Submandibular salivary gland infections are uncommon. For this reason, neonatal acute suppurative submandibular sialadenitis non-coincident with parotitis is considered as a rare entity. The aim of this work was to verify if there have been changes in the clinical and microbiological aspects of this infection from its first description.
MethodsA review of the international literature from different sources was performed since the earliest reports until April, 2016, in order to collect data of all the cases reported with this infection up to the present date.
ResultsFor this review, data on 39 neonate patients reported in 30 articles were collected. Most articles came from the United States of America and Europe, whereas in Latin America there was only one case declared in Cuba. Some of the clinical aspects of the presentation and evolution of this infection in the reported patients were described.
ConclusionsNeonatal acute suppurative submandibular sialadenitis is an uncommon infection that presents similar clinical features throughout the years. As a microbiological feature, this infection is usually caused by Staphylococcus aureus, although some methicillin-resistant Staphylococcus aureus have been implicated during the last 16 years. In general, it presents a satisfactory evolution with an early and appropriate antibiotic treatment.
La infección de la glándula salivar submandibular es poco común, por lo que la sialadenitis submandibular supurativa aguda neonatal no coincidente con parotiditis se considera una entidad de rara presentación. El objetivo de esta revisión fue verificar si ha habido cambios en los aspectos clínicos y microbiológicos de esta infección desde sus primeras descripciones.
MétodosSe realizó una revisión de la literatura internacional en distintas fuentes de información desde los primeros reportes y hasta abril del 2016, para tratar de recopilar todos los casos publicados con esta infección hasta la fecha actual.
ResultadosEl total de pacientes reportados en esta revisión fue de 39 neonatos en 30 artículos. Las publicaciones provinieron fundamentalmente de los Estados Unidos y Europa, mientras que en América Latina solo ha sido declarado un caso en Cuba. Se describen distintos aspectos clínicos de presentación y evolución de esta infección en los pacientes reportados.
ConclusionesLa sialadenitis submandibular supurada aguda neonatal es una infección poco común que presenta características clínicas similares a través de los años. Como característica microbiológica, esta infección es habitualmente ocasionada por Staphylococcus aureus aunque en los últimos 16 años involucra algunos Staphylococcus aureus resistentes a meticilina. Por lo general, evoluciona de manera favorable con tratamiento temprano y apropiado.
Every salivary gland inflammation is labeled as sialadenitis regardless of the origin agent. Salivary glands inflammation can be caused by bacterial agents, which produce a suppurative sialadenitis. This infection is unilateral, in which the parotid gland is more commonly affected than the submandibular glands. Exclusive submandibular gland infection is rare. Therefore, neonatal suppurative submandibular sialadenitis non-coincident with parotitis is considered as a rare entity.1,2 It is commonly associated with prematurity, dehydration, and orogastric or nasogastric tube enteral feeding.3,4 Several reports have found Staphylococcus aureus as the responsible pathogen. It has been suggested that an ascending infection from the oral cavity to the salivary glands is the most common way of infection. Diagnosis and early antibiotic treatment may prevent complications, such as the formation of abscesses and septicemia.3,4
Regarding the above mentioned, a review of the international literature was conducted to collect published case reports with these infections of scarce presentation in the neonatal period. The aim of this study was to confirm if the clinical and microbiological aspects of this infection have changed since it was first described until the present date.
2MethodsA descriptive and retrospective study of case reports on neonatal patients with the diagnosis of acute suppurative submandibular sialadenitis was conducted, from the earliest publication found until April 2016.
A review of the literature in medical journals kept in institutional libraries and the private library of the author of correspondence was conducted. Furthermore, an online medical search was conducted on databases and other sites to cover the gray literature (conference proceedings, research reports, memoirs). The following keywords were used: [“suppurative submandibular sialadenitis” OR “bacterial submandibular sialadenitis” OR “purulent submandibular sialadenitis”] AND [neonatal OR newborn] for every year. The databases consulted were PubMed-Medline (http://www.ncbi.nlm.nih.gov/pubmed/), Embase (http://www.embase.com), Scielo (http: //www.scielo.org), Worldcat (http://www.Worldcat.org), LILACS (http://regional.bvsalud.org), ClinicalKey (https://www.clinicalkey.com), Google (http: // www.google.com), OpenGrey (http://www.opengrey.eu/).
