Introducción: La notificación espontánea depende de la capacidad de los médicos de detectarlas reacciones adversas a medicamentos (RAM) y del hábito de reportarlas. En 2008 y 2009, la frecuencia de reportes de RAM al Programa Electrónico de Farmacovigilancia (SISFAR) del total de egresos del Hospital Infantil de México Federico Gómez fueron bajas (0.44 y 0.20%, respectivamente). Por esta razón, en el 2010 se decidió evaluar la capacidad de los médicos de identificar las RAM utilizando como estrategia la revisión de los expedientes clínicos.
Métodos: Se llevó a cabo un estudio observacional, descriptivo, transversal y retrospectivo en el Departamento de Urgencias (DU), del 1 de marzo al 31 de agosto del 2010. Se clasificaron y cuantificaron como RAM identificadas por los médicos cuando existió evidencia por escrito en el expediente clínico de que ellos habían asociado una manifestación clínica con una RAM, incluyendo además la evaluación del número de reportes al SISFAR. Se realizó el análisis descriptivo con SPSS versión 18.
Resultados: La frecuencia de RAM de los pacientes que ingresaron al DU fue del 21.8%. El 86% de ellas fueron identificadas por los médicos en el expediente clínico y el 14% por el farmacéutico. Se reportó solamente el 6.1% al SISFAR.
Conclusiones: Aunque fue elevada la identificación de las RAM en el expediente clínico, es posible que existan algunas que no se hayan detectado. Por otra parte, se confirmó el elevado grado de subreporte al SISFAR, por lo que se requieren acciones para fomentar el hábito del reporte.
Introduction: Spontaneous notification depends on the ability of pediatricians to identify adverse drug reactions (ADRs) along with their habit of reporting these incidents. During the years 2008 and 2009, the frequency of reports of ADRs to the Electronic Program of Pharmacovigilance (SISFAR) in the Hospital Infantil of Mexico Federico Gomez (HIMFG) was low (0.44% and 0.20%, respectively). Because of the above, the ability of pediatricians from the Emergency Department (ED) to identify ADRs using the clinical chart review was evaluated in 2010 in this study.
Methods: A descriptive, observational, cross-sectional retrospective study was conducted in the ED from March 1 to August 31. ADRs were classified and quantified as ‘‘ADRs identified by pediatricians’’ when there was evidence in the clinical chart that pediatricians associated a clinical sign, symptom and laboratory value with an ADR. The numbers of notifications reported in SISFAR were quantified. Descriptive analysis was done using SPSS v.18.
Results: Considering patients who were admitted to the ED, the frequency of ADRs was 21.8%. The frequency of ADRs identified by physicians in clinical charts was 86%. The pharmacist detected 14% of ADRs. The frequency of ADRs reported by physicians was 6.1%.
Conclusions: Although identification of ADRs in the clinical charts by pediatricians was high, it is possible that some ADRs were undetected. Because underreporting was very high, it is necessary to take actions to improve the reporting process.
