In the steroid-sensitive nephrotic syndrome (SSNS), the prolonged treatment with steroids could decrease the frequency of relapses. A comparative study of a prolonged steroid scheme and the usual treatment of primary SSNS were conducted to assess the number of patients with relapses, the mean time to treatment initiation, until remission and until first relapse, total number of relapses, total cumulative dose of steroids, and the steroid toxicity.
MethodsPatients were divided into two groups: group A (27 patients) received 16-β-methylprednisolone for 12 weeks, reducing the steroid until week 24. Group B (29 patients) received 16-β-methylprednisolone for 12 weeks and placebo until week 24.
ResultsThe cumulative incidence rate of relapses (person/year) for group A was of 36/100 and for group B, 66/100 (p=0.04). The mean elapsed time to first relapse was 114 days for group A and 75 days for group B (p=0.01). The difference in time for the initial response to treatment and up to achieve remission between both groups was not significant. Total cumulative relapses were nine for group A and 17 for group B (p=0.04). Total patients with relapses were three for group A and seven for group B (p=0.17). The cumulative average dose per patient was 5,243mg/m2 for group A and 4,306mg/m2 for group B (p=0.3), and serum cortisol was 14μg/dl for group A and 16μg/dl for group B (p=0.4). There were no steroid toxicity differences between both groups.
ConclusionsThe duration of the treatment had an impact on the number of relapses without increasing steroid toxicity.
En el síndrome nefrótico cortico-sensible (SNCS), la corticoterapia prolongada podría reducir la frecuencia de recaídas. El objetivo de este trabajo fue la comparación de un esquema corticoide prolongado frente al tratamiento habitual del SNCS primario, con la evaluación de los siguientes parámetros: el número de pacientes con recaídas, el número total de recaídas, el tiempo medio transcurrido al iniciar el tratamiento, hasta la remisión y hasta la primera recaída, la dosis acumulada de corticosteroides y la toxicidad esteroide.
MétodosLa población se dividió en dos grupos: el grupo A (27 pacientes) recibió 16-β-metilprednisona durante 12 semanas, reduciendo la dosis hasta la semana 24; y el grupo B (29 pacientes) recibió 16-β-metilprednisona durante 12 semanas, y placebo hasta la semana 24.
ResultadosLa tasa de incidencia acumulada de recaídas (persona/año) fue de 36/100 en el grupo A y de 66/100 (p=0.04) en el grupo B. El tiempo medio transcurrido (días) hasta la primera recaída fue de 114 en el grupo A y 75 en el grupo B (p=0.01). Las diferencias de tiempo transcurrido al iniciar tratamiento y hasta la remisión no fueron significativas entre ambos grupos. El total acumulado de recaídas fue de nueve en el grupo A y 17 en el grupo B (p=0.04), y el total de pacientes con recaídas fue de tres (grupo A) y siete (grupo B) (p=0.17). La dosis media acumulada (mg/m2) por paciente fue de 5,243 en el grupo A y de 4,306 en el grupo B (p=0.3), y el cortisol sérico (μg/dl) final fue de 14 en el grupo A y 16 en el grupo B (p=0.4). La toxicidad esteroide fue similar entre ambos grupos.
ConclusionesLa duración del tratamiento disminuyó el número de recaídas, sin incrementar la toxicidad esteroide.
Nephrotic syndrome is defined as the presence of proteinuria>40mg/m2/h and serum albumin <2.5g/ml. Hypercholesterolemia and the presence of edema are frequent findings but not required for diagnosis. In pediatrics, the incidence of its primary form is 2/100,000 children1.
Initially, the International Study of Kidney Disease in Children (ISKDC) established a daily 8-week corticosteroid regimen. Later recommendations prolonged treatment up to 12 weeks2,3. With these schemes, up to 53% of patients will suffer disease relapse at some point during their evolution4,5, with the need of restarting glucocorticoid administration and the subsequent risk of developing associated adverse effects6,7. For this reason, 6-month corticosteroid therapeutic schemes have been described8–10. In the present work, a comparative study between a prolonged corticosteroid scheme and usual steroid treatment for primary steroid-sensitive nephrotic syndrome (SSNS) was conducted. General objectives included evaluation of the following parameters:
- 1)
The number of patients with relapses within 12, 24 and 36 months after completion of either treatment
- 2)
Number of relapses throughout the duration of the study
- 3)
The time from diagnosis to the initiation of therapy, the time from the initiation of therapy to the initial remission and the time from remission to the first relapse
- 4)
The accumulated dose of corticosteroids
The specific objective was to evaluate the occurrence of steroid toxicity:
- •
Cushingoid appearance
- •
Increased HOMA index (>3)
- •
Decrease growth rate
- •
Arterial hypertension
- •
Acne, striae
- •
Cataract/increased intraocular pressure
- •
Osteoporosis/osteopenia
A controlled, randomized, double-blind (patients and physicians), non-inferiority clinical trial was implemented in patients with new-onset primary SSNS.