Once the articles were collected and after being reviewed, different clinical aspects and laboratory tests showing the particular characteristics of the patients were obtained, and a table with the identified cases was created. When the full article was not available, the summary was used to get as much information as possible.
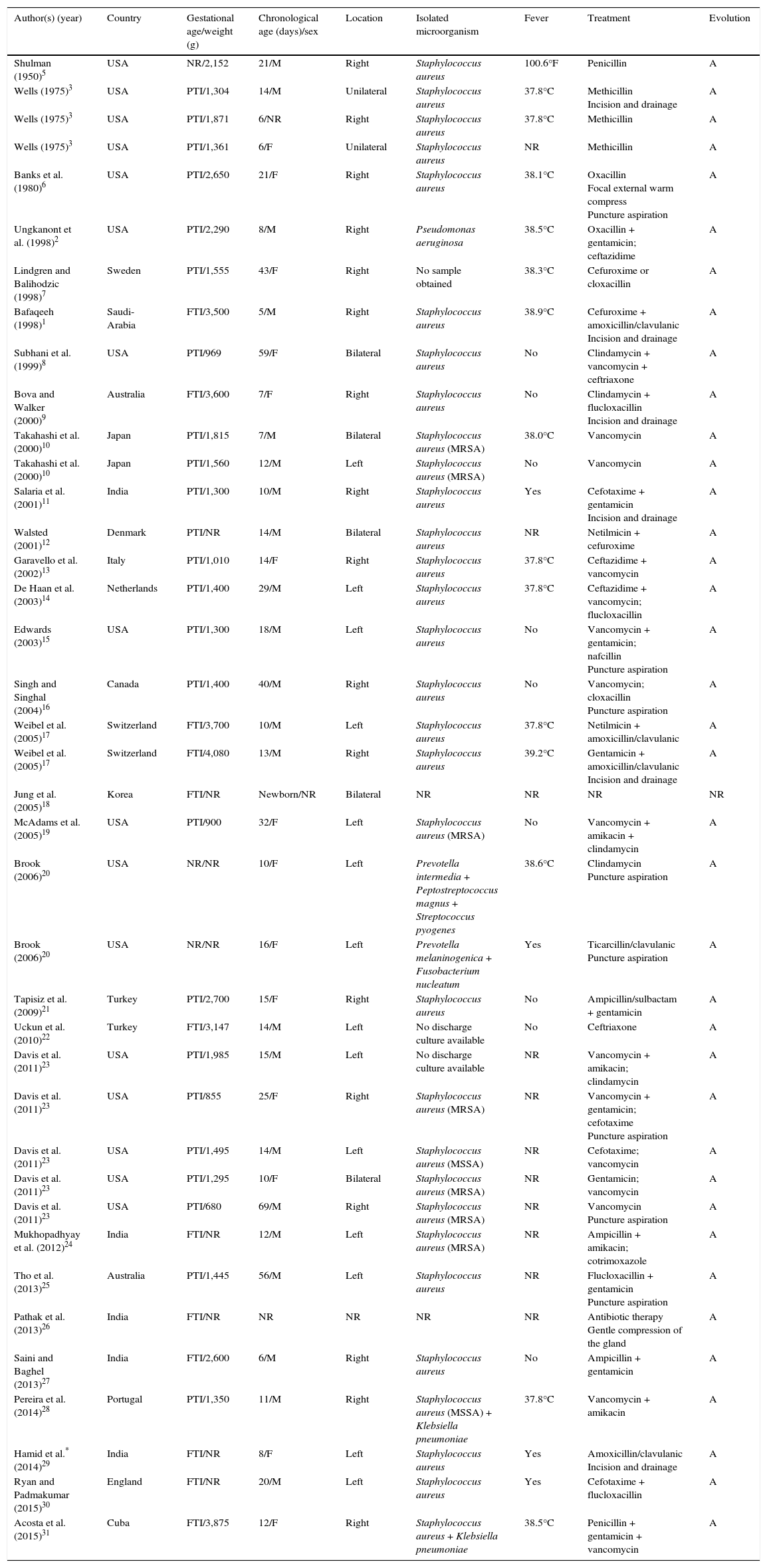
3ResultsA total of 30 papers with reports of neonatal patients with acute suppurative submandibular sialadenitis from different countries (39 cases) were found. From 1950 (BH Shulman in the United States)5 until the most recent in 2015 (same team of authors who carried out this review, in Havana, Cuba), well-documented reports were included. Different aspects of the clinical presentation of this infection are described (Table 1).
