Oral mucositis (OM) is an inflammatory reaction of the oropharyngeal mucosa to cumulative chemotherapy (CT) and radiation therapy (RT), affecting one or more parts of the digestive tract along with the quality of life (QoL) of the patient. The goal of this study was to identify valid and reliable tools to evaluate QoL related to OM.
MethodsA systematic review of the literature was conducted up to May 2016. Articles were selected by peers using the PubMed database through a search following the inclusion and exclusion criteria and STAndards for the Reporting of Diagnostic accuracy studies (STARD) checklist with a cut-off point ≥ 70%.
ResultsWe identified four relevant articles that described instruments to assess the QoL related to OM in patients undergoing cancer treatment.
ConclusionsThe evaluation of the QoL in patients with OM is a difficult scenario because of its multiple variables. The knowledge of this relationship is limited because general instruments of oral health or cancer therapy are commonly used for evaluation. However, valid instruments are already available for estimating the impact of OM on the QoL from the patient's perspective.
La mucositis oral (MO) es una reacción inflamatoria de la mucosa orofaríngea a la quimio y radioterapia acumulativa, que afecta una o varias partes del tracto digestivo, además de la calidad de vida (CV) del paciente. El objetivo de este estudio fue identificar instrumentos válidos y confiables para evaluar la CV relacionada con MO.
MétodosSe realizó una revisión sistemática de la literatura hasta mayo del 2016. Se realizó una selección por pares de los artículos a través de una búsqueda en PubMed, siguiendo los criterios de inclusión y exclusión y la lista de los estudios de precisión diagnóstica STARD (STAndards for the Reporting of Diagnostic) con un punto de corte ≥ 70%.
ResultadosSe identificaron cuatro artículos relevantes que describen instrumentos para evaluar la CV relacionada con MO de pacientes que reciben tratamiento contra el cáncer.
ConclusionesEl escenario para la evaluación de la CV de pacientes con MO resulta complicado debido a las múltiples variables. El conocimiento de la relación entre la CV y la MO es limitado porque los instrumentos generales son comúnmente utilizados tanto para la evaluación de la salud oral como para la terapia contra el cáncer. Sin embargo, ya se cuenta con instrumentos válidos para la evaluación del impacto de la MO sobre la CV desde la perspectiva del paciente.
Anticancer therapy-induced toxicity is a current significant clinical problem.1 The quality of life (QoL) is an area of growing interest that has evolved as a multidimensional construct. It integrates the perception of the patient to the impact of the disease and its treatment, as well as its performance concerning various aspects of life, including physical, psychological, and social health.2 Since the evaluation of the QoL includes subjective elements, a consistent method is required to gather information on the individual. The evolution of the study of the QoL has allowed instruments used for its evaluation to become more precise to understand and compare the health status of populations, as well as for evaluating the impact of certain medical interventions, symptoms, and physical function over time.3 Currently, there are generic and specific instruments. Generic instruments are used in healthy or sick subjects for comparing the QoL related to health under different conditions and times. On the other hand, specific instruments evaluate the QoL based on the disease characteristics and its treatment, such as nausea, pain, and anxiety, as well as the ability to observe areas with greater problems to provide specific therapies.4 In order to consider a questionnaire to be valid, it should be reliable and able to detect and measure changes throughout time. It has to be adequate for measuring the phenomenon that it intends to measure, and reflect the underlying theory in the phenomenon or concept that is desired to be measured.5
