaluated the safety and efficacy of the addition of granulocyte colony-stimulating factor (G-CSF) or granulocyte-macrophage-colony-stimulating factor (GM-CSF) to the standard antibiotic treatment for febrile neutropenia induced by chemotherapy in people diagnosed with cancer.17 In this review, 14 RCTs with 1,553 participants were included. From these trials, one included children and adults with hematological malignancies; three included children with mixed tumors, and the rest of the trials included adult participants presenting both mixed tumors and hematological malignancies (10 trials, 71%). The studies compared the use of a CSF plus antibiotics versus antibiotics alone. Seven studies evaluated the effects of G-CSF, six studies evaluated the effects of GM-CSF and one study evaluated G-CSF, GM-CSF, and placebo. According to the outcomes, a meta-analysis that grouped 13 trials (1,335 participants) showed that CSFs do not diminish the overall mortality (HR 0.74, 95% CI 0.47 to 1.16; p=0.19), or the mortality associated with infection (10 trials with 897 participants, HR 0.75, 95% CI 0.47 to 1.20; p=0.23). However, a meta-analysis of eight trials (1,221 participants) showed that CSF plus antibiotics reduced the number of patients hospitalized for more than ten days (RR 0.65, 95% CI 0.44 to 0.95; p=0.03). Similarly, the analysis of five trials (794 participants) for neutrophil recovery time showed a statistically significant effect of the CSF plus antibiotics compared to antibiotics alone (RR 0.52, 95% CI 0.34 to 0.81; p=0.004). For the duration of the neutropenia grade IV, time recovery from fever and the duration of antibiotics use, data analysis showed a statistically significant effect in favor of the CSF plus antibiotics compared to antibiotics alone. However, patients that received CSF plus antibiotics presented a higher incidence of symptoms such as bone and joint pain compared with patients treated with antibiotics alone. The review concludes that the use of CSF plus antibiotics in individuals with chemotherapy-induced febrile neutropenia has no effect on overall mortality, but reduces the time of hospital stay and improves neutrophil recovery.
Sasse et al. (2005)20 conducted a systematic review to evaluate the safety and effectiveness of the addition of CSF for the prevention of febrile neutropenia induced by myelosuppressive chemotherapy in children with acute lymphoblastic leukemia (ALL). The review included six RCTs with 332 participants: five trials examined the effects of G-CSF, and one trial analyzed the effects of GM-CSF. The majority of the studies used CSF as primary prophylaxis; only one study used CSF as secondary prophylaxis. These results reported that only two studies where patients received 451 cycles of treatment presented data on febrile neutropenia episodes, where the CSF group (226 cycles) reported 68 episodes compared with the control group (228 cycles) that presented 112 episodes (RR -0.47, 95% CI -0.77 to -0.16). These results showed a risk reduction of 47% of presenting febrile neutropenia with the use of CSF. Only two studies reported deaths: one by septic shock and three more by treatment-related toxicity. Three studies (134 participants) reported data on the duration of neutropenia. The combined data from the studies suggested that patients who received CSF had a shorter duration of neutropenia compared with patients who did not receive CSF (weighted mean difference (WMD)=- 3.44, 95% CI - 4.76 to - 2.12; p<0.00001). For the length of hospitalization, the meta-analysis of three clinical trials (134 participants) showed a significant benefit with the use of CSF compared with the control group (WMD=- 1.58, 95% CI - 3.00 to - 0.15; p=0.03). The combined analysis of five studies showed a significant reduction in the incidence of infections in the CSF group (45 episodes with 476 cycles) compared with the control group (91 episodes with 482 cycles) (RR=0.56, 95% CI 0.39 to 0.80, p=0.002). Among the adverse effects described were redness or swelling at the site of injection, fever, muscle aches, skin rash, urticaria, and bone pain. According to the evidence, the authors of this review concluded that the prophylactic administration of CSF reduces the length of the hospitalization and the rate of infection during the treatment. However, no evidence demonstrated that the use of CSF reduces the duration of neutropenia episodes or delays cycles of treatment in ALL pediatric patients receiving myelosuppressive chemotherapy.