Neonatal acute suppurative submandibular sialadenitis literature reports (1950-2015).
| Author(s) (year) | Country | Gestational age/weight (g) | Chronological age (days)/sex | Location | Isolated microorganism | Fever | Treatment | Evolution |
|---|---|---|---|---|---|---|---|---|
| Shulman (1950)5 | USA | NR/2,152 | 21/M | Right | Staphylococcus aureus | 100.6°F | Penicillin | A |
| Wells (1975)3 | USA | PTI/1,304 | 14/M | Unilateral | Staphylococcus aureus | 37.8°C | Methicillin Incision and drainage | A |
| Wells (1975)3 | USA | PTI/1,871 | 6/NR | Right | Staphylococcus aureus | 37.8°C | Methicillin | A |
| Wells (1975)3 | USA | PTI/1,361 | 6/F | Unilateral | Staphylococcus aureus | NR | Methicillin | A |
| Banks et al. (1980)6 | USA | PTI/2,650 | 21/F | Right | Staphylococcus aureus | 38.1°C | Oxacillin Focal external warm compress Puncture aspiration | A |
| Ungkanont et al. (1998)2 | USA | PTI/2,290 | 8/M | Right | Pseudomonas aeruginosa | 38.5°C | Oxacillin + gentamicin; ceftazidime | A |
| Lindgren and Balihodzic (1998)7 | Sweden | PTI/1,555 | 43/F | Right | No sample obtained | 38.3°C | Cefuroxime or cloxacillin | A |
| Bafaqeeh (1998)1 | Saudi-Arabia | FTI/3,500 | 5/M | Right | Staphylococcus aureus | 38.9°C | Cefuroxime + amoxicillin/clavulanic Incision and drainage | A |
| Subhani et al. (1999)8 | USA | PTI/969 | 59/F | Bilateral | Staphylococcus aureus | No | Clindamycin + vancomycin + ceftriaxone | A |
| Bova and Walker (2000)9 | Australia | FTI/3,600 | 7/F | Right | Staphylococcus aureus | No | Clindamycin + flucloxacillin Incision and drainage | A |
| Takahashi et al. (2000)10 | Japan | PTI/1,815 | 7/M | Bilateral | Staphylococcus aureus (MRSA) | 38.0°C | Vancomycin | A |
| Takahashi et al. (2000)10 | Japan | PTI/1,560 | 12/M | Left | Staphylococcus aureus (MRSA) | No | Vancomycin | A |
| Salaria et al. (2001)11 | India | PTI/1,300 | 10/M | Right | Staphylococcus aureus | Yes | Cefotaxime + gentamicin Incision and drainage | A |
| Walsted (2001)12 | Denmark | PTI/NR | 14/M | Bilateral | Staphylococcus aureus | NR | Netilmicin + cefuroxime | A |
| Garavello et al. (2002)13 | Italy | PTI/1,010 | 14/F | Right | Staphylococcus aureus | 37.8°C | Ceftazidime + vancomycin | A |
| De Haan et al. (2003)14 | Netherlands | PTI/1,400 | 29/M | Left | Staphylococcus aureus | 37.8°C | Ceftazidime + vancomycin; flucloxacillin | A |
| Edwards (2003)15 | USA | PTI/1,300 | 18/M | Left | Staphylococcus aureus | No | Vancomycin + gentamicin; nafcillin Puncture aspiration | A |
| Singh and Singhal (2004)16 | Canada | PTI/1,400 | 40/M | Right | Staphylococcus aureus | No | Vancomycin; cloxacillin Puncture aspiration | A |
| Weibel et al. (2005)17 | Switzerland | FTI/3,700 | 10/M | Left | Staphylococcus aureus | 37.8°C | Netilmicin + amoxicillin/clavulanic | A |