Head and neck chemotherapy (CT) and radiation therapy (RT) usually cause severe damage to the epithelial layer of oral mucosa.1 Therefore, oropharyngeal mucositis (OM) has become a common secondary effect,6 being one of the main complications related to cancer treatment.7 The incidence of OM varies depending on the type of therapy, prior number of cycles, and episodes of OM. It occurs between ∼30% and 75% of patients,8 specifically in 20% of patients with CT for colorectal cancer, 50% of patients with breast cancer, ∼97% of patients who receive conventional RT, and in 89% of patients with CT for head and neck cancer. Patients subjected to high doses of myeloablative CT with or without concomitant total body radiation before hematopoietic stem cell transplantation (HSCT) have an incidence of ∼75-100%.1
OM manifests as erythema and edema in the oropharyngeal mucosa until ulcers appear.7 It occurs after 3-5 days or 7-10 days from the initiation of CT or RT, respectively. Labial and buccal mucosa, tongue, the floor of the mouth and soft palate are more affected than the more keratinized tissues.9 During the most symptomatic phase of OM, high levels of pain and regional dysfunction including swallowing, chewing, drinking and speaking may cause profound effects on the patient's daily life. It is estimated that ∼38% of the patients with OM suffer from depression.1 Pain is the most distinctive symptom of OM. Although it is not a fatal complication, it is very distressing for patients, particularly those who are subjected to HSCT. OM aggravates the clinical status and causes oral symptoms due to an inadequate consumption of food and fluids. This insufficient ingestion leads to periods of malnutrition, dehydration, and weight loss, which represent changes in physical and mental health that alter the QoL of patients.10–12 Despite the fact that there is no definitive treatment for preventing or treating OM, clinical improvement is related to neutrophil recovery, implementation of therapies, and protocols based on guidelines for its management that have shown good results through nutritional support, pain management, control of bleeding, disinfection of the buccal cavity, and palliative care.13
Currently, the patient outcomes that some tools report are increasingly used to measure symptom burden, functionality, and the QoL related to OM. These tools capture the perception of the severity of the symptoms without the interpretation of a third party directly. Since patient-reported symptoms tend to be different from those recorded by physicians, there are instruments designed to measure this association. These tools evaluate the severity of symptoms, pain, emotions, and physical limitations that together interfere with activities of daily living.14 For this reason, the purpose of this study was to identify specific, valid, and reliable instruments to evaluate the QoL of patients with OM secondary to cancer treatment and to determine their content and psychometric properties. Therefore, the research question we wanted to answer in this systematic review was the following: What are the specific and valid instruments for the evaluation of the QoL of cancer patients with OM?
2MethodsWe conducted a systematic search in PubMed until May 2016. The search strategy included the following MeSH (Medical SubHeadings) terms: stomatitis, quality of life, questionnaire, and the text words oral mucositis and oropharyngeal mucositis.
2.1Inclusion criteriaInclusion criteria involved specific instruments for evaluation of the QoL in patients with OM secondary to cancer treatment (CT, RT or HSCT), patients of any age and either gender, and that the studies presented the methodology and statistical tests to determine the reliability, accuracy, and validity or reproducibility of the instrument. The report of internal consistency and test-retest was considered as reliability. For validity, the report of content, convergence and of the construct, description of the target population (demographic and clinical characteristics of the study group), calculation of sample size or sampling, ethical aspects, and the description of the scale used to determine the degree of OM.
2.2Exclusion criteriaFor the purpose of this study, exclusion criteria were the following: other languages different from English or Spanish, instruments for the general evaluation of cancer treatment, generic QoL instruments for the cancer patient, and tools that exclusively measured symptomatology and severity of OM. Independent peer reviewers performed article selection (titles and summaries) and evaluation of the complete text. This last step was performed following the STAndards for the Reporting of Diagnostic accuracy studies (STARD)15 with a cut-off point ≥ 70%.