2.1Patient criteria2.1.1Inclusion- •
Patients from 1 year to 11 years-old, eutrophic and normotensive
- •
Serum creatinine within normal range for age and gender
- •
Urinary sediments without hematuria
- •
Normal renal ultrasound
- •
Approved informed consent. In adolescents, assent was required according to the basic principles of the Declaration of Helsinki
- •
Intolerance to corticoisteroid therapy
- •
Contraindication to the use of steroids (e.g., acute or chronic active infections)
- •
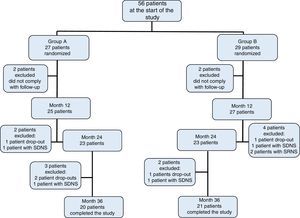
Patients who developed either steroid-dependent nephrotic syndrome (SDNS) or steroid-resistant nephrotic syndrome (SRNS), those who did not comply with the indications or did not attend to at least 80% of the programmed follow-up appointments, and those who developed uncontrolled steroid toxicity were removed from the study
The total population was randomly divided into two groups. In group A, a prolonged steroid treatment with 16-β-methylprednisolone was administered at a dose of 48mg/m2/day during 6 weeks; afterwards, a dose of 33mg/m2/day on alternate days was administered for 6 weeks. As of week 13, the dose was reduced to 24mg/m2/day on alternate days until week 16; thereafter, 18mg/m2/day on alternate days until week 20; and finally, 9mg/m2/day on alternate days until week 24.
In group B, the usual treatment consisting of 16-β-methylprednisolone at a dose of 48mg/m2/day during 6 weeks was administered; afterwards, a dose of 33mg/m2/day on alternate days was administered until week 12; and finally, placebo was given on alternate days until week 24 of treatment.
Placebo properties regarding flavor, external appearance and presentation were similar to 16-β-methylprednisolone.
Steroid doses were prescribed once daily, during the morning, on an empty stomach and not exceeding 60mg/day. Adherence to treatment was controlled by counting the medication at each visit, excluding patients who did not comply with the indications.
The dose of 48mg/m2/day of 16-β-methylprednisolone is equivalent to 60mg/m2 of prednisone, and the dose of 33mg/m2 of 16-β-methylprednisolone is equivalent to 40mg/m2 of prednisone.
In both groups, vitamin D at 1,500 IU/day, calcium carbonate at 50mg/kg/day (maximum dose of 2g/day) and a hyposodic diet were prescribed during the 24 weeks of treatment.
2.3Blinding and randomizationBoth corticoids and placebo were given as tablets in indistinguishable submissions. Outside the group of investigators, one medical professional knew the content of these drugs (with this specific function previously delegated), who gave the tablet to each patient according to their assigned number with the randomization sequence. Randomization was obtained with the statistical program GraphPad InStat.
2.4Controls2.4.1At admission- •
Data as weight and height with growth rate calculation, blood pressure control (oscillometric and auscultatory methods), pubertal development stage (Tanner), evaluation of bone age (left hand radiograph) and ophthalmologic control (eye fundoscopy, tonometry) were registered
- •
Renal ultrasound and Mantoux-PPD reaction
- •
Control blood tests included VDRL, serology for HIV, hepatitis A, B, C, toxoplasmosis and Chagas, and serum complement (C3 and C4)
- •
Uremia, venous acid-base status, protein electrophoresis, total cholesterol, LDL-C, HDL-C, triglycerides, creatinine, ionogram, blood calcium, phosphorus, alkaline phosphatase (ALP), uric acid, glycemia, and basal insulin levels
- •
25-OH vitamin D levels (cholecalciferol)
- •
Urine 24 hour-protein determination (expressed in mg/m2/h) or the proteinuria/creatininuria index in a single sample (normal value <0.2) were performed. For operative purposes, reactive strips were delivered to the patient's proxy to measure urine protein; they were instructed to be able to communicate with the researcher
- •
Calculation of the estimated glomerular filtration rate was used, expressed as the creatinine/height index and Barrat's constant according to gender and age
- •
Calculation of the growth rate obtained from the measure of height in cm. For operative reasons, the first measure corresponded to the first nephrologic control, and the following measures to the annual height until the end of the study. To calculate growth rate, results were expressed as cm/year
- •
In stool samples, a full parasitoscopic study was performed (direct, serial, and Graham's test)
- •
Clinical follow-up including blood pressure measurement
- •
Blood protein electrophoresis, total cholesterol, LDL-C, HDL-C, triglycerides, creatinine, ionogram, calcium, phosphorus, alkaline phosphatase, glycemia and basal insulin were performed
- •
Growth rate percentile, cardiovascular evaluation, ophtalmologic follow-up
- •
Bone age. In patients older than 6 years, a dual-photon absorptiometry was indicated (lumbar spine bone mineral densitometry) to measure bone mineral content (g/cm2) and a Z score. The registered values for this population, which were provided by studies conducted in local controls, were employed for the calculations. The interpretation of data was based on the comparison of the results with normal values adjusted for gender, age and stage of puberty11
- •
At the end of the study, morning serum cortisol level (08:00 AM) was measured
- •
A renal biopsy was performed in patients with SDNS and SRNS
The duration of the study was 36 months.