| Weibel et al. (2005)17 | Switzerland | FTI/4,080 | 13/M | Right | Staphylococcus aureus | 39.2°C | Gentamicin + amoxicillin/clavulanic Incision and drainage | A |
| Jung et al. (2005)18 | Korea | FTI/NR | Newborn/NR | Bilateral | NR | NR | NR | NR |
| McAdams et al. (2005)19 | USA | PTI/900 | 32/F | Left | Staphylococcus aureus (MRSA) | No | Vancomycin + amikacin + clindamycin | A |
| Brook (2006)20 | USA | NR/NR | 10/F | Left | Prevotella intermedia + Peptostreptococcus magnus + Streptococcus pyogenes | 38.6°C | Clindamycin Puncture aspiration | A |
| Brook (2006)20 | USA | NR/NR | 16/F | Left | Prevotella melaninogenica + Fusobacterium nucleatum | Yes | Ticarcillin/clavulanic Puncture aspiration | A |
| Tapisiz et al. (2009)21 | Turkey | PTI/2,700 | 15/F | Right | Staphylococcus aureus | No | Ampicillin/sulbactam + gentamicin | A |
| Uckun et al. (2010)22 | Turkey | FTI/3,147 | 14/M | Left | No discharge culture available | No | Ceftriaxone | A |
| Davis et al. (2011)23 | USA | PTI/1,985 | 15/M | Left | No discharge culture available | NR | Vancomycin + amikacin; clindamycin | A |
| Davis et al. (2011)23 | USA | PTI/855 | 25/F | Right | Staphylococcus aureus (MRSA) | NR | Vancomycin + gentamicin; cefotaxime Puncture aspiration | A |
| Davis et al. (2011)23 | USA | PTI/1,495 | 14/M | Left | Staphylococcus aureus (MSSA) | NR | Cefotaxime; vancomycin | A |
| Davis et al. (2011)23 | USA | PTI/1,295 | 10/F | Bilateral | Staphylococcus aureus (MRSA) | NR | Gentamicin; vancomycin | A |
| Davis et al. (2011)23 | USA | PTI/680 | 69/M | Right | Staphylococcus aureus (MRSA) | NR | Vancomycin Puncture aspiration | A |
| Mukhopadhyay et al. (2012)24 | India | FTI/NR | 12/M | Left | Staphylococcus aureus (MRSA) | NR | Ampicillin + amikacin; cotrimoxazole | A |
| Tho et al. (2013)25 | Australia | PTI/1,445 | 56/M | Left | Staphylococcus aureus | NR | Flucloxacillin + gentamicin Puncture aspiration | A |
| Pathak et al. (2013)26 | India | FTI/NR | NR | NR | NR | NR | Antibiotic therapy Gentle compression of the gland | A |
| Saini and Baghel (2013)27 | India | FTI/2,600 | 6/M | Right | Staphylococcus aureus | No | Ampicillin + gentamicin | A |
| Pereira et al. (2014)28 | Portugal | PTI/1,350 | 11/M | Right | Staphylococcus aureus (MSSA) + Klebsiella pneumoniae | 37.8°C | Vancomycin + amikacin | A |
| Hamid et al.* (2014)29 | India | FTI/NR | 8/F | Left | Staphylococcus aureus | Yes | Amoxicillin/clavulanic Incision and drainage | A |
| Ryan and Padmakumar (2015)30 | England | FTI/NR | 20/M | Left | Staphylococcus aureus | Yes | Cefotaxime + flucloxacillin | A |
| Acosta et al. (2015)31 | Cuba | FTI/3,875 | 12/F | Right | Staphylococcus aureus + Klebsiella pneumoniae | 38.5°C | Penicillin + gentamicin + vancomycin | A |
USA, United States; MSSA, Methicillin-sensitive S. aureus; MRSA, Methicillin-resistant S. aureus; FTI, full-term infant; PTI, premature infant; M, male; F, female; A, alive; NR: not reported.