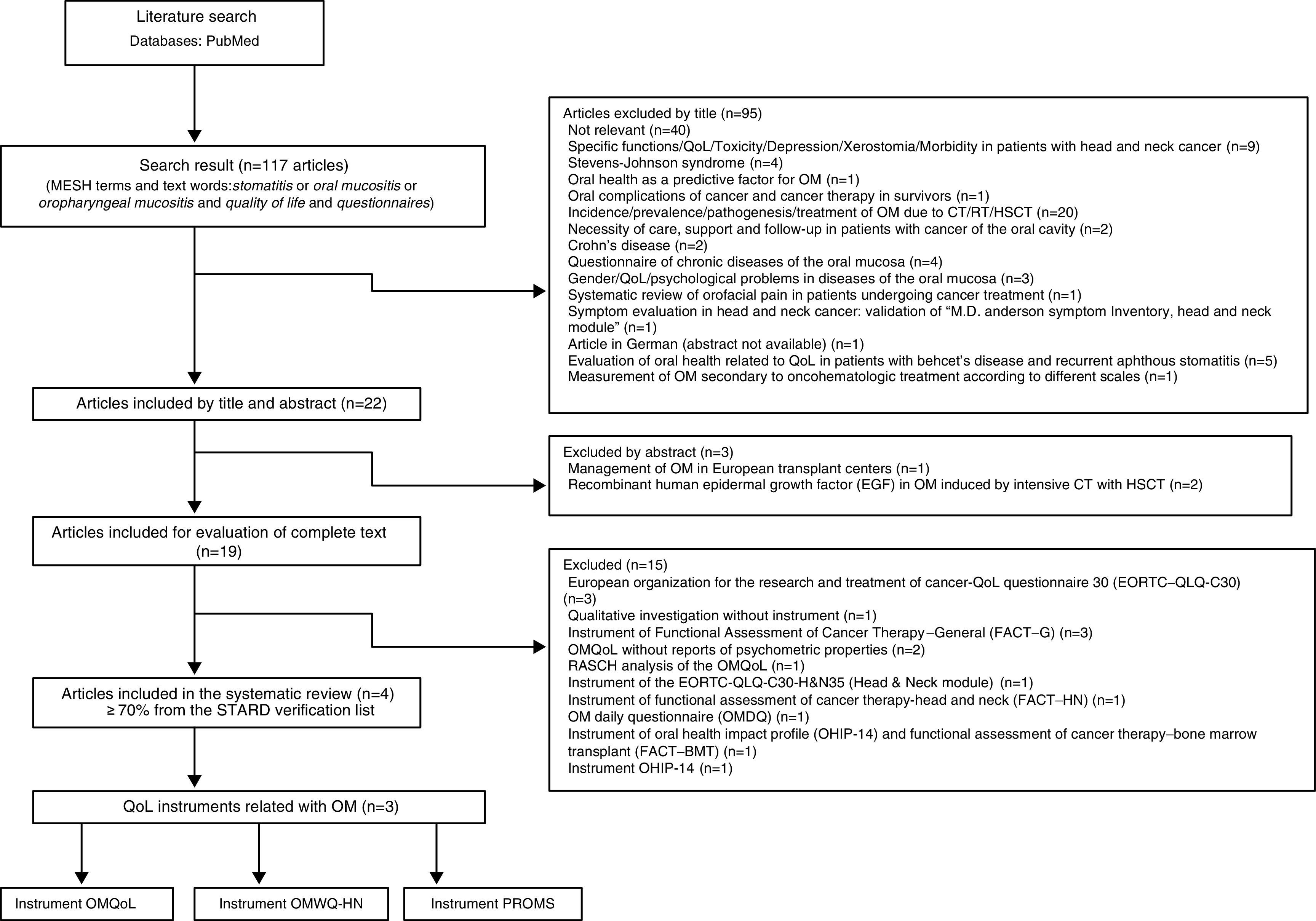
3ResultsOne-hundred seventeen titles of scientific articles were found using the search strategy mentioned above. We selected 22 titles with a summary for their review. Three of them (clinical management of OM) were excluded, and 19 articles were included for complete text review. From these, 15 articles were excluded because they used instruments from the European Organisation for the Research and Treatment of Cancer−QoL (EORTC-QoL) for evaluating the QoL in patients with OM. We excluded any qualitative investigation and several articles that used instruments for functional evaluation of general cancer treatment, or instruments for functional evaluation of treatment of chronic diseases. The following articles, which reported the QoL of patients with OM using the OMQoL instrument without the psychometric properties, were also excluded: one article with Rasch analysis of the OMQoL, a questionnaire of daily evaluation of OM, and articles that used instruments from the profile of the impact of oral health (one of them together with the instrument for functional evaluation of cancer treatment in bone marrow transplants).1,16–26,28
Only four potentially relevant articles were included (Figure 1). Within the four articles selected, we found three specific instruments for the evaluation of the relationship of symptoms of OM regarding the QoL of patients affected by the cancer treatment. Among these tools was the Patient-Reported Oral Mucositis Symptom (PROMS) scale, which is based on the perception of the impact of OM on the oral health of patients who are candidates for bone marrow transplant and other therapies against cancer, and that theoretically have a negative impact on the QoL. PROMS scale consists of a 100-mm visual analogue scale (VAS) that evaluates ten elements: mouth pain; difficulty speaking; restriction of speech; difficulty eating hard foods and soft foods; restriction of eating; difficulty of drinking; restriction of drinking; difficulty swallowing; and changes in taste. It also presents an adequate internal consistency and a discriminating validity with a depression scale.11