Recently, Cheuk et al. conducted a systematic review, which objective was to compare the effect of medical interventions on the cardiotoxicity induced by anthracyclines in childhood cancer patients and survivors.15 The review included two RCTs with 203 participants, of which 135 were childhood cancer survivors and 68 were children with acute leukemia. In one of the clinical trials, enalapril was compared with placebo, whereas a 14-day treatment of phosphocreatine and vitamin C combined with adenosine triphosphate (ATP), vitamin E, and coenzyme Q10 was assessed in the other trial. In the latter trial, children showed symptomatic cardiotoxicity. For overall survival and mortality due to heart failure, this review reported that there were no deaths in neither of the compared groups nor the trials. The enalapril clinical trial reported on the occurrence of clinical heart failure, which was one case (1%) in the intervention group and six cases (9%) in the placebo group (RR 0.16, 95% CI 0.02 to 1.29; p=0.09). Both studies reported the emergence of adverse events; dizziness, hypotension, and fatigue presented a greater incidence in the enalapril group. In the phosphocreatine study, the type of events evaluated was not specified. In the enalapril trial, no statistically significant differences were detected in the rate of change of the parameters of heart function tests between comparison groups. Moreover, in the phosphocreatine trial, all participants had normal echocardiograms before and at the end of the treatment. None of the studies reported data on the duration of the hospital stay for heart failure. The review concluded that although there is some evidence that enalapril temporarily improves some parameters of cardiac function, it is not clear whether it improves clinical outcomes. Enalapril was associated with higher risk of dizziness and hypotension. Therefore, the possible benefits with these side effects in childhood cancer survivors with asymptomatic anthracycline-induced cardiotoxicity should be evaluated. Regarding to the effect of phosphocreatine in childhood cancer patients with anthracycline-induced cardiotoxicity, data were limited and with a high risk of bias. Thus, it was not possible to establish any conclusion.
van As et al. (2012) evaluated medical interventions for the prevention of platinum-induced hearing loss in children with cancer.21 This review included three clinical trials with 149 participants of ages ranging between 0 and 22 years. In two RCTs, participants were diagnosed with osteosarcoma, and in the other study, with hepatoblastoma. The three trials used amifostine as a possible otoprotective intervention. None of the studies reported a follow-up. For the loss of the hearing or tinnitus in a study with 28 participants who received intra-arterial platinum chemotherapy, the review reported that the group that received amifostine (15 patients) presented asymptomatic or symptomatic ototoxicity, as well as 10 of the 13 patients of the control group. Data analysis from the study showed no significant differences between the comparison groups (RR 1.29, 95% CI 0.94 to 1.77; p=0.11). In another trial with 39 participants where patients received intravenous platinum chemotherapy, 14/17 patients in the amifostine group and 15/19 patients in the control group presented symptomatic or asymptomatic ototoxicity. However, data analysis showed no significant differences between comparison groups (RR 1.04, 95% CI 0.76 to 1.44; p=0.80). A study with 28 participants who received intra-arterial platinum chemotherapy reported that tumor response showed no significant differences between the treatment groups (RR 1.60, 95% CI 0.97 to 2.63; p=0.06). There were 14 remissions among 15 patients from the amifostine group and seven remissions in the control group (12 patients). For adverse events, vomiting grade 3 or 4 and renal toxicity were reported. There was no difference in the number of events between different groups of treatment. The review concludes that there is no evidence from individual studies in children with osteosarcoma or hepatoblastoma treated with various analogs of platinum that demonstrates some benefit of the amifostine as an otoprotective intervention in comparison with no additional treatment. Since other studies for otoprotective medical interventions, and other types of malignant tumors do not exist, it is not possible to establish conclusions about their effectiveness in the prevention of ototoxicity in children treated with platinum.
Riley et al. (2015) developed a systematic review, which objective was to evaluate the effects of oral cryotherapy to prevent oral mucositis in patients receiving anticancer treatment.19 This review included studies that compared oral cryotherapy versus standard care, no treatment, or any other treatment to prevent oral mucositis produced by chemotherapy, radiotherapy, and targeted therapy. It included 14 RCTs with 1,316 participants aged between 8 and 85 years. However, only one study reported the inclusion of children. In eight studies, the participants presented a type of solid cancer, while the participants presented hematological cancers, such as multiple myelomas, Hodgkin lymphoma, or non-Hodgkin lymphoma in five studies. In most of the studies included in the review, pieces of ice to cool the oral cavity were used. The duration of the treatment varied according to the chemotherapy regime, being the longest of seven hours. The combined analysis of five studies showed the following results in patients with solid cancer who received fluorouracil (5FU) (444 participants). Oral cryotherapy reduced the risk of developing oral mucositis compared with the standard treatment or no treatment (RR 0.61, 95% CI 0.52 to 0.72; p<0.00001); i.e., oral cryotherapy decreased the risk of developing oral mucositis in 39%. The combined analysis of five studies in patients who received treatment with melphalan before stem cell transplantation (270 participants) showed that oral cryotherapy reduced the risk of developing oral mucositis compared with the standard treatment or no treatment (RR 0.59, 95% CI 0.35 to 1.01; p=0.05). Three trials showed that oral cryotherapy reduced the duration and severity of oral pain when compared with the control group. In addition, only one study reported that oral cryotherapy reduced the length of time of parenteral nutrition by 2.18 days compared with the control group (95% CI 0.03 to 4.33 days; p=0.05). The most common adverse events reported in the study were of mild intensity, such as headaches, chills, numbness, taste disturbance, shooting pain from the teeth and coldness. The review concluded that cryotherapy could be useful in the reduction of the incidence of oral mucositis in patients that receive 5FU-based treatment for solid cancers.
