ENSANUT 2012 showed a combined prevalence of overweight and obesity of 34.4% in Mexican children. Single nucleotide polymorphisms (SNPs) of the ADIPOQ and ADIPOR2 genes have been reported in many populations, but their association with obesity has not been confirmed in other studies. Our aim was to determine the association of SNPs from ADIPOQ and ADIPOR2 genes with obesity in Mexican children.
MethodsA total of 2,634 children from 6 to 12 years old were enrolled in the study from four IMSS Units in Mexico City. We selected 1,469 unrelated children (745 normal weight and 724 overweight/obese). Phenotype characterization included anthropometric measurements, blood pressure, biochemical parameters, insulin concentrations and presence of acanthosis nigricans (AN). Analysis of the SNPs rs182052, rs266729, rs2241766, rs822393 of ADIPOQ and rs11061971 of ADIPOR2 was carried out in the DNA samples.
ResultsThe study showed significant differences (p <0.05) between groups in waist circumference, blood pressure, presence of AN, insulin concentrations, HOMA-IR, fasting glucose and lipid parameters, being higher in obese children. No associations in ADIPOQ variants with the presence of overweight/obesity were found. The presence of the variant rs11061971 of ADIPOR2 in children had a significant association with protection of overweight/obesity (OR 0.79, 95% CI 0.68-0.93, p = 0.003). Also, the log-additive model confirmed the association by codominant and dominant models (p <0.05).
ConclusionsThe presence of rs11061971 of ADIPOR2 variant confers protection against obesity and could be used as a marker in Mexican children.
ENSANUT 2012 mostró una prevalencia combinada de sobrepeso y obesidad en el 34.4% en niños mexicanos. Se han reportado polimorfismos de un solo nucleótido (SNP) de los genes ADIPOQ y ADIPOR2 en varias poblaciones, pero su asociación con la obesidad ha sido controversial. El objetivo de este trabajo fue determinar la asociación de SNP de ADIPOQ y ADIPOR2 con obesidad en una muestra de niños mexicanos.
MétodosUn total de 2,634 niños de 6-12 años se inscribieron en el estudio en cuatro unidades del Instituto Mexicano del Seguro Social en la Ciudad de México. Se seleccionaron 1,469 niños no emparentados (745 peso normal y 724 sobrepeso/obesidad). Se les tomaron medidas antropométricas, presión arterial, parámetros bioquímicos, insulina y presencia de acantosis nigricans (AN). El análisis de los SNP (rs182052, rs266729, rs2241766, rs822393 de ADIPOQ y rs11061971 de ADIPOR2) se realizó en muestras de ADN.
ResultadosSe observaron diferencias significativas (p < 0.05) entre los grupos en la circunferencia de cintura, presión arterial, AN, insulina, HOMA-IR, glucosa en ayunas y parámetros lipídicos siendo elevados en los niños obesos. No se encontró asociación en variantes ADIPOQ con la presencia de sobrepeso/obesidad. La presencia de rs11061971 de ADIPOR2 tuvo una asociación significativa con la protección de sobrepeso/obesidad (OR de 0.79; IC95% 0.68 a 0.93, p = 0.003). El modelo Log-aditivo confirmó la asociación de los modelos codominante y dominante (p < 0.05).
ConclusionesLa presencia de la variante rs11061971 de ADIPOR2 confiere protección contra la obesidad, y podría utilizarse como marcador en niños mexicanos.
Obesity is a public health problem that has increased dramatically in recent years, with epidemic proportions worldwide.1–4 Obesity in Mexico is increased on average of 1.1 percentage points per year. The ENSANUT 2012 showed a combined prevalence of overweight and obesity of 34.4% in both sexes in children. It represents about 5,664,870 overweight and obese children in a national population.5
Many factors are associated with obesity in childhood, for example, lack of physical activity, diets with high content of carbohydrates and the genetic predisposition.6,7 Previous studies have concluded that high concentration of adiponectin predict a lower prevalence of type 2 diabetes (T2D) in Mexican children8 and that serum adiponectin can be a biomarker to predict metabolic syndrome in eutrophic and obese children.9
Adiponectin binds to its receptors (AdipoR1 and AdipoR2) for signaling actions. Adiponectin exhorts its effects through the sensitization of the body to the insulin10,11 by activating numerous signaling molecules including adenosine monophosphate-activated protein kinase (AMPK), p38-MAPK, JNK, PPARα transcription factor and NF-κB in multiple tissues. These signals are transduced via the AdipoRs.12,13 Mechanisms regulating the expression of AdipoRs appear to be complex and are governed by numerous factors.