The Oropharyngeal Mucositis-specific Quality-of-Life (OMQoL) instrument particularly measures the QoL of patients with OM from the patient's perspective, using 31 elements grouped into four dimensions that evaluate the symptomatology, nutrition, social function, and symptomatology for swallowing with a 4-point Likert-type scale (1=not at all; 2=a little; 3=quite a bit; 4=very much). Construction of the instrument was done in two phases. The first was a qualitative phase for the elaboration of the elements through patient and family interviews and evaluators (apparent validity and content validity). The second phase consisted of a factorial analysis presenting an internal consistency, a test-retest, and an adequate intraclass correlation coefficient (ICC), in addition to a concurrent validity, a convergent validity, and known groups validity by correlation with a scale of OM symptoms along with the simultaneous application of the Chinese version of the EORTC-QoLQ−Core 30 and the WHO scale for OM where the correlations were adequate.1,29
The Oral Mucositis Weekly Questionnaire−Head and Neck Cancer (OMWQ−HN) is the result of the patient's report that measures the symptoms of OM, including mouth and throat pain and its impact on well-being and function. The OMWQ−HN was developed in two phases: the qualitative phase, through patient interviews and the reformulation of questions based on the Functional Assessment of Cancer Treatment−Head and Neck Cancer (FACT−HNC) and Performance Status Scale−Head and Neck (PSS−HN), resulting in 12 elements with an internal consistency and ICC. The quantitative phase was adequately correlated with the Functional Assessment of Cancer Treatment–Physical Wellbeing (FACT−PWB) subscale and Emotional Wellbeing (FACT−EWB) subscale from the Functional Assessment of Cancer Treatment−General (FACT−G). These scales were adequate, demonstrating to be valid and reliable instruments for evaluating the impact of OM in patients who receive RT with or without CT for head and neck cancer (Table 1).30
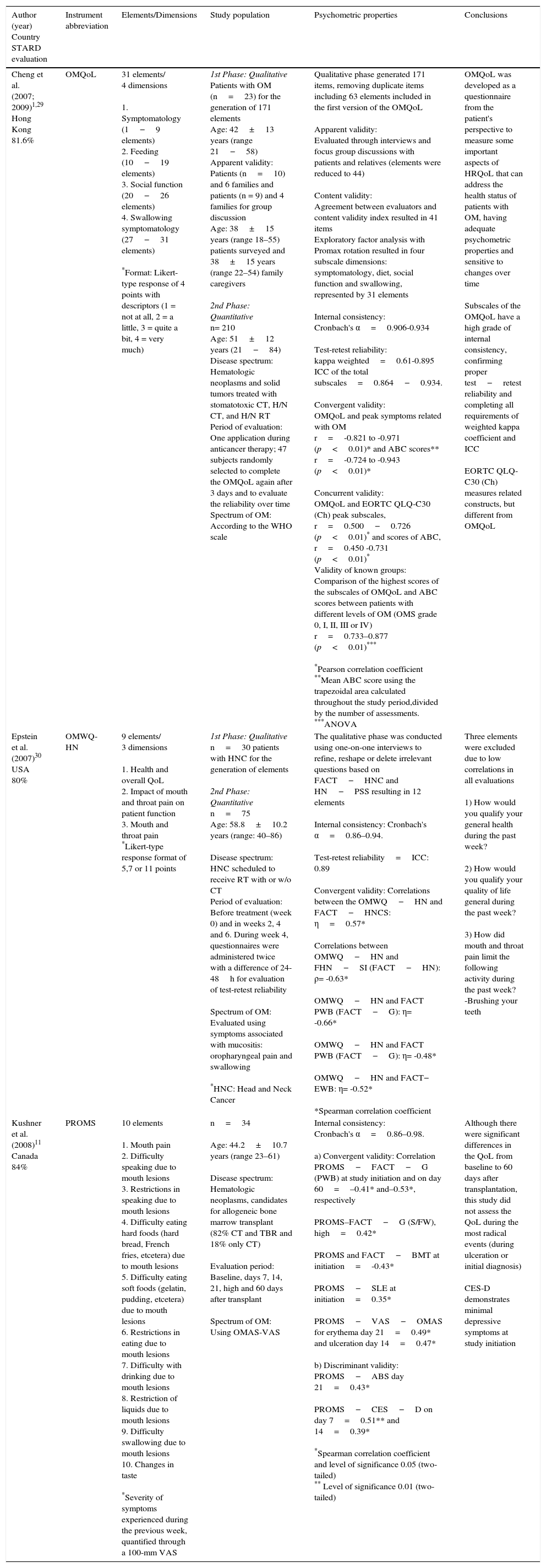
Review of the instruments that evaluate the impact of oral mucositis on QoL.