The patients reported in this review (39) came mainly from the United States (16) and Europe (10), while in Latin America, only the aforementioned report in Cuba was described.
This infection was observed in 24 preterm (66.6%) and in 12 term neonates. In three cases, the gestational age was not specified.
According to the reviewed cases, 22 male and 14 female patients were registered. Although there were some imprecise data in three newborns, the male gender presentation was predominant. The age of presentation was variable, from as early as five days of life until the end of the neonatal period. In six patients, who were neonates with very low birth weight (< 1500g), the infection presented after the first 30 days of life, for which it was inferred that probably they had about 30 to 32 gestational weeks of age and had not exceeded the neonatal period from a postconceptional age view.
Suppurative submandibular sialadenitis was bilateral in five patients, unilateral in 33 patients and the location could not be determined in one case. The one-sided infection appeared at the right submandibular gland in 17 patients, at the left side in 14 patients, and it was described as unilateral in two other infants. Hence, the unilateral presentation was observed to be the most common, although the involvement of both submandibular glands can occur simultaneously.
The most common pathogen was Staphylococcus aureus, which was the case in 31 patients (88.5%), from which two cases presented together with Klebsiella pneumonia. The first reported cases of infection with methicillin-resistant Staphylococcus aureus dated back to the year 2000. In three neonates, no sample of secretions was obtained for the bacteriological culture, and in one patient, the result was not specified in the article. Other microorganisms were less relevant due to the low frequency, although they were considered as microorganisms from the normal oral flora.
In this case series, fever was present in 19 neonates, not detailed in the clinical description in nine patients, and not specified in the rest of the reviewed publications.
Considering a neonate with no treatment description, all the patients received a parenteral antibiotic treatment. Variations in the type of antibiotics were found due to the different dates of the cases and the treatment protocols of each institution, among other aspects.
It is important to mention that all the patients described in this review, survived.
4DiscussionIn this study, the clinical and microbiological characteristics of 39 cases of patients with neonatal submandibular suppurative sialadenitis were analyzed.1–3,5–31 Some authors have recognized that D. H. Wells was the first to describe a case of acute neonatal submandibular suppurative sialadenitis in 1975.1,2,6 More recently, some authors21,28 give the credit to B. H. Shulman, who described a neonate with this infection in the right submandibular gland without the involvement of the parotid glands5 in 1950. Since then, it is possible to find reports on specific cases, a series of five cases,23 and the most recent paper published on the subject, a neonate in Havana, Cuba.31
Acute suppurative submandibular sialadenitis is more common in preterm neonates in contrast with what is described for the acute suppurative parotitis, which occurs primarily in term neonates.32 Prematurity is a clear condition of risk for this infection in the neonatal period, even within the first months of life.13 However, it is important to consider that these infants frequently need nasogastric or orogastric feedings. The long-term tube feeding can decrease the saliva secretion by the lack of oropharyngeal stimulation, slowing or producing a functional blockage of saliva ducts. The stasis favors bacterial colonization and subsequent infection of the gland.7,13 Similarly, dehydration can decrease the secretion of saliva by thickening and slowing its the flow in Wharton's duct.13 Although salivary, parotid, and submandibular glands flow through their respective ducts into the mouth, the quality of their secretions differs. Accordingly, acini are primarily serous in the parotid glands, while serous and mucinous in the submandibular and sublingual glands.33 Mucinous secretions contain more IgA and other enzymes, which protect the submandibular glands from infections. For this reason, it may be possible that the parotid glands are more commonly affected.33 The reason why Staphylococcus aureus is the most universal causal agent is not clear since it is not part of the normal oral flora in the newborn. It usually consists of Streptococcus, lactobacillus, and anaerobes.34 Therefore, it is understandable that the use of baby bottles, a poor breast hygiene in feeding the infant, the use nasogastric or orogastric devices, or endotracheal intubation can carry this organism to the oral cavity of the neonates.