The polygenic nature of obesity and the interactions of single nucleotide polymorphisms (SNPs) in overweight/obesity are not clear.14 SNPs of the ADIPOQ gene have been reported in many populations; however, some of these associations with obesity could not be confirmed in other studies. The human ADIPOR2 gene is generally not associated with serum adiponectin but is associated with insulin resistance and T2D risk in genetic association studies, but the mechanisms are not yet clear.15–19
The aim of present study was to determine the association of obesity with SNP variants rs182052, rs266729, rs2241766, rs822393 of ADIPOQ and the rs11061971 of ADIPOR2 genes in Mexican children.
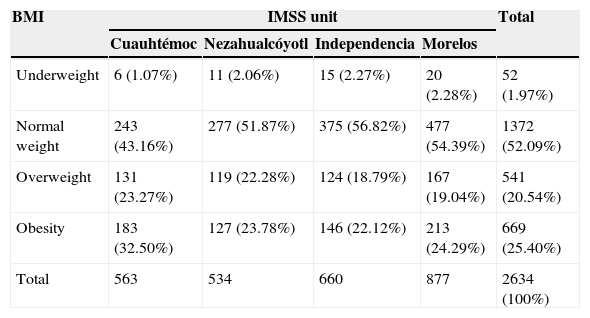
2Methods2.1ParticipantsA total of 2,634 children from 6–12 years old were enrolled in the study from four IMSS Units in Mexico City: Cuauhtémoc, Nezahualcóyotl, Independence and Morelos (north, east, south, and northeast corresponding areas). A general description of the children studied is shown in Table 1. Children were randomly selected to participate in a cross-sectional study between July 2011 and July 2012.
General characteristics of the four units from the IMSS (n = 2634).
| BMI | IMSS unit | Total | |||
|---|---|---|---|---|---|
| Cuauhtémoc | Nezahualcóyotl | Independencia | Morelos | ||
| Underweight | 6 (1.07%) | 11 (2.06%) | 15 (2.27%) | 20 (2.28%) | 52 (1.97%) |
| Normal weight | 243 (43.16%) | 277 (51.87%) | 375 (56.82%) | 477 (54.39%) | 1372 (52.09%) |
| Overweight | 131 (23.27%) | 119 (22.28%) | 124 (18.79%) | 167 (19.04%) | 541 (20.54%) |
| Obesity | 183 (32.50%) | 127 (23.78%) | 146 (22.12%) | 213 (24.29%) | 669 (25.40%) |
| Total | 563 | 534 | 660 | 877 | 2634 (100%) |
Data represented in frequency and percentages by IMSS unit studied.
Unrelated children (n = 1,469) were classified into normal weight (n = 745) and overweight/obesity (n = 724) groups according to their body mass index (BMI) percentile described by the U.S. Centers for Disease Control and Prevention (CDC). Children with autoimmune diseases, cancer or other diseases related to obesity were excluded. The study was conducted after informed consent was signed by parents and children, respectively. The National Committee and the Ethics Committee Board from the IMSS National Research Commission approved the protocol.
2.2ProcedureInvitation for the study was conducted in each unit mentioned. The personnel enrolled in the project explained the benefits of participation in the study. Participants were evaluated with questionnaires and anthropometric measurements, and biochemical and genetic studies were applied. Each of the steps was performed according to related norms and clinical guidelines.
2.3Clinical recordsAll participants were weighed using a digital scale (Seca, Hamburg, Germany). Height was measured with a portable stadiometer (Seca 225) and waist circumference (WC) was measured at the midpoint between the lowest rib and the iliac crest after a normal exhalation with children in the standing position. Body mass index (BMI) was calculated and classified according to the CDC 2000 (Atlanta, GA, USA) references (eutrophic children 10th to <85th percentile, overweight children ≥85th to <95th percentile and obese children ≥95th percentile in BMI). Blood pressure was measured by auscultatory method using a mercurial sphygmomanometer (ALPK2, Tokyo, Japan) with appropriate cuff size for arm length following North American guidelines issued in 2004. Blood pressure readings were taken for each participant twice, on the right arm in a sitting position, resting 5min between each measurement, and considering the level of blood pressure as the mean of the readings. The presence of acanthosis nigricans (AN) was recorded in the neck, armpit, under the breast or a skin crease and is described as positive if presented in any of the areas mentioned.