Pagina nueva 1
1. Introduction
The international program for medication monitoring of the WHO defines pharmacovigilance as the science and activities related to the detection, measurement, understanding and prevention of adverse effects or any other problem associated with medications.1 According to the methodology used for its search, adverse drug reactions (ADR) occur with a frequency from 0.14-36.6% in children2-4 with a high impact on morbidity and mortality5,6 and on costs.7 Within the existing methodologies, the one recommended by the International Program for Drug Monitoring is noteworthy, which is the spontaneous notification (SN) that must be carried out voluntarily by health professionals and the pharmaceutical industry to the health authorities.1
SN allows for early detection of new signals as well as severe ADR and those that are infrequent.8 According to what has been published by the International Center for Drug Monitoring in Uppsala, Sweden,9 physicians are the health professionals who have the greatest participation in that program sponsored by the WHO, with 55% of the notifications in patients from 0-17 years and 49% in those >18 years of age. Notwithstanding the foregoing, there are causes attributed to physicians that potentially decrease the effectiveness of the SN such as a low capacity for identifying the ADR or the habit of not reporting to the regulatory agencies those that have been detected.10 With respect to the identification of ADR, it is essential in the first place to suspect that a sign, symptom or abnormality in laboratory tests in a patient may be caused by a medication. For this, the physician has to develop the ability that must become a habit to detect, in all patients who are receiving one or various medications, signs and symptoms that are not directly related with the disease. But it may also happen that the physicians, having identified an ADR, do not report it to the corresponding regulatory agencies, so that underreporting of the numbers occurs, which varies between 6 and 100%.10 Various studies have attempted to identify the personal and professional characteristics as well as attitudes for the physicians associated with underreporting.11 However, there are scant publications that have evaluated the ability of physicians to identify ADR in pediatric patients.12-14
The drug monitoring system of the Hospital Infantil de Mexico Federico Gomez (HIMFG), which is a tertiary care hospital, is based on the SN. Initially, it was done by manually completing reporting forms. However, it was substituted by an electronic program called SISFAR developed by the Institutional Center for Drug Monitoring (CIF) of the HIMFG and based on the Official Mexican Standard for Pharmacovigilance (NOM-220-SSA1-2012).15 It was installed in ten areas of hospitalization including the emergency department.16
SISFAR has the advantage of easy access and completion so the capture of information takes less time compared with the manual method. Upon receiving the electronic information, a pharmacist analyzes it according to the guidelines of the NOM-220-SSA1-201215 and sends it to the National Center of Pharmacovigilance of the Mexican Department of Health.
Despite having this system in the HIMFG, during 2008 and 2009 the frequency of ADR reports to the SISFAR from the total number of discharges was low (0.44 and 0.20%, respectively).17,18 With the purpose of finding an explanation for the low reporting of the ADR to SISFAR, it was decided to investigate whether this phenomenon could be attributed the physicians not identifying the ADR or if they identified them and chose not to report them to the SISFAR.
2. Methods
The study was carried out in the emergency department of the HIMFG. The design was observational, descriptive, cross-sectional and retrospective. It began March 1, 2010 and concluded on August 31 of the same year. Definition of an ADR was based on that given by the WHO: a drug response that is harmful, unintended and occurs at doses normally used for prophylaxis, diagnosis, treatment or modification of a physiological function.1
Before starting the present study and as part of the daily routines of the Institutional Center of Pharmacovigilance, training directed exclusively at emergency department medical personnel was undertaken, which included training in the electronic documentation of SISFAR. Any doubts the staff may have had regarding the program were answered and in-service training regarding the importance of drug monitoring in adults and children was included. Once this training was completed, notifications sent to the CIF during the study period by the physicians were quantified. Affiliated physicians were four permanent staff members in addition to five subspecialty residents of pediatric emergencies (two from the first year, two from the second year and a chief resident) who remained during the entire study period, and five pediatric residents (two from the first year, one from the second year and one from the third year) who on average had a 1-month stay and were substituted by new residents who were also trained.
Patients were classified with a “physician-identified ADR” when, according to the pharmacist trained in pharmacovigilance, there was written evidence that the physician had associated a clinical manifestation with an ADR. In order to do this, the clinical charts of each patient admitted to the emergency department were reviewed, looking at the clinical notes from physicians, nurse’s log, and laboratory and imaging tests, evidence of a possible ADR including search of the terms and actions such as “due to medication”, “related with medications”, “associated with the administration of medications”, “adverse drug reaction”, “patient diagnosis”, “if the physician discontinued the suspected medication”, and “if the physician prescribed some medication to treat an ADR.” ADRs were classified with respect to the severity, seriousness and causality according to the Official Mexican Standard for Pharmacovigilance (NOM-220-SSA1-2012).15 Using MICROMEDEX 2.0, the pharmacist consulted the clinical manifestations of the ADR of each of the medications given to the patients.19
When there was a question regarding a possible cause-effect relationship of the medication with the clinical manifestations, it was discussed with a pediatrician with expertise in pharmacovigilance. For data collection from the clinical charts, a database was designed using Microsoft Office Excel 2007 and included capture of patient demographics (chart number, name, date of birth, age, gender, weight, height), admission diagnosis, date of admission, laboratory results, relevant medical history and medications taken before or during admission as well as the discharge date from the emergency department.