| Author (year) Country STARD evaluation | Instrument abbreviation | Elements/Dimensions | Study population | Psychometric properties | Conclusions |
|---|---|---|---|---|---|
| Cheng et al. (2007; 2009)1,29 Hong Kong 81.6% | OMQoL | 31 elements/ 4 dimensions 1. Symptomatology (1−9 elements) 2. Feeding (10−19 elements) 3. Social function (20−26 elements) 4. Swallowing symptomatology (27−31 elements) *Format: Likert-type response of 4 points with descriptors (1 = not at all, 2 = a little, 3 = quite a bit, 4 = very much) | 1st Phase: Qualitative Patients with OM (n=23) for the generation of 171 elements Age: 42±13 years (range 21−58) Apparent validity: Patients (n=10) and 6 families and patients (n = 9) and 4 families for group discussion Age: 38±15 years (range 18–55) patients surveyed and 38±15 years (range 22–54) family caregivers 2nd Phase: Quantitative n= 210 Age: 51±12 years (21−84) Disease spectrum: Hematologic neoplasms and solid tumors treated with stomatotoxic CT, H/N CT, and H/N RT Period of evaluation: One application during anticancer therapy; 47 subjects randomly selected to complete the OMQoL again after 3 days and to evaluate the reliability over time Spectrum of OM: According to the WHO scale | Qualitative phase generated 171 items, removing duplicate items including 63 elements included in the first version of the OMQoL Apparent validity: Evaluated through interviews and focus group discussions with patients and relatives (elements were reduced to 44) Content validity: Agreement between evaluators and content validity index resulted in 41 items Exploratory factor analysis with Promax rotation resulted in four subscale dimensions: symptomatology, diet, social function and swallowing, represented by 31 elements Internal consistency: Cronbach's α=0.906-0.934 Test-retest reliability: kappa weighted=0.61-0.895 ICC of the total subscales=0.864−0.934. Convergent validity: OMQoL and peak symptoms related with OM r=-0.821 to -0.971 (p<0.01)* and ABC scores** r=-0.724 to -0.943 (p<0.01)* Concurrent validity: OMQoL and EORTC QLQ-C30 (Ch) peak subscales, r=0.500−0.726 (p<0.01)* and scores of ABC, r=0.450 -0.731 (p<0.01)* Validity of known groups: Comparison of the highest scores of the subscales of OMQoL and ABC scores between patients with different levels of OM (OMS grade 0, I, II, III or IV) r=0.733–0.877 (p<0.01)*** *Pearson correlation coefficient **Mean ABC score using the trapezoidal area calculated throughout the study period,divided by the number of assessments. ***ANOVA | OMQoL was developed as a questionnaire from the patient's perspective to measure some important aspects of HRQoL that can address the health status of patients with OM, having adequate psychometric properties and sensitive to changes over time Subscales of the OMQoL have a high grade of internal consistency, confirming proper test−retest reliability and completing all requirements of weighted kappa coefficient and ICC EORTC QLQ-C30 (Ch) measures related constructs, but different from OMQoL |
| Epstein et al. (2007)30 USA 80% | OMWQ-HN | 9 elements/ 3 dimensions 1. Health and overall QoL 2. Impact of mouth and throat pain on patient function 3. Mouth and throat pain *Likert-type response format of 5,7 or 11 points | 1st Phase: Qualitative n=30 patients with HNC for the generation of elements 2nd Phase: Quantitative n=75 Age: 58.8±10.2 years (range: 40–86) Disease spectrum: HNC scheduled to receive RT with or w/o CT Period of evaluation: Before treatment (week 0) and in weeks 2, 4 and 6. During week 4, questionnaires were administered twice with a difference of 24-48h for evaluation of test-retest reliability Spectrum of OM: Evaluated using symptoms associated with mucositis: oropharyngeal pain and swallowing *HNC: Head and Neck Cancer | The qualitative phase was conducted using one-on-one interviews to refine, reshape or delete irrelevant questions based on FACT−HNC and HN−PSS resulting in 12 elements Internal consistency: Cronbach's α=0.86–0.94. Test-retest reliability=ICC: 0.89 Convergent validity: Correlations between the OMWQ−HN and FACT−HNCS: η=0.57* Correlations between OMWQ−HN and FHN−SI (FACT−HN): ρ= -0.63* OMWQ−HN and FACT PWB (FACT−G): η= -0.66* OMWQ−HN and FACT PWB (FACT−G): η= -0.48* OMWQ−HN and FACT− EWB: η= -0.52* *Spearman correlation coefficient | Three elements were excluded due to low correlations in all evaluations 1) How would you qualify your general health during the past week? 2) How would you qualify your quality of life general during the past week? 3) How did mouth and throat pain limit the following activity during the past week? -Brushing your teeth |
| Kushner et al. (2008)11 Canada 84% | PROMS | 10 elements 1. Mouth pain 2. Difficulty speaking due to mouth lesions 3. Restrictions in speaking due to mouth lesions 4. Difficulty eating hard foods (hard bread, French fries, etcetera) due to mouth lesions 5. Difficulty eating soft foods (gelatin, pudding, etcetera) due to mouth lesions 6. Restrictions in eating due to mouth lesions 7. Difficulty with drinking due to mouth lesions 8. Restriction of liquids due to mouth lesions 9. Difficulty swallowing due to mouth lesions 10. Changes in taste *Severity of symptoms experienced during the previous week, quantified through a 100-mm VAS | n=34 Age: 44.2±10.7 years (range 23–61) Disease spectrum: Hematologic neoplasms, candidates for allogeneic bone marrow transplant (82% CT and TBR and 18% only CT) Evaluation period: Baseline, days 7, 14, 21, high and 60 days after transplant Spectrum of OM: Using OMAS-VAS | Internal consistency: Cronbach's α=0.86–0.98. a) Convergent validity: Correlation PROMS−FACT−G (PWB) at study initiation and on day 60=–0.41* and–0.53*, respectively PROMS–FACT−G (S/FW), high=0.42* PROMS and FACT−BMT at initiation=-0.43* PROMS−SLE at initiation=0.35* PROMS−VAS−OMAS for erythema day 21=0.49* and ulceration day 14=0.47* b) Discriminant validity: PROMS−ABS day 21=0.43* PROMS−CES−D on day 7=0.51** and 14=0.39* *Spearman correlation coefficient and level of significance 0.05 (two-tailed) ** Level of significance 0.01 (two-tailed) | Although there were significant differences in the QoL from baseline to 60 days after transplantation, this study did not assess the QoL during the most radical events (during ulceration or initial diagnosis) CES-D demonstrates minimal depressive symptoms at study initiation |
OM, oral mucositis; CT, chemotherapy; RT, radiation therapy, H/N, head and neck; OMQoL, Oropharyngeal Mucositis-specific Quality-of-Life; OMWQ−HN, Weekly Questionnaire−Head and Neck; HRQoL, Health-related Quality-of-Life; EORTC QLQ-C30 (Ch), European Organization for Research and Therapy of Cancer Quality of Life Questionnaire−30 (Chinese version); ABC, area below the curve; HNC, head and neck cancer; TBR, total body radiation; ICC, intraclass correlation coefficient; VAS, visual analogue scale; FACT−G, Functional Assessment of Cancer−General (FACT−HNC, Head and Neck Cancer; FACT−HNPSS, Head and Neck Performance Status Scale; FACT−FHN-SI, Head and Neck−Symptom Index; FACT−BMT, Bone Marrow Transplant; FACT−PWB, Physical Well-being; FACT−SW/F, Social and Family Well-being, FACT−EWB, Emotional Well-being); PROMS, Patient-Reported Oral Mucositis Symptoms (PROMS−ABS, Affect Balance Scale; PROMS−SLE, Stressful Life Events; PROMS−VAS−OMAS, Visual Analogue Scale−Oral Mucositis Assessment Scale; PROMS−CES−D, Center for Epidemiologic Studies−Depression).