2.4Biochemical studiesBlood samples were taken in children after a 12-h fast. Biochemical analysis included fasting glucose, total cholesterol, HDL-C and LDL-C and triglycerides using the ILab 350 Clinical Chemistry System (Instrumentation Laboratory IL, Barcelona, Spain). Insulin (μU/mL) was measured by chemiluminiscence (IMMULITE) and the HOMA-IR was calculated for insulin resistance.
2.5Genotyping and genetic analysisGenomic DNA was isolated from peripheral blood using a standard protocol for DNA extraction with a FLEX STAR Autogen (Holliston, MA). All samples were run in 0.8% agarose gels stained with ethidium bromide to verify the integrity and purity by 260/280 DO. We selected the most promising SNPs of the adiponectin gene and the receptor and minor allele frequencies ≥10% in the Mexican population according to the HapMap database. SNPs were rs182052, rs266729, rs2241766, and rs822393 of ADIPOQ and rs11061971 of ADIPOR2. Genotyping was performed using the TaqMan OpenArray Real-Time PCR System (Life Technologies, Carlsbad, CA) following the manufacturer's instructions. The genotype success rate was at least 98%, and no deviation (p ≥0.05) from Hardy-Weinberg equilibrium was observed in the analysis. Thirty random samples were done in duplicate for genotype quality control with 100% concordance.
2.6Statistical analysisTests were performed to check whether there were significant differences between cases and controls for the anthropometric and biochemical variables. Comparison between groups was done using the t test for continuous variables. For categorical data we used χ2 test. Logistic regression models were performed to evaluate the association of overweight/obesity in different genotypes. We obtained odds ratios (OR) with 95% confidence intervals (CI) in the three main inheritance models: codominant, dominant and recessive, adjusted by age, gender and WHR. Statistical analysis was performed using STATA 11 software (Stata Corp LP, College Station, TX). We used the Stata program to estimate the statistical significance of p <0.05 and 95% CI in the study for each SNP with an expected OR of 1.2, 1.5, 2, 2.5; alpha = 0.05 and the minor allele frequency (MAF) for each SNP and each population in the study.
3ResultsTable 1 shows the general characteristics of the 2634 children studied. The presence of obesity ranged from 22-32% in the four units. Overweight and obesity was higher in Cuauhtémoc Unit, 23.27% and 32.50%, respectively, whereas the Independencia Unit showed the lowest percentage.
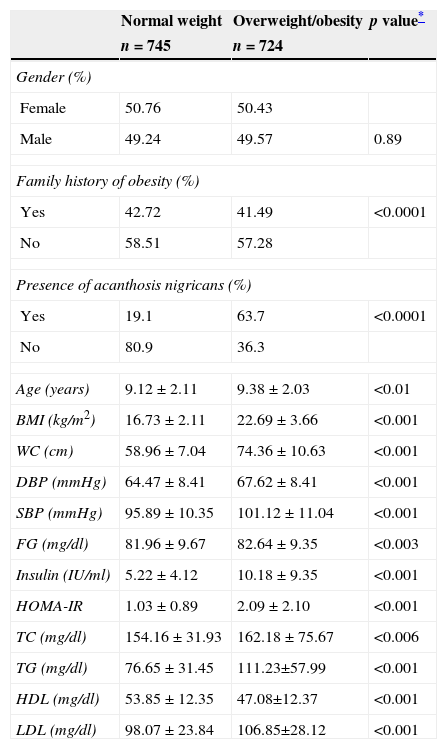
Table 2 shows the characteristics of the unrelated children (n = 1,469). The analysis comparison of normal weight children vs. overweight/obesity according to gender was similar in both groups. Family history of obesity was similar in children with normal weight and overweight/obese groups. The presence of AN was higher in the overweight/obesity group (p <0.0001). Waist circumference was significant (p <0.001) when comparing the groups and was 14cm higher in children with overweight/obesity. Systolic and diastolic blood pressures were significant in children with overweight/obesity (p <0.001). Biochemical parameters showed statistical significance in fasting glucose and insulin measured by HOMA-IR in overweight/obese children. Lipids were elevated in children with overweight/obesity compared to normal weight children. Triglyceride concentration increased to 30mg/dL in children with overweight and obesity.