2.1. Statistical analysis
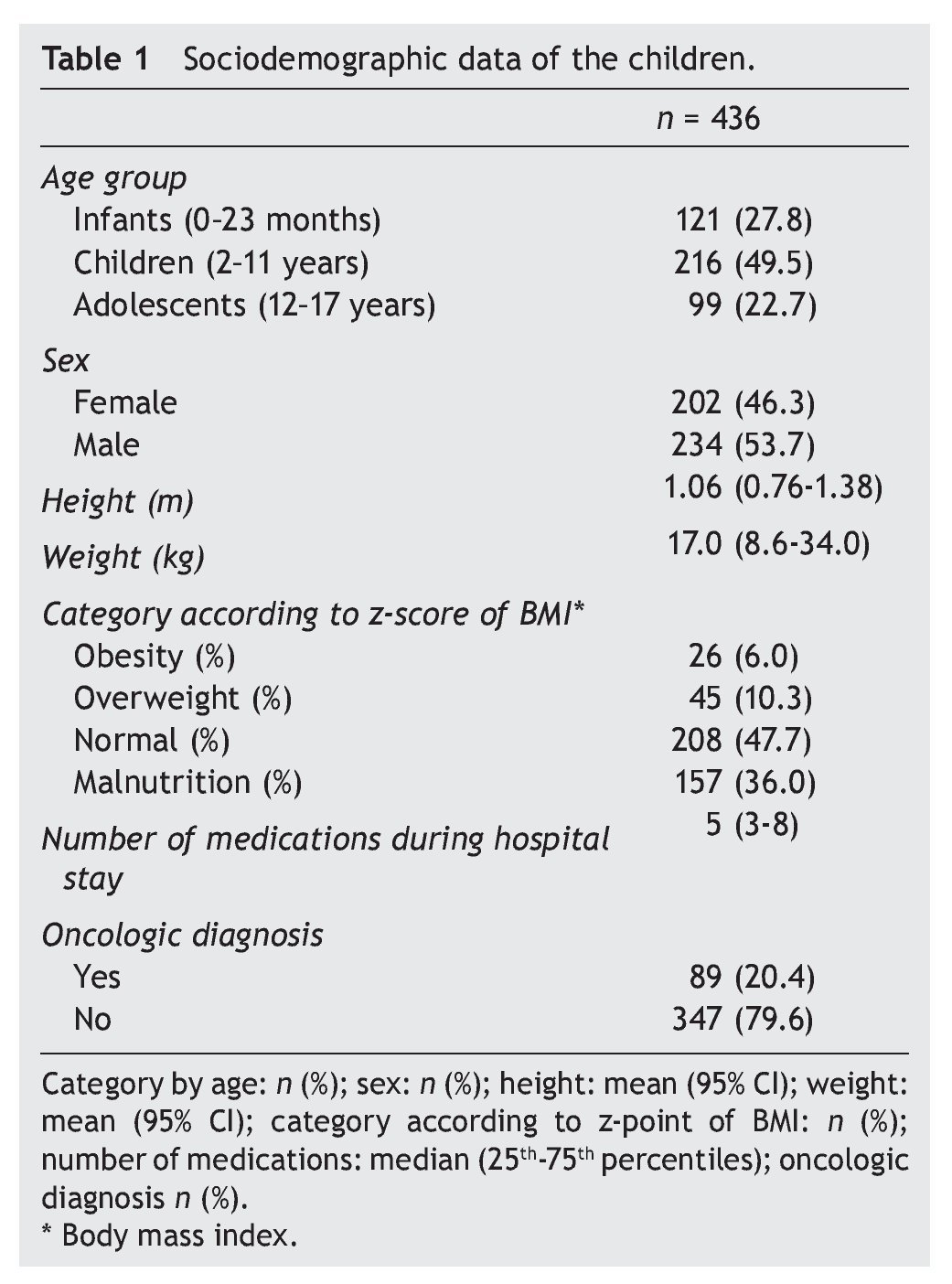
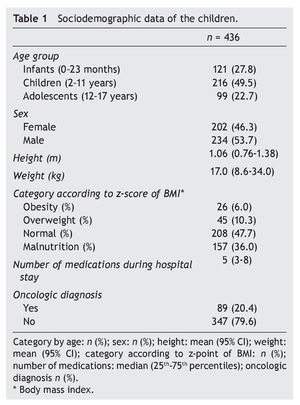
Sample size was determined by convenience. Frequency of ADR in the emergency department was calculated as well as the frequency of the ADR identified by the physicians in the clinical chart and those reported to SISFAR. Patient’s age was categorized according to the Guide of the International Conference on Harmonisation on the Clinical Investigation of Medicinal Products in the Paediatric Population20 and the Z-score of the body mass index was calculated with the WHO AnthroPlus program.21 Descriptive analysis included central tendency and dispersion as well as the number of cases (%), mean (95% CI) and median (25th-75th percentiles). Statistical analysis was done with SPSS v.18.
2.2. Ethical considerations
The research protocol was approved by the Research Commission and the Committee on Ethics and Biosafety of the HIMFG.
3. Results
During the study period, the first ten causes of admission to the emergency department classified according to the ICD-10 22 were pneumonia (J18.9) (8.97%), agranulocytosis (D70) (5.42%), convulsions (R56.8) (4.73%), hemarthrosis due to hemophilia (M25.0) (4.26%), gastroenteritis (A09) (3.17%), septicemia (A41.9) (3.05%), head trauma (S06.9) (2.98%), epistaxis (R04.0) (2.89%), asthma (J46) (2.11%) and acute lymphoblastic leukemia (C91.0) (1.79%).
From a total of 436 cases who were admitted to the emergency department from March 1 to August 31, 2010, there were 95 patients documented to have an ADR (21.8%). Of these, 82 were identified and documented in the clinical chart by the physicians (86%) and the remaining 13 were identified by the pharmacist alone (14%). From the 82 patients who were identified and documented by the physicians, only five were also reported to SISFAR (6.1%). In order to describe the demographic characteristics of the patients included in the study, Table 1 was generated, which shows that 49.5% of the children were 2-11 years of age, 6% had obesity and 36.0% malnutrition and that the average number of medicines received during their stay was five. In addition, 20.4% had an oncological disease.
4. Discussion
