Background: Histamine is widely used as a pharmacological tool for the evaluation of airway responsiveness. Nevertheless, undesirable and contradictory effects have been described after histamine provocation tests. In previous evaluations of airway responsiveness in a guinea pig asthma model, the control groups consistently showed high neutrophil counts in bronchoalveolar lavage fluid (BALF) immediately after the histamine challenge. The changes in cytokine and chemokine levels in guinea pig lung associated with histamine induced-neutrophilia are described in this paper.
Methods: Immediately and 24 h after histamine challenge, airway wall and BALF eosinophil and neutrophil counts as well as lung cytokines (IL-5, IL-10, IL-17A, TNF-α and TGF-β) and chemokines (CCL11 and CXCL8) levels were evaluated.
Results: Histamine inhalation generated an all-or-none bronchial response, and the dose inducing airway obstruction was similar in all guinea pigs. Immediate increases in neutrophil counts in airway wall and BALF and in IL-5, IL-10 and IL-17A levels in the lung homogenate were observed after histamine challenge. Significant correlations were found between neutrophil counts from airway wall and IL-5, IL-10 and IL-17A levels in the lung homogenate.
Conclusions: Histamine inhalation induced rapid neutrophil LBA and airway wall infiltration that was not associated with CXCL8 expression but with a Th2 and Th17 cytokines that probably are involved in the recruitment and activation of neutrophils.
1. Introduction
Histamine is a valuable tool to determine nonspecific bronchial responsiveness.1,2 However, the few studies that have evaluated the effects of inhaled histamine in the lungs have shown side effects such as an increase of lymphocytes, mast cells and neutrophils in bronchoalveolar lavage fluid (BALF) after histamine challenge3 although opposite effects such as a reduction of neutrophil chemotaxis have also been described.4 Our research group was interested in the evaluation of airway responsiveness to histamine after antigen challenges in guinea pig and consistently in control groups (nonsensitized) high BALF neutrophil counts occur immediately after histamine challenges.5-7 This phenomenon is not observed 24 h after the histamine provocation (unpublished data). As far as we know, histamine-induced transient increment in BALF neutrophil counts has not been described in guinea pigs. We showed the effect on cytokine and chemokine levels induced by histamine challenge and its association with transient neutrophilia in guinea pig.
2. Methods
Male, outbred guinea pigs (350-400 g; strain HsdPoc:DH), handled according to protocols approved by the Scientific and Bioethics Committee at the Instituto Nacional de Enfermedades Respiratorias, were used.
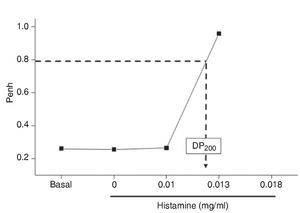
After an initial obstructive index (Penh) baseline acquisition, guinea pigs were exposed to increasing noncumulative doses of histamine (0 to 0.32 mg/ml; Sigma Chemical Co., St. Louis, MO) aerosols until a broncho-obstructive response triplicating the basal Penh value was observed. Each histamine dose was delivered over 1 min, and the average Penh value over the following 5 min was obtained. The interval between doses was 10 min. The airway obstructive response to histamine aerosol delivery was recorded using a whole-body, single-chamber barometric plethysmograph for freely moving animals (Buxco Electronics Inc., Troy, NY) as previously described.6 Afterward, two independent groups of animals were studied, i.e., immediately and 24 h after the inhalation of histamine. At these time points, the animals received an i.p. injection of sodium pentobarbital (65 mg/kg), and the trachea was cannulated to obtain BALF as previously described.6 Cell counts were expressed as the number of cells per ml of BALF. All smears were coded, and the cells were counted in a blinded fashion. Control animals were handled with sham maneuvers with saline solution.
Left caudal lung lobe from each guinea pig group was dissected and fixed intra-arterially to obtain lung fragments to be embedded in paraffin. Lung sections were stained with hematoxylin-eosin and eosinophils and neutrophils were counted as previously described.6
Anti-human interleukin 5 (IL-5; clone TRFK58; BD Pharmingen, Franklin Lakes, NJ), tumor necrosis factor (TNF)-α (epitope corresponding to amino acids 77-233 representing mature TNF-α, rabbit polyclonal IgG9; Santa Cruz Biotechnology, Santa Cruz, CA), IL-10 (clone 127107; [PDB:P22301], R&D Systems, Minneapolis, MN), IL-17A (clone 41809, [PDB:Q16552], R&D Systems), transforming growth factor (TGF)-β1 ([PDB:P01137], R&D Systems), CCL11 (clone 43911, [P51671], R&D Systems) or CXCL8 (clone 6217,10 R&D Systems) antibodies were used in lung homogenates to measure cytokines and chemokines by ELISA as previously described.7 We corroborated that IL-10, IL-17A and TGF-β1 are expressed at gene level in guinea pig by comparing asthma