Clinical characteristics of unrelated children studied (n = 1469).
| Normal weight | Overweight/obesity | p value* | |
|---|---|---|---|
| n = 745 | n = 724 | ||
| Gender (%) | |||
| Female | 50.76 | 50.43 | |
| Male | 49.24 | 49.57 | 0.89 |
| Family history of obesity (%) | |||
| Yes | 42.72 | 41.49 | <0.0001 |
| No | 58.51 | 57.28 | |
| Presence of acanthosis nigricans (%) | |||
| Yes | 19.1 | 63.7 | <0.0001 |
| No | 80.9 | 36.3 | |
| Age (years) | 9.12 ± 2.11 | 9.38 ± 2.03 | <0.01 |
| BMI (kg/m2) | 16.73 ± 2.11 | 22.69 ± 3.66 | <0.001 |
| WC (cm) | 58.96 ± 7.04 | 74.36 ± 10.63 | <0.001 |
| DBP (mmHg) | 64.47 ± 8.41 | 67.62 ± 8.41 | <0.001 |
| SBP (mmHg) | 95.89 ± 10.35 | 101.12 ± 11.04 | <0.001 |
| FG (mg/dl) | 81.96 ± 9.67 | 82.64 ± 9.35 | <0.003 |
| Insulin (IU/ml) | 5.22 ± 4.12 | 10.18 ± 9.35 | <0.001 |
| HOMA-IR | 1.03 ± 0.89 | 2.09 ± 2.10 | <0.001 |
| TC (mg/dl) | 154.16 ± 31.93 | 162.18 ± 75.67 | <0.006 |
| TG (mg/dl) | 76.65 ± 31.45 | 111.23±57.99 | <0.001 |
| HDL (mg/dl) | 53.85 ± 12.35 | 47.08±12.37 | <0.001 |
| LDL (mg/dl) | 98.07 ± 23.84 | 106.85±28.12 | <0.001 |
Results are shown as percentages for categorical variables and mean ± standard deviation for continuous variables.
BMI, body mass index; WC, waist circumference; DBP, diastolic blood pressure; SBP, systolic blood pressure; FG, fasting glucose; HOMA-IR, Homeostasis Model Assessment-Insulin Resistance; TC, total cholesterol; TG, triglycerides; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
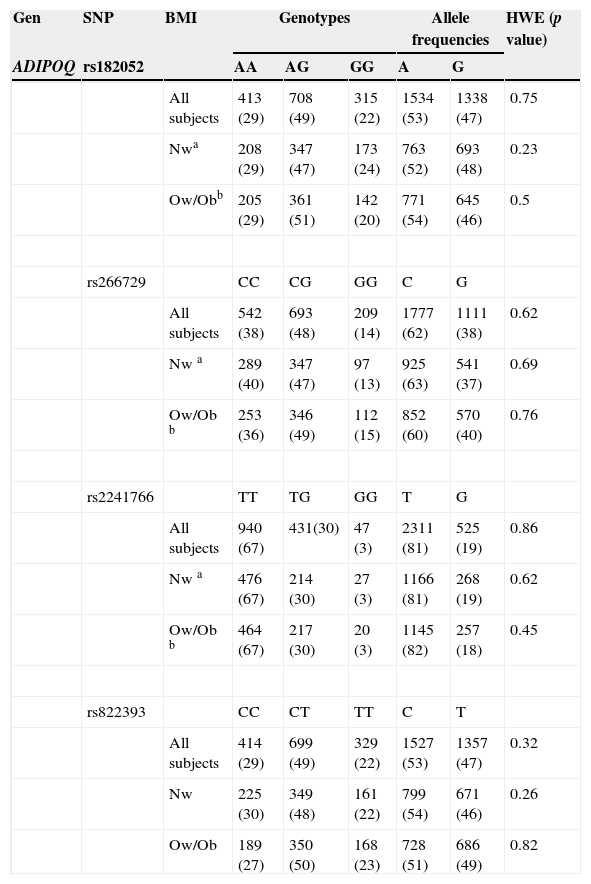
Table 3 shows the allele frequencies and genotypes rs182052, rs266729, rs2241766, rs822393 of ADIPOQ and rs11061971 of ADIPOR2 SNPs being displayed according to BMI children. All polymorphisms studied were in Hardy-Weinberg equilibrium (HWE).