The independent variables evaluated were gender, age at onset of the nephrotic syndrome (years), time until the beginning of treatment (from the onset of therapy until remission and from remission to the first relapse).
The dependent variables evaluated were accumulated steroid dose, number of diagnosed relapses, adherence to treatment, final serum cortisol level, steroid toxicity.
2.5Diagnosis and treatment definitions- 1)
New-onset or early primary nephrotic syndrome. First episode with proteinuria greater than 40mg/m2/h, serum albumin less than 2.5g/ml and, eventually, hypercholesterolemia above the percentile 95, without an infectious or immune concurrent disease.
- 2)
Relapse. Proteinuria>40mg/m2/h during three consecutive days (ISKDC).
- 3)
Relapse treatment. With 48mg/m2/daily of 16-β-methylprednisolone until achievement of remission during three or more consecutive days, followed by four weeks of 16-β-methylprednisolone.
- 4)
Frequent relapses. Two or more relapses within six months or four relapses within 12 months (ISKDC).
- 5)
Partial remission. Proteinuria of <2+, proteinuria-creatinuria index of>0.2 and <3.5g/mg or>4mg/m2/h and <40mg/m2/h and an increment of serum albumin levels of>2.5g/ml (ISKDC).
- 6)
Complete remission. Absence of edema and proteinuria <1+on a urine test strip, proteinuria-creatinuria <0.2g/mg or <4mg/m2/h during three consecutive days.
- 7)
Steroid-dependent nephrotic syndrome. Reappearance of proteinuria>40mg/m2/h with a reduction of the steroid dose during or within 20 days of completion of treatment.
- 8)
Steroid-resistant nephrotic syndrome. Persistence of proteinuria>40mg/m2/h or>3.5g/mg after two series of steroid administration of at least 12-weeks duration each, or after four weeks of daily methylprednisolone treatment plus three consecutive methylprednisolone pulses.
- 9)
Steroid toxicity. Diabetes, gastrointestinal bleeding, cerebral edema, glaucoma, hypertension, fluid retention, obesity, insulin resistance, growth retardation, increased intra-ocular pressure, cataract, psychological and behavioral changes, cushingoid habitus (dorso-cervical fat pad, abdominal striae, skin thinning, ecchymosis, hirsutism, acne).
The statistical test suite EPIDAT 3.0 was used for subject simple randomization and assignment (two groups), randomization was done in a 1:1 ratio.
To evaluate the number of relapses in nephrotic syndrome, a percentage between 30-50% during the first three years was assumed. The sample size calculation was performed considering a desired potency of 80% for a two-tailed alpha=0.05 (a confidence Z value of 1.65) and an estimated SE of 10% of 22 patients. Anticipating 20% of patient loss, a total sample 27 patients was planned for recruitment. To avoid selection bias, both groups were arbitrarily stratified according to age groups: 1-year-old to 6 years, 11 months and 29 days old; and 7-years-old to 11 years, 11 months and 29 days old.
Cumulative incidence rate (CIR) was used to evaluate the proportion of individuals with an event (relapse) during the entire follow-up period (person-time).
The heterogeneity of the final accumulated dose was analyzed with a non-paired t-test with Welch correction.
Relative risk (RR) was used to evaluate the appearance of steroid toxicity related to both treatments.
Considering sample size and the number of events, Fisher's exact test was used to evaluate if the total cohort was behaving as two distinct populations regarding the total accumulated relapses and the total number of patients with relapses.
A logarithmic ordinal calculation (log-rank) was used to compare the distribution for the actuarial survival between patients in group A and B.
A p value of <0.05 was established as statistically significant.
An intention-to-treat strategy was used to analyze the results.
The results are presented as mean+SD and the statistical packages GraphPad InStat and EPIDAT 3.0 were used.
The protocol was approved by the Ethics and Research Committee of the Children's Hospital Ricardo Gutiérrez.