A relevant and unexpected finding in the present study was that the physicians identified and noted in the clinical chart a high percentage of the ADRs found in children, even when the majority did not send an electronic notification to the CIF. It should be noted that identification of the ADR by the physicians in all 95 patients was 86% and the lack of reporting to CIF was very high (93.9%). Another unexpected finding was that actions were undertaken with the patients in whom the physician identified an ADR such as suspension of the suspected medication or, in some cases, initiation of a specific treatment to control adverse effects, actions which were not quantified in the study. It should be noted that identification of 14% of the additional ADR cases found in the present study was done by the pharmacist, which led to a more effective identification. This finding is consistent with other studies that include pharmacists as clinical chart reviewers because they detect higher rates of ADRs than other health professionals.23
With respect to the identification of the ADR, there are findings similar to those in the present study. Such is the case of an adult intensive care unit in which the physicians identified and recorded in the clinical chart 70% of the ADRs presented.24 In the case of the pediatric patients, Neubert et al. performed a study in which the clinical chart was reviewed by a team of experts (clinical pharmacist, pharmacist and pediatrician) as the gold standard and found that the physicians recognized 50% of the ADR presented by the children admitted to that pediatric hospital.12 Oehme et al. in 1999 found that the physicians identified in the clinical chart 45.7% of the ADRs. This number increased to 96.2% for the year 2008, which was attributed to a greater sensitization by the medical personnel. In another study in children in which the clinical chart was also used to search for ADR, it was found that 91.1% of the children had been identified by the physician.14 In Mexico, in a study performed on adult patients, the physicians identified and recorded in the clinical chart 76% of the ADRs.25 As mentioned in the Results, frequency of the ADR in the emergency department was 21.8%, which can be considered one of the highest reported in hospitalized pediatric patients2,3 and can be explained in good measure by the methodology used or by the characteristics of the pediatric emergency department.