Genotypic, allelic frequencies and HWE of ADIPOQ and ADIPOR2 genes.
| Gen | SNP | BMI | Genotypes | Allele frequencies | HWE (p value) | |||
|---|---|---|---|---|---|---|---|---|
| ADIPOQ | rs182052 | AA | AG | GG | A | G | ||
| All subjects | 413 (29) | 708 (49) | 315 (22) | 1534 (53) | 1338 (47) | 0.75 | ||
| Nwa | 208 (29) | 347 (47) | 173 (24) | 763 (52) | 693 (48) | 0.23 | ||
| Ow/Obb | 205 (29) | 361 (51) | 142 (20) | 771 (54) | 645 (46) | 0.5 | ||
| rs266729 | CC | CG | GG | C | G | |||
| All subjects | 542 (38) | 693 (48) | 209 (14) | 1777 (62) | 1111 (38) | 0.62 | ||
| Nw a | 289 (40) | 347 (47) | 97 (13) | 925 (63) | 541 (37) | 0.69 | ||
| Ow/Ob b | 253 (36) | 346 (49) | 112 (15) | 852 (60) | 570 (40) | 0.76 | ||
| rs2241766 | TT | TG | GG | T | G | |||
| All subjects | 940 (67) | 431(30) | 47 (3) | 2311 (81) | 525 (19) | 0.86 | ||
| Nw a | 476 (67) | 214 (30) | 27 (3) | 1166 (81) | 268 (19) | 0.62 | ||
| Ow/Ob b | 464 (67) | 217 (30) | 20 (3) | 1145 (82) | 257 (18) | 0.45 | ||
| rs822393 | CC | CT | TT | C | T | |||
| All subjects | 414 (29) | 699 (49) | 329 (22) | 1527 (53) | 1357 (47) | 0.32 | ||
| Nw | 225 (30) | 349 (48) | 161 (22) | 799 (54) | 671 (46) | 0.26 | ||
| Ow/Ob | 189 (27) | 350 (50) | 168 (23) | 728 (51) | 686 (49) | 0.82 | ||
| ADIPOR2 | rs11061971 | TT | TA | AA | T | A | ||
|---|---|---|---|---|---|---|---|---|
| All subjects | 589 (41) | 661 (46) | 186 (13) | 1839 (64) | 1033 (36) | 1 | ||
| Nw | 275 (38) | 350 (48) | 104 (14) | 900 (62) | 558 (38) | 0.7 | ||
| Ow/Ob | 314 (44) | 311 (44) | 82 (12) | 939 (66) | 475 (34) | 0.74 |
The results are shown as numbers of patients for each genotype and allele frequency. Data in parentheses are given in percentages.
HWE, Hardy-Weinberg equilibrium; BMI, body mass index; Nw, normal weight; Ow,Ob, overweight/obesity.