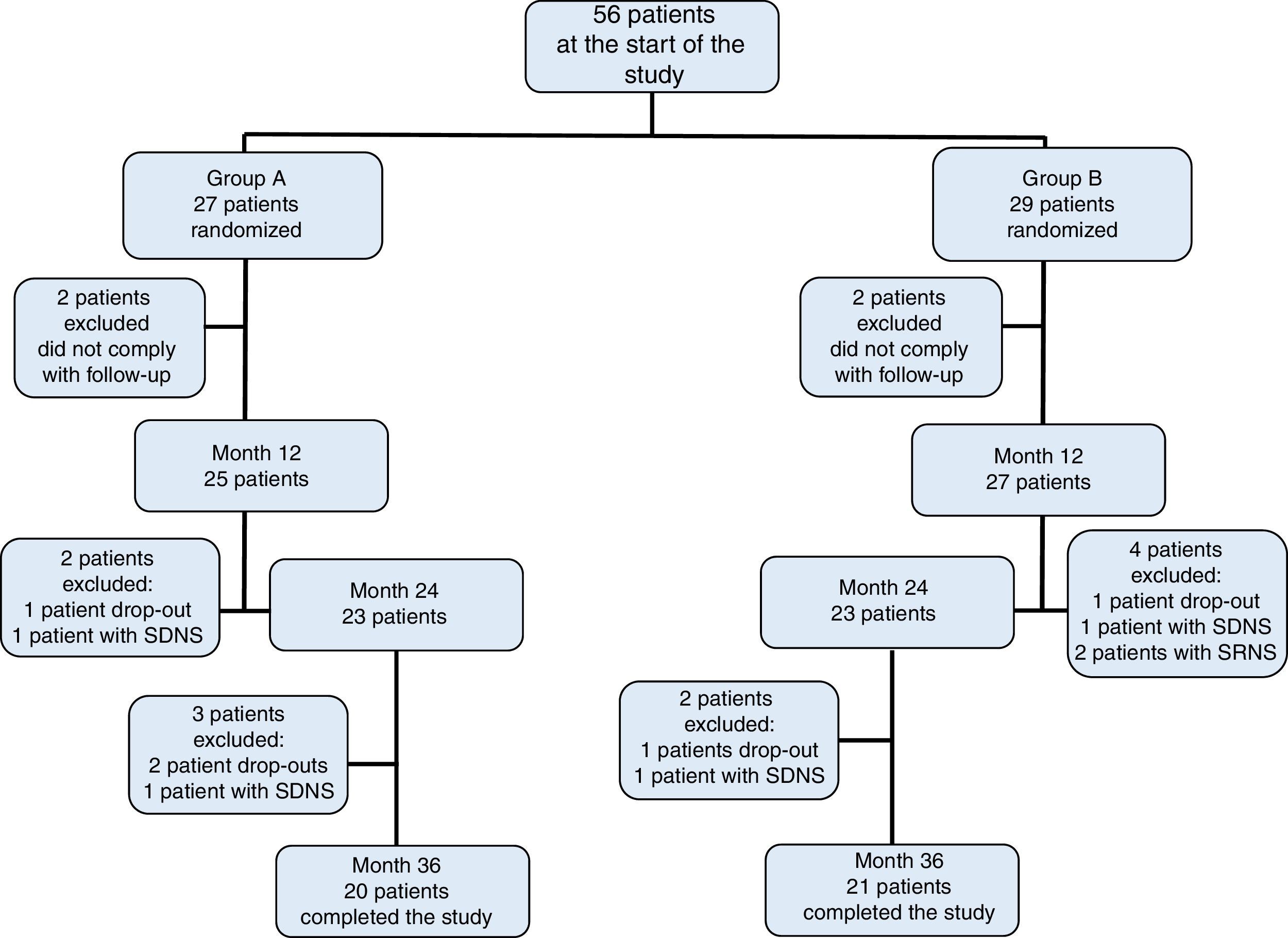
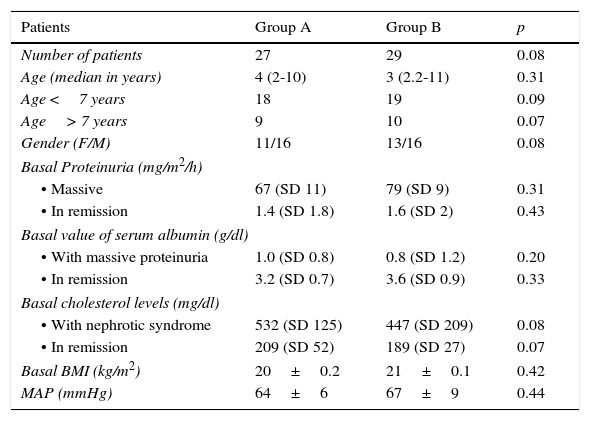
3ResultsOf the initially included 56 patients, 41 completed the 36-month duration of the study and were distributed in the following fashion: 27 patients in group A, of which 11 were women (median age 4 years, r: 2-10 years); and 29 patients in group B, of which 13 were women (median age 3 years, r: 2-11 years) (Table 1). Adherence to treatment in the total population was 73%; therapeutic adherence by group was 74% in group A and 72% in group B (p=0.04) (Figure 1).
Characteristics of the population during the study.
| Patients | Group A | Group B | p |
|---|---|---|---|
| Number of patients | 27 | 29 | 0.08 |
| Age (median in years) | 4 (2-10) | 3 (2.2-11) | 0.31 |
| Age <7 years | 18 | 19 | 0.09 |
| Age> 7 years | 9 | 10 | 0.07 |
| Gender (F/M) | 11/16 | 13/16 | 0.08 |
| Basal Proteinuria (mg/m2/h) | |||
| • Massive | 67 (SD 11) | 79 (SD 9) | 0.31 |
| • In remission | 1.4 (SD 1.8) | 1.6 (SD 2) | 0.43 |
| Basal value of serum albumin (g/dl) | |||
| • With massive proteinuria | 1.0 (SD 0.8) | 0.8 (SD 1.2) | 0.20 |
| • In remission | 3.2 (SD 0.7) | 3.6 (SD 0.9) | 0.33 |
| Basal cholesterol levels (mg/dl) | |||
| • With nephrotic syndrome | 532 (SD 125) | 447 (SD 209) | 0.08 |
| • In remission | 209 (SD 52) | 189 (SD 27) | 0.07 |
| Basal BMI (kg/m2) | 20±0.2 | 21±0.1 | 0.42 |
| MAP (mmHg) | 64±6 | 67±9 | 0.44 |
F, female; M, male; BMI, body mass index; MAP, mean arterial pressure; SD, standard deviation.