Some authors13,26 reported that the clinical chart review can be considered the gold standard. However, they also note that it is a method that consumes time and human resources and, therefore, is costly. There is one more condition, if applied in medical departments where the clinical chart is incomplete, results may be unreliable. With respect to computerized methods,14,26-28 these allow for analyzing the possibility of a causal relationship through the generation of algorithms and are considered a low-cost and practical tool that consumes fewer resources and increases the rate of ADR detection compared with the SN and manual review of the clinical charts. However, a limitation of the computerized method is that there are hospitals without a complete electronic record of the clinical files that include the clinical history, physician and nursing notes, and laboratory and imaging tests. A procedure that could perhaps increase the number of reports and improve the identification of the ADR would be mandatory reporting instead of voluntary reporting as was recently implemented in the Official Mexican Standard for Pharmacovigilance.15 However, as was shown in a systematic review,11 this is not necessarily useful in practice because there are professional and personal factors that commonly have a role in underreporting of the ADR such as the medical specialty, age, place and workload, specific training in drug monitoring, lack of knowledge about the requirements, fear of ridicule, lack of interest, insecurity, complacency and fear of legal problems.
In future studies related to ADR, definition of what constitutes an ADR must be specified, whether it is the definition recommended by the WHO or the new definition which, in December 2010,29 was approved by Parliament and the European Council and which has been in force since July 2012 in the regulation of human medical products in the European Union. It defines an ADR as a “drug response that is harmful and unintended.” This change was aimed at ensuring that the harmful and not intended effects would be covered, not only from the authorized use of a medication at normal dosages but also as a consequence of medication errors and applications outside the terms of authorization, including misuse and abuse of drugs.
Within the limitations of this study, the following might be considered:
• The findings reflect only what happens in the emergency department and cannot be extrapolated to the clinical and surgical departments of the hospital being studied.
• Despite training of emergency department personnel, it can be considered that this was not effective either because it had no impact on physicians or because it was not sufficiently didactic as can be inferred by the low percentage of spontaneous reporting of the ADR to the CIF.
• The reasons for not reporting an identified ADR by the physician to the CIF through the structured questionnaire was not explored as several of the reasons previously mentioned.11
• Sometimes, in the clinical chart, physician’s clinical notes are not clearly identified because they were handwritten or at other times because they were in disorder or were missing some laboratory tests or treatment data.
• It was not able to be ruled out that, in some cases, the physician may have considered the presence of an ADR but opted to not document it in the clinical chart.
• The cases in which the physician did not consider the association of a medication with the presence of certain signs, symptoms and alterations of the laboratory tests were not investigated.
In conclusion, the present study demonstrates that physicians at this hospital identified ADRs that occur in children hospitalized in the emergency department; however, the main problem is that they did not report these events. It is necessary to implement strategies to substantially improve ADR reporting to the CIF; therefore, minimizing underreporting. It is advisable to intentionally incorporate the pharmacists in the surveillance of drug monitoring activities.
Ethical responsibilities
Protection of persons and animals. The authors declare that all procedures were carried out in accordance with the ethical norms of the responsible Committee of Human Experimentation and in accordance with the World Medical Association and the Declaration of Helsinki.
Confidentiality of Data. The authors declare that no patient information appears in this article and all data were kept confidential.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Funding
None.
Conflict of interest
The authors declare no conflict of interest of any nature.
Received 16 February 2015;
accepted 21 April 2015
http://dx.doi.org/10.1016/j.bmhimx.2015.04.003
* Corresponding author.
E-mail:magdalaqfb@yahoo.com.mx (O. Morales-Ríos).