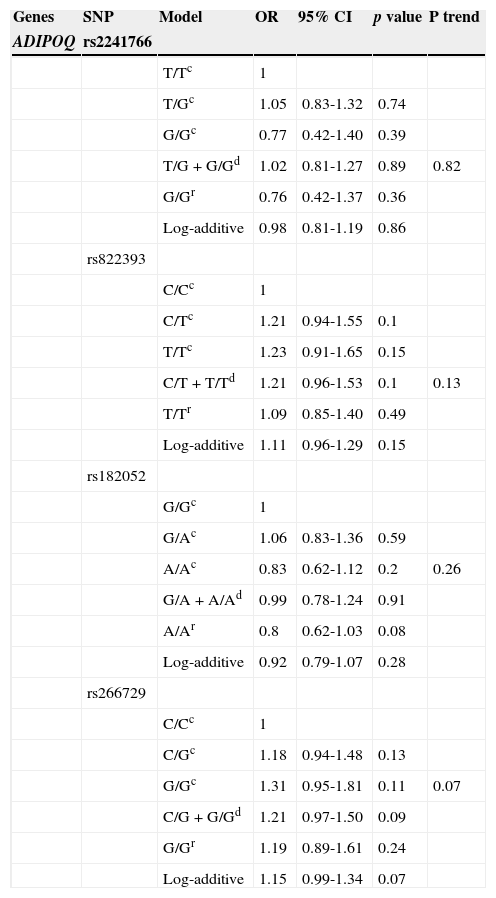
Table 4 shows the effect of the five polymorphisms and the risk of overweight/obesity with ORs estimated through logistic regression adjusted by age, gender and family history of obesity. No association with obesity was shown in ADIPOQ SNPs, whereas the ADIPOR2 rs11061971 polymorphism had a significant protective effect (OR 0.79, 95% CI 0.68-0.93, p = 0.003) in the log-additive model. This association was confirmed by codominant and dominant model (p <0.05).
Estimated effect of association by Mendelian inheritance model of ADIPOQ and ADIPOR2 polymorphism with overweight/obesity.
| Genes | SNP | Model | OR | 95% CI | p value | P trend |
|---|---|---|---|---|---|---|
| ADIPOQ | rs2241766 | |||||
| T/Tc | 1 | |||||
| T/Gc | 1.05 | 0.83-1.32 | 0.74 | |||
| G/Gc | 0.77 | 0.42-1.40 | 0.39 | |||
| T/G + G/Gd | 1.02 | 0.81-1.27 | 0.89 | 0.82 | ||
| G/Gr | 0.76 | 0.42-1.37 | 0.36 | |||
| Log-additive | 0.98 | 0.81-1.19 | 0.86 | |||
| rs822393 | ||||||
| C/Cc | 1 | |||||
| C/Tc | 1.21 | 0.94-1.55 | 0.1 | |||
| T/Tc | 1.23 | 0.91-1.65 | 0.15 | |||
| C/T + T/Td | 1.21 | 0.96-1.53 | 0.1 | 0.13 | ||
| T/Tr | 1.09 | 0.85-1.40 | 0.49 | |||
| Log-additive | 1.11 | 0.96-1.29 | 0.15 | |||
| rs182052 | ||||||
| G/Gc | 1 | |||||
| G/Ac | 1.06 | 0.83-1.36 | 0.59 | |||
| A/Ac | 0.83 | 0.62-1.12 | 0.2 | 0.26 | ||
| G/A + A/Ad | 0.99 | 0.78-1.24 | 0.91 | |||
| A/Ar | 0.8 | 0.62-1.03 | 0.08 | |||
| Log-additive | 0.92 | 0.79-1.07 | 0.28 | |||
| rs266729 | ||||||
| C/Cc | 1 | |||||
| C/Gc | 1.18 | 0.94-1.48 | 0.13 | |||
| G/Gc | 1.31 | 0.95-1.81 | 0.11 | 0.07 | ||
| C/G + G/Gd | 1.21 | 0.97-1.50 | 0.09 | |||
| G/Gr | 1.19 | 0.89-1.61 | 0.24 | |||
| Log-additive | 1.15 | 0.99-1.34 | 0.07 |
| ADIPOR2 | rs11061971 | |||||
|---|---|---|---|---|---|---|
| T/Tc | 1 | |||||
| T/Ac | 0.74 | 0.59-0.93 | 0.01 | |||
| A/Ac | 0.67 | 0.48-0.93 | 0.01 | 0.004 | ||
| T/A + A/Ad | 0.72 | 0.58-0.90 | 0.003 | |||
| A/Ar | 0.78 | 0.57-1.07 | 0.12 | |||
| Log-additive | 0.79 | 0.68-0.93 | 0.003 |
Results are described according to the models: ccodominant, ddominant, rrecessive and log-additive; adjusted by age, gender and family history of obesity.
SNP, single nucleotide polymorphisms; OR, odds ratio; CI, confidence interval.