The mean time of control of the disease for the total population was 36 months (r: 34-38 months). During the entire protocol, the presence of edema coincided with relapses, although its magnitude and, eventually, its disappearance had no relationship with disease control, and did not always coincide with remission.
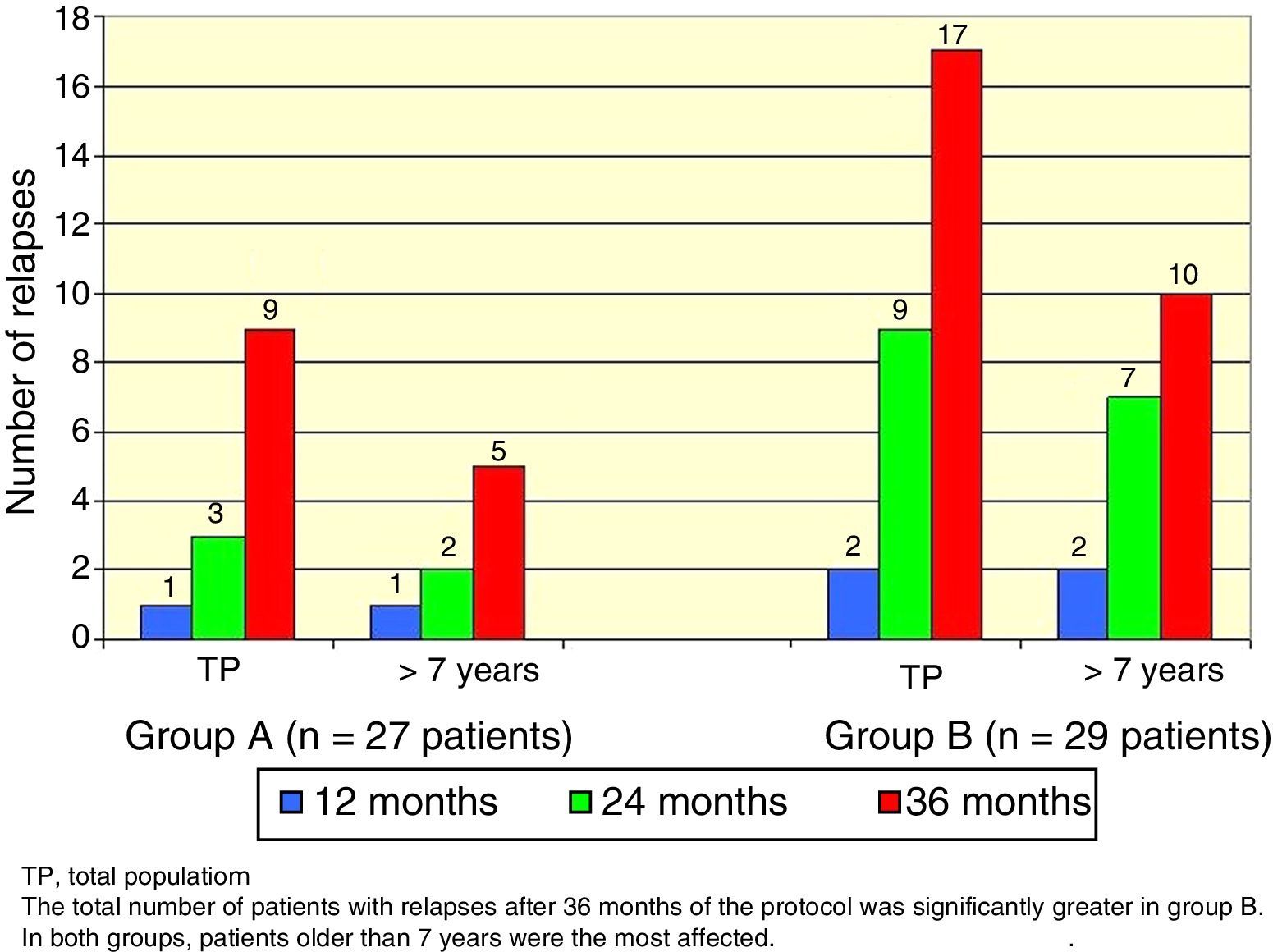
Mean age of onset of nephrotic syndrome in group A was 3.7 years (SD 1.6) and relapse CIR was 36/100 person/year. In group B, mean age of onset was 4.2 years (SD 2.1) and relapse CIR was 66/100 person/year (p=0.04).