One of the characteristics of the neonatal suppurative submandibular sialadenitis is the acute onset, with signs of submental inflammation that sometimes spreads underneath the neck. It is easily palpable, presenting a firm consistency usually painful to pressure; the overlying skin is usually erythematous, warm and slightly thickened.35 These characteristics were universal in every case described. Fever was frequent, and sometimes high.
The characteristic sign of acute suppurative submandibular sialadenitis, and for some the most specific diagnostic clinical sign is Wharton's duct discharge of purulent secretion on the floor of the mouth, under the tongue, more evident when applying pressure on the inflamed gland.13,35 The opening of the excretory duct may be red, or in some cases, dilated. The infection can spread and cause bacteremia. Consequently, some patients may present a systemic inflammatory response, reaching a sepsis condition.19,35
The evolution to an abscess formation in the submandibular gland may also occur in the neonate.1,9,25,27 It may be identified by a fluctuant sensation when palpating, which was not present at the beginning of the infection, that can be corroborated by imaging studies such as an ultrasound.1,9
This infection must be differentiated from suppurative adenitis, which may exhibit a submental location but is characterized by a firm, painful, and mobile mass associated with chin inflammation, or intraoral, which shows no pus output through the Wharton's duct when pressed. Other conditions easy to exclude are lymphangioma, teratoma, and dermoid cysts, or cystic hygroma lymphangioma due to the palpation characteristics and by not showing inflammatory signs.
Currently, the infection with methicillin-resistant Staphylococcus aureus is common. In this review, it was noted that, in some cases, the pathogen isolation corresponded to methicillin-resistant Staphylococcus aureus, and in other cases, it was sensitive to methicillin. Due to these epidemiological aspects, the use of methicillin-resistant anti-staphylococcus antibiotics is recommended in such cases.19,21,24,28 Moreover, the combination of antibiotics that cover the spectrum of Gram-negative is suggested, as these microorganisms are also among the causative agents of neonatal suppurative submandibular sialadenitis.19,21,28 Thus, empiric therapy should begin with penicillin for methicillin-resistant Staphylococcus plus an aminoglycoside or penicillin for methicillin-resistant Staphylococcus plus a cephalosporin.
The duration of the antibiotic therapy has not been precisely determined but is normally agreed that a minimum of 7 to 10 days is necessary, or at least until the infection is solved.19,35 It is also possible to consider a sequential oral-parenteral scheme to avoid a long parenteral treatment.
Some patients with abscess formation may require surgical treatment by incision and drainage.1,9 External compression of the gland to facilitate pus drainage, as well as a guidance in the diagnosis may help to a faster resolution of the infection. However, this procedure should be performed by a gentle manipulation to prevent the hematogenous spread of the infection.26
To the present day, the course of this infection is favorable with the appropriate antibiotic treatment.
It is concluded that the acute neonatal suppurative submandibular sialadenitis is a rare infection, which has maintained similar clinical and microbiologic features throughout the years: it mainly affects preterm neonates, presents local inflammatory manifestations in the submandibular region, it is usually unilateral, with purulent secretion by the Wharton duct on the floor of the mouth as a characteristic sign. It is usually caused by Staphylococcus aureus, although strains of methicillin-resistant Staphylococcus aureus have been identified recently. In general, this infection progresses favorably with a parenteral wide-spectrum antibiotic treatment. However, sometimes an incision and drainage are necessary in the case of an abscess.
FundingNo funding was received from any institution or organization. The work was carried out at the expense of the authors themselves.
Conflict of interestThe authors declare no conflict of interest of any nature.
Please cite this article as: Díaz Álvarez M, Acosta Batista B, Rivera Alés L. Sialadenitis submandibular supurada aguda neonatal. Reportes de la literatura, periodo 1950-2015. Bol Med Hosp Infant Mex. 2016;73:302–308.