Adiponectin plays a role in obesity displaying anti-inflammatory and anti-atherogenic actions.20–22 Several SNPs were reported associated in obese children; however, only few studies in Mexican children have been reported. We investigated the distribution of ADIPOQ and ADIPOR2 polymorphisms in children from Mexico City. Our study showed no association between ADIPOQ gene variants in obesity. Only ADIPOR2, rs11061971 showed a protective association against obesity (Table 4).
Several reports showed an association of ADIPOQ gene variants with obesity or its comorbidities such as MetS. However, these results have not been convincing in replication studies in other populations; therefore, it seems to be playing an important role in the ancestry of the populations studied. Our study includes four different geographical regions of Mexico City where we observed differences in BMI prevalence.
Significant associations between obesity and polymorphisms in the gene coding for ADIPOQ have been reported in other studies. 23,24 Guzman-Ornelas et al. suggested that polymorphisms in the gene coding for ADIPOQ could be associated with distribution of body fat storage in adult obesity. On the other hand, no association was observed between gene polymorphisms and obesity in a Mexican-Mestizo population.25
The rs2241766 polymorphism of ADIPOQ has not been associated with obesity but has had significant associations in comorbidities such as MetS.26 However, few studies have investigated the relationship between rs266729 polymorphism and risk of obesity or MetS. Regarding links between rs266729 and MetS, early studies reported varying results.27,28 The rs182052 was associated with an increased risk of the prevalence of obesity in adult Korean women, but there were no significant interactions observed between the genotype of ADIPOQ rs182052 and dietary intake on BMI and body fat mass. These findings suggest that the obesity-related variables may be more dominantly affected by the genotype of ADIPOQ rs182052 than dietary intake in middle-aged Korean women. MAF in a Korean population (0.49) was different from European (0.40), Chinese (0.42) and Mexican (0.22) populations.29 No study has reported the relationship between rs822393 of ADIPOQ and obesity. Ramya et al. in 2013 described that there was no association with T2D but there was an association with decreased adiponectin values.30 On the other hand, the rs822393 of ADIPOQ was not associated in Mexican children.
The role of ADIPOR2 in obesity could arise from the unique function of this receptor in mediating the effects of adiponectin in the liver. Adiponectin influences fat metabolism, increasing fatty acid oxidation through activation of AMP-activated protein kinase which, in turn, phosphorylates acetyl CoA carboxylase.10 Lui et al. demonstrated a role of ADIPOR2 in the pathogenesis of insulin resistance (IR) syndrome and T2D and suggest ADIPOR2 as a promising target for the treatment of T2D patients, particularly those who have adiposity, IR and dyslipidemia.18
There are few studies of variant rs11061971 of ADIPOR2 gene. No data exist in the National Center for Biotechnology Information (NCBI) for the Mexican or Latino population. Studies in the Russian population showed that ADIPOR2, rs11061971 has an association with T2D.19 Similarly, Damcott et al. reported that the T allele of rs11061971 was significantly associated with a higher risk of T2D.31 The alleles of ADIPOR2 forming the common haplotype AG were associated with a reduced risk of T2D. The protective role of this haplotype in the development of T2D could be attributed to its association with a decreased HOMA-IR value and, therefore, with reduced IR. In addition, the AG haplotype showed an association with lower serum concentrations of triglycerides. Other studies also found the relationship between multiple ADIPOR2 variants and triglyceride levels but not with obesity.32,33ADIPOR2 variant was observed as being protective for obesity in Mexican children.
The Mexican population is at relatively high risk for obesity, IR and T2D. The genetic variation in candidate genes of ADIPOQ and ADIPOR2 could influence variation in such disease conditions and related traits. In the present study we provide evidence that the ADIPOR2 polymorphism is associated with protection for obesity in Mexican children. Therefore, the presence of rs11061971 polymorphism of ADIPOR2 could be used as a protective marker for obesity in children.
Conflict of interestThe authors declare there are no conflicts of interests of any nature.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Source of fundingThis work was supported by the SSA/IMSS/ISSSTE-CONACYT 2013 SALUD-2013-01-201471.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
The authors wish to thank all the individuals who participated and contributed to the successful completion of the present study. They would also like to thank IMSS Foundation and Department of Economic and Social benefits together with IMSS sports units for allowing them to collect samples.