Regarding the instruments analyzed in this review, the PROMS scale was designed to evaluate symptoms reported by patients with OM that may have an adverse impact on the QoL. However, the authors recognized that this study did not evaluate the QoL at the most significant time (during ulceration or the initial diagnosis) because it was not designed for this purpose, resulting in an insufficient evaluation of the construct of the health-related (HR) QoL in OM.
Similarly, the Oral Mucositis Weekly Questionnaire−Head and Neck Cancer (OMWQ−HN) only measures OM symptoms, specifically mouth and throat pain related to oral disorders, such as drinking, swallowing, speaking, and sleep disturbances, excluding a whole range of characteristics secondary to OM, such as inflammation, bleeding, expression, and depression, among other activities of the daily life of the patient. In its further development, elements were eliminated for presenting low correlations with OM such as overall health and the QoL in general, aside from being directed exclusively to patients with head and neck cancer.
So far, it was found that the Oropharyngeal Mucositis–specific Quality-of-Life (OMQoL) instrument covers most of the dimensions (symptomatology, nutrition, social function and symptoms of swallowing) for evaluating the QoL in patients with OM secondary to cancer treatment.
The creation of instruments to assess the QoL in patients with OM secondary to other cancer treatments is a giant step for health professionals in the area of Oncology and Stomatology; however, available knowledge regarding this relationship is still limited. During the development of this review, we discovered that a large number of studies that evaluate the QoL construct related with OM use general tools of oral health together with instruments for overall assessment of cancer treatment. Such is the case of the Oral Health Impact Profile-14 (OHIP-14) that evaluates oral health-related QoL,8 measuring the perception of the social impact that oral disorders have on patients’ well-being.31 It uses seven dimensions: functional limitation, physical pain, psychological disorder, physical disability, psychological disability, social disability and deficiency. OHIP-14 has frequently been used for patients with Behçet disease, recurrent aphthous stomatitis, lichen planus, candidiasis, burning mouth syndrome, and temporomandibular disorders, among others. However, Barkokebas et al.8 evaluated 60 patients who developed OM during cancer treatment using the OHIP-14 for assessing the impact of OM on the QoL as it relates to oral health where pain, physical limitations, and psychological disorders were the predominant dimensions. Bezilleni et al.32 used the OHIP-14 to determine the QoL related to oral health together with the PROMS scale for evaluating OM symptoms in patients subjected to HSCT and low-level laser treatment, demonstrating a correlation and high scores in both scales. In a similar fashion, Silva et al.27 evaluated the impact of low-level laser treatment on OM and the QoL through the OHIP-14 and the FACT−BMT (Functional Assessment of Cancer Therapy–Bone Marrow Transplant) in 31 patients with HSCT, where both instruments showed deterioration of the QoL. Despite this, the OHIP-14 instrument does not include dimensions that describe signs and symptoms that patients with OM commonly report, such as bleeding and oral burning, the perception of saliva, phonation, swallowing, dysgeusia and throat pain.