Phillips et al. (2016) assessed the efficacy and safety of pharmacological interventions for the prevention of nausea and vomiting in children and young people (<18 years) that received chemotherapy.18 Thirty-four RCTs, which in turn included 2,023 participants, were included in the review, where standard antiemetic drugs versus placebo or any active intervention were compared. The trials examined a group of different antiemetic drugs, used different doses and comparators, and reported a variety of outcomes. Neither data on any outcomes beyond the first 24hours of chemotherapy was reported, nor was the time of duration of the different treatments evaluated. According to the results of the review, it was reported that it was not possible to describe the interventions separated by groups of age, type of tumor or chemotherapy received. The results on the efficiency of the cannabinoids in comparison with antiemetic alternatives reported a beneficial effect of tetrahydrocannabinol compared with prochlorperazine for the complete control of acute nausea (RR 20.7, 95% CI 17.2 to 36.2) and complete control of vomiting (RR 19.0, 95% CI 13.7 to 26.3). Similarly, another trial that compared nabilone versus domperidone demonstrated a reduction of nausea. Two trials examined steroids as antiemetics; one of them assessed the efficacy of dexamethasone versus metoclopramide, demonstrating that dexamethasone was significantly better for the complete control of vomiting (RR 2.10, 95% CI 1.77 to 2.50). The other trial evaluated methylprednisolone compared with chlorpromazine for the control of vomiting, but no statistically significant differences among treatments were found (RR 1.0, 95% CI 0.54 to 1.86). Three studies comparing ondansetron with granisetron were identified; the combined analysis of the data found no statistically significant differences between the treatments for the prevention of acute nausea or vomiting retardation. However, regarding the prevention of acute vomiting, granisetron was significative better that ondansetron (RR 2.26, 95% CI 2.04 to 2.51). Four other studies compared different doses of granisetron (10α/4g/kg versus 40α/4g/kg); nevertheless, the results demonstrated no significant differences between doses for complete control of acute vomiting (RR 0.88, 95% CI 0.70 to 1.10). The review also reported the results of seven studies that evaluated different agents, such as chlorpromazine, metoclopramide, hydroxyzine, fentanyl, combined formulas of drugs (‘cocktails tm)) that contained lorazepam, dexamethasone, metoclopramide, and benztropine (LDMB), or granisetron, dexamethasone, midazolam, and diphenhydramine (GDMD), and a study that evaluated an intervention with traditional Chinese herbal medicine. From these studies, two showed that both chlorpromazine and domperidone are more effective than metoclopramide for the control of nausea and vomiting. The most common adverse effects reported with the use of 5-HT3 antagonists were sedation, somnolence, headaches, and abdominal pain, and with cannabinoids were drowsiness and alteration of mood. The review concluded that 5-HT3 antagonists (granisetron or palonosetron) were effective for the control and the prevention of nausea and vomiting in patients that receive emetogenic chemotherapy. Furthermore, adding dexamethasone improved the control of vomiting; however, adjunctive steroid risk-benefit profile remains uncertain.