In group A, the mean time between the diagnosis of nephrotic syndrome and the onset of treatment was 10+3 days and in group B was 13+2 days (p=0.07). At the same time, the mean time between the onset of treatment and remission in group A was 14+3 days and 11+2 days in group B (p=0.08). Finally, the mean time since completion of the initial treatment until first relapse in group A was 114+7 days for the total population and 102+5 days for those> 7 years old, and in group B it was 75+9 days for the total population and 67+3 for those> 7 years old (p=0.01).
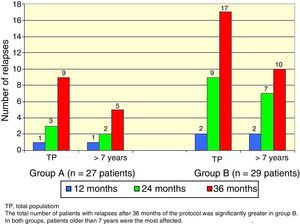
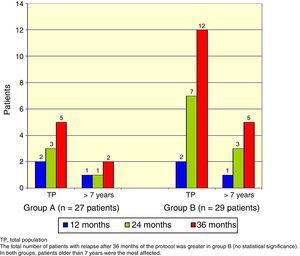
There was a total of nine cumulative relapses in group A and 17 in group B (Fisher's exact test, p=0.048). In both groups, patients> 7 years old were the most commonly affected (Figure 2).
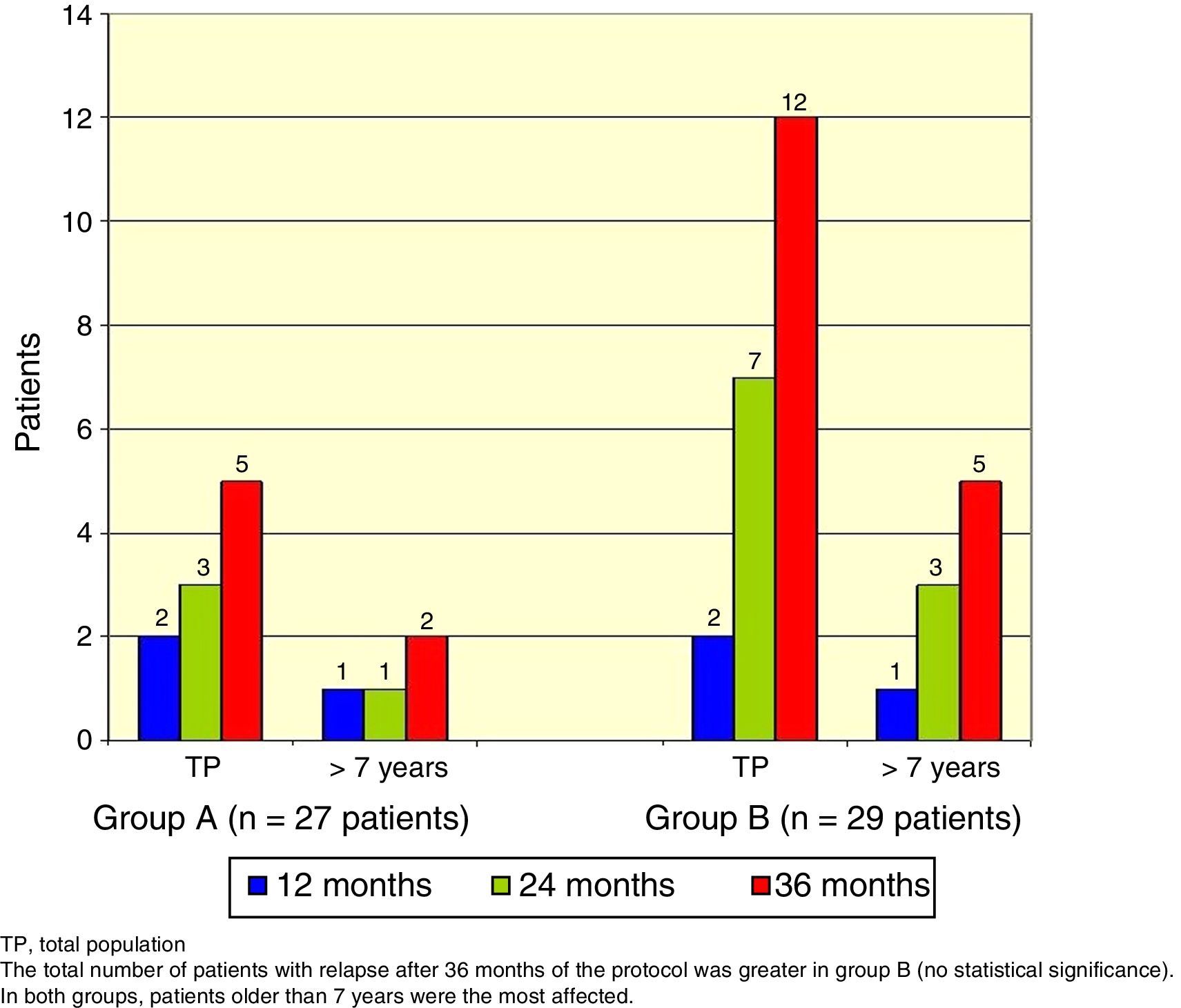
The total number of patients with relapses was lower in group A (three patients) than group B (seven patients), but this difference was not statistically significant (Fisher's exact test, p=0.18). Once again, patients> 7 years old were the most commonly affected (Figure 3).