Another instrument frequently reported in studies that evaluates the QoL in patients with OM is the Functional Assessment of Cancer Therapy−General (FACT−G), developed by Cella et al. This is a multidimensional instrument designed to evaluate the QoL and severity of pain in patients with chronic diseases such as cancer. It comprises five subscales that evaluate physical well-being, social/family well-being, emotional well-being, functional well-being, and physician/patient relationship.33,34 Sakellari et al. evaluated the severity of mouth pain, oral limitations, and the QoL through the application of an oral and throat pain scale and the FACT−G to patients subjected to autologous HSCT who received palifermin post-transplant and developed OM.20 The most significant limitations were swallowing, drinking, eating, and speaking (reported on the scale of limitations). The QoL related to health was worse on day +7 compared to day +1 according to FACT−G. Low incidences and severity of pain associated with OM were also noted, suggesting a favorable influence of palifermin. Similarly, Elting et al.21 measured the severity of OM through the application of the Oral Mucositis Daily Questionnaire (OMDQ), a mouth and throat pain scale evaluating the QoL through the FACT−G and the Functional Assessment of Chronic Illness Therapy (FACIT), before, during, and 4 weeks after RT. A decrease in QoL during RT associated with mouth and throat pain and with a reduction in the median scores of the FACT−G was reported. Although both studies provided relevant results—a decline in functional status mainly according to the subscale of limitation associated with OM and an increase of pain symptomatology even with analgesics—the use of general scales to measure the QoL makes it even side aspects related to damage of the oropharyngeal mucosa, such as presence of erythema, ulcers, bleeding, social isolation, sadness or preoccupation secondary to OM, among others.
In the same way, the EORTC-QLQ-C30 has been applied. This basic questionnaire incorporates a range of health, physical, emotional, and social problems. For a broad spectrum of patients with cancer, this strategy attempts to evaluate the QoL mainly in clinical trials.35 The v.3.0 version of this instrument and its specific module for head and neck cancer (EORTC QLQ−H&N35), developed by Bjordal et al.,36 has an additional oral evaluation that provides a measure of the QoL in patients with head and neck cancer. It demonstrates the oral impact of RT on the functional domains and symptom scales despite having elements related to oral dysfunction, swallowing and speaking. This evaluation is focused on defects associated with RT and surgical treatment of head and neck cancer like facial disfigurement, and permanent deterioration of the vasculature, connective tissue, salivary glands, muscles, and bones. However, it does not fully address the various problems arising from acute erythema and ulcerative disorders to swallowing or social function of the mucosa with OM during CT and RT. Moreover, in a clinical trial of 138 patients, Duncan et al. assessed the utilization of an antimicrobial vs. placebo before, during and after head and neck RT.16 Using the EORTC−QLQ−C30 and an oral-specific checklist for trials to measure the QoL, OM, and xerostomia, these authors demonstrated that the antimicrobial did not have an impact on the QoL, and the OM evaluation was responsible for the OMAS scale. Oral pain occurred in >90% of the patients, the functional role being the most affected; there was an increase in fatigue, appetite, and persistence of dry mouth; however, this study focused more on the evaluation of the QoL in general and symptoms in patients with head and neck cancer.
Physical, functional, nutritional, and psychological impact, including the aesthetics perceived by the patient with OM, should be a major cornerstone in the entire treatment spectrum because it may be the key to a torpid evolution from the medical point of view due to the risk of local infection that could evolve to systemic infection, involvement of the oral intake impacting the nutritional status, the notorious oral ulcers that are not only painful but also distressing when these increase in size and number along with the perception of patients who question whether their whole body is also involved and if this indicates that their treatment is not going well.
The questionnaire selected for measuring the impact of OM on the construct of the QoL should be methodically and psychometrically robust. Stewart et al.31 described the conceptual, statistical and pragmatic requirements that the health questionnaires should have, including the following characteristics:
- 1.
Questionnaires should represent multiple health concepts and a range of health states related to general functioning and well-being.
- 2.
Questionnaires should have excellent psychometric properties (reliability, validity, and precision).
- 3.
According to the configuration of the clinic, it should be simple and easy to use.
QoL measurements may be a difficult scenario for the investigation of OM due to its heterogeneous symptomatology that varies with the type of diagnosis, type of cancer, and treatment received to attenuate it as well as by the patient.1 The results of these measurements may be useful for decision making in comprehensive patient care, thereby facilitating activities of daily living.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare no conflicts of interest of any nature.