A review of Ward et al. (2015), whose objective was to determine the effects of nutritional support in children and young people with cancer undergoing chemotherapy, included 14 RTC with a total of 562 participants under 21 years of age.22 The studies included children and young participants with leukemia or solid tumors that received chemotherapy, radiotherapy or both. The studies evaluated different interventions such as parenteral nutrition (peripheral or central parenteral nutrition); enteral nutrition (usual or nasogastric food intake); fluid therapy; energy-dense fructooligosaccharide (fibre) supplementation; glutamine supplementation of enteral or parenteral nutrition; value of different lipid formulations, and calory and nutritional intake, in order to establish different comparisons. For the parenteral nutrition (PN) compared with enteral nutrition (EN) (usual food intake) outcomes, a clinical trial with 25 participants reported that the PN group had an average increased weight of 12.9% in comparison with the usual food intake group. A trial with ten participants found changes in weight in favor of the NP group (mean difference 4.12, 95% CI 1.91 to 6.33; p=0.0003). Another clinical trial with 23 participants, where measures were taken from the beginning to the end of radiation therapy, found that the mean percentage of change of the cutaneous or triceps skinfold thickness increased 13.9% in the PN group in comparison with the usual food intake group where no change was observed. The same trial reported that the mean percentage of change in the arm circumference increased 2.65% for the PN group compared with no changes in the usual food intake group. Finally, for the arm muscle circumference, the mean percentage of change decreased 0.2% in the PN group in comparison with an increase of 0.4% in the usual food intake group. The adverse events reported in these studies were sepsis, pneumonia, infection of the urinary tract without significant differences in the number of infections presented in either of the comparison groups. For mortality at the end of the study, the combined analysis of three trials with 52 participants reported no differences in the number of deaths between the PN group compared with the usual food intake group (RR 1.19, 95% CI 0.32 to 4.39; p=0.80). For the rest of the outcomes, no significant differences were observed in the various comparisons of the trials. The review concluded that the evidence of individual studies is limited and there are no tests to suggest that PN is more efficient that EN in well-nourished children and young people with cancer undergoing chemotherapy. There is limited evidence suggesting that enteral or parenteral glutamine supplementation has no significant effect on the mucositis, infection rates or length of hospital stay. Evidence of other comparisons in patients with malnutrition remains unclear.
The above results should be interpreted cautiously since they mostly derive from low-quality evidence based on few studies and small-sized samples.
4DiscussionThe majority of cancer patients undergoing treatment develop side effects to the therapy. Many of these effects tend to be symptomatic; however, they may also occur in an asymptomatic way. Some of these are potentially deadly complications; therefore, it is important to have effective medical interventions that will help in the prevention and treatment of the symptoms induced by anticancer therapy in these patients. These complications can also lead to interruptions or alterations in the cancer therapy, which can reduce the survival rate. Similarly, cancer survivors face problems that can affect their organic function, causing diseases that become limiting to life or that produce a low quality of life. Therefore, physicians who face patients with cancer and cancer survivors should be capable of taking well-informed decisions on the risks and benefits of the different treatment options regarding the side effects of cancer therapies.
This review summarizes the evidence found on the current medical interventions for the management of cancer treatment-induced symptoms in pediatric patients. Revisions developed by the Cochrane methodology were selected since they are considered as high quality because of the methodological rigor by which they are carried out.
Only a small number of systematic reviews developed in children populations were identified, maybe because there are still few studies (clinical trials) in children. It was also observed that the evaluated interventions are still scarce and do not include all the possible symptoms or effects developed by the different therapies that cancer patients receive. Within the evaluated interventions listed in the review, the vast majority showed no beneficial effects on the patients tm) symptoms (i.e., they did not decrease the risk of presenting symptoms or events secondary to the treatment). The most reported outcomes were that there was no difference in the effects when comparing the interventions evaluated with placebo, standard treatment, no treatment or any other active intervention. Similarly, the results that showed the effectiveness of any intervention were based on individual studies or the combination of results from a limited number of clinical trials (two to four studies). The great majority of the studies included in the systematic reviews were of low quality, with a high risk of bias and with small-sized samples.
To our knowledge, no other similar study that identified and described these interventions exists. Thus, this review is original and allows to demonstrate in a succinct way what are the best interventions for the prevention and management of the treatment-induced symptoms in children with cancer.
4.1Implications for clinical practiceThis review includes very recent data and even some unpublished data. The information presented offers some guidance that allows supporting the clinical practice of health care professionals in addition to strengthening the international recommendations on the use of interventions for the management of patients with cancer.
4.2Implications for researchFurther research focused on children populations is required, especially in childhood cancer. More long-term studies with representative sample sizes of this population to assess the effects of medical interventions for the management of cancer treatment-induced symptoms should be undertaken. It is essential to make trials with a greater statistical power and an adequate methodological quality.
So far, there is not enough information about the efficiency of the interventions for the prevention and management of the treatment-induced symptoms in children with cancer. The results show that only a small number of clinical trials have evaluated these interventions.
The current evidence is limited and of low quality, for which it is not possible to determine the security, efficiency, and utility of the interventions for the prevention and management of the treatment-induced symptoms in children with cancer conclusively.
In our opinion, at present, this review is the most comprehensive proof on this topic, and while there are some important implications for future research, these findings provide timely information to guide the clinician on the effectiveness of interventions, and thereby contribute to the proper use of these interventions.
FundingThis project did not receive any funding.
Conflict of interestThe authors declare no conflicts of interest of any nature.