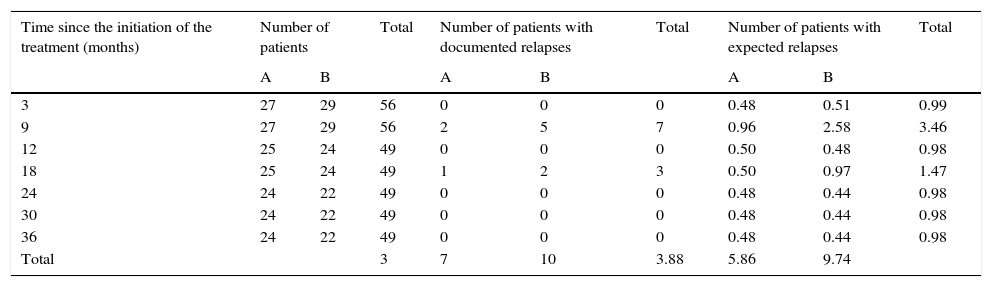
Logarithmic ordinal calculation (χ2=4.1, p<0.05) showed significant differences in the distribution of relapses for patients in group A vs. group B (Table 2).
Calculation for the logarithmic ordinal tests (log-rank) for comparison of actuarial survival distributions between patients in group A and group B.
| Time since the initiation of the treatment (months) | Number of patients | Total | Number of patients with documented relapses | Total | Number of patients with expected relapses | Total | |||
|---|---|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | ||||
| 3 | 27 | 29 | 56 | 0 | 0 | 0 | 0.48 | 0.51 | 0.99 |
| 9 | 27 | 29 | 56 | 2 | 5 | 7 | 0.96 | 2.58 | 3.46 |
| 12 | 25 | 24 | 49 | 0 | 0 | 0 | 0.50 | 0.48 | 0.98 |
| 18 | 25 | 24 | 49 | 1 | 2 | 3 | 0.50 | 0.97 | 1.47 |
| 24 | 24 | 22 | 49 | 0 | 0 | 0 | 0.48 | 0.44 | 0.98 |
| 30 | 24 | 22 | 49 | 0 | 0 | 0 | 0.48 | 0.44 | 0.98 |
| 36 | 24 | 22 | 49 | 0 | 0 | 0 | 0.48 | 0.44 | 0.98 |
| Total | 3 | 7 | 10 | 3.88 | 5.86 | 9.74 | |||
X2=4.1 (p<0.05). The decimal extension explains the minimal difference among the totals.
Upon study completion, the mean accumulated total dose per patient in group A was 5,243mg/m2 and 4,306mg/m2 (p=0.3) in group B, adding the administered steroid during the initial scheme (group A, 4,802mg/m2 and group B, 3,402mg/m2) and the treated relapses (group A, 441mg/m2 and group B, 904mg/m2).
The final value of morning serum cortisol was 14μg/dl in group A and 16μg/dl in group B (p=0.4).
In group A, a renal biopsy was performed in three children. From these patients, two with SDNS were histopathologically diagnosed with minimal change disease (MCD), while the third patient with SRNS was diagnosed with mesangial proliferation.
In group B, renal biopsy was performed to four children: in two patients with SRNS, the histopathological diagnosis was focal and segment sclerosis, and mesangial proliferation, respectively. In the other two patients with SDNS, the histopathological diagnosis was MCD.
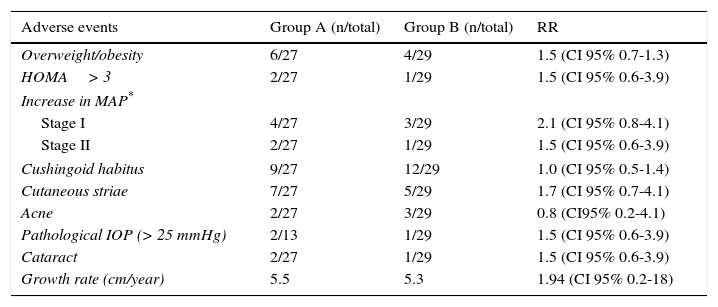
The risk of developing steroid toxicity was low and there were no significant differences between both groups (Table 3).
Steroid toxicity in both groups.
| Adverse events | Group A (n/total) | Group B (n/total) | RR |
|---|---|---|---|
| Overweight/obesity | 6/27 | 4/29 | 1.5 (CI 95% 0.7-1.3) |
| HOMA> 3 | 2/27 | 1/29 | 1.5 (CI 95% 0.6-3.9) |
| Increase in MAP* | |||
| Stage I | 4/27 | 3/29 | 2.1 (CI 95% 0.8-4.1) |
| Stage II | 2/27 | 1/29 | 1.5 (CI 95% 0.6-3.9) |
| Cushingoid habitus | 9/27 | 12/29 | 1.0 (CI 95% 0.5-1.4) |
| Cutaneous striae | 7/27 | 5/29 | 1.7 (CI 95% 0.7-4.1) |
| Acne | 2/27 | 3/29 | 0.8 (CI95% 0.2-4.1) |
| Pathological IOP (> 25 mmHg) | 2/13 | 1/29 | 1.5 (CI 95% 0.6-3.9) |
| Cataract | 2/27 | 1/29 | 1.5 (CI 95% 0.6-3.9) |
| Growth rate (cm/year) | 5.5 | 5.3 | 1.94 (CI 95% 0.2-18) |
HOMA, homoeostasis model assessment; MAP, mean arterial pressure; IOP, intraocular pressure; RR, relative risk.
Phosphate/calcium metabolic derangement were not significant: for group A, calcium levels were 7.9+0.9mg/dl, and for group B, 8.2+2.1mg/dl (p=0.6). Phosphate levels in group A were 4.8+1.1mg/dl, and 5.2+1.8mg/dl in group B (p=0.09).
In group A, alkaline phosphatase levels were 339+132mg/dl; in group B, 430+112mg/dl (p=0.06).
Parathyroid hormone (PTH) levels in group A were 41+16 pg/ml and 32+11 pg/ml in group B (p=0.08). In group A, the concentration of 25-OH vitamin D was 26+4 ng/ml, and 29+5 ng/ml in group B (p=0.29).
Finally, bone mineral content was 0.76+0.11g/cm2 with and Z score of 1+1 in group A (nine patients), and in group B, the bone mineral content was 0.80+0.08g/cm2 (p=0.26) with a Z score of -1+0.5 (p=0.9). Specific genetic studies were not evaluated in any patient.
4DiscussionThe rationale behind this research was to justify the use of a prolonged therapeutic scheme by comparing it with the usual treatment. It was shown that patients in group A presented fewer relapses, without a significant increase in the accumulated doses of corticosteroids. Although no significant differences were observed regarding the number of patients with relapses between the two randomized populations, the final value of serum cortisol in both groups indicated an adequate steroid suppression12.
The ISKDC established a daily 8-week regimen of corticosteroids. Later schemes were extended for up to twelve weeks13,14, and although no consensus has been reached regarding treatment duration15, it was observed in this work that a prolonged scheme of more than 3 months reduces relapse rate, which coincides with the reports from Bagga et al. (1999)16 and Hiraoka et al. (2000)17. Furthermore, according to Ueda et al.18 and Ksiazek and Wyszynska19, a significant remission index was achieved since the first episode. Interestingly, although the follow-up in the studies of Sinha et al.20, Yoshikawa et al.21 and Hoyer22 was different to those established in the present work, the variation in the size of the studied sample could be responsible for the discrepancies of the final results. In this regard, it is ostensible that sample size showed substantial variations in relation to the present experience.
Regarding gender and age as influencing variables over the evolution of SSNS23,24, gender was not significantly related to relapse incidence unlike the study of Takeda et al.25 In contrast, a relationship with average age was observed. In both groups, patients> 7 years had an increased frequency of relapses.
Steroid toxicity is known to be related to the accumulated dose26,27. However, no significant differences were found between both groups regarding ophthalmologic damage28,29, central obesity and insulin resistance30; and although several patients developed cushingoid facies, this event was transitory and reversible31.
Steroid toxicity over mineral metabolism (osteopenia) was not found either32, and although it is known that the increase of both the bone content and density is linear in the pre-pubertal population, this bias was lessened in the included pre-pubertal females and males by separately studying bone density and mineral content and adjusting the results to anthropometric and gender variables. Moreover, to attenuate the eventual bone mass reduction, the studied population received calcium carbonate and vitamin D supplements according to established guidelines14.
Finally, registries on arterial hypertension, which were probably associated to the corticosteroid effect on endogenous angiotensin II (patients in group A had a percentage increase of up to 30% for stage I), were transitory and responded to nifedipine administration33.
Behavioral changes in patients receiving steroid therapy were not evaluated (mild euphoria, insomnia, increased appetite) despite these symptoms can have a frequency up to 25% according to some references34.
This work had several limitations. Firstly, although sample size was the result of pre-established statistical guidelines, it is evident that the number of patients with relapse was relatively small in each group, which limited the observation of differences.
Similarly, the lack of histopathological diagnosis in every patient may have covered up different etiologies of the primary nephrotic syndrome35,36. However, established international guidelines concerning indications for initial renal biopsy in pediatric patients with SSNS were followed37,38.
In conclusion, although prolonged steroid treatments have been documented,2,3 the increase in the number of observed relapses in children> 7 years allows considering whether this group would require the implementation of prolonged therapeutic regimes, according to the experience obtained from this study.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare no conflict of interests.
Please cite this article as: Liern M, Codianni P, Vallejo G. Estudio comparativo entre el esquema convencional y el tratamiento prolongado con esteroides en el síndrome nefrótico cortico-sensible primario en Pediatría. Bol Med Hosp Infant Mex. 2016;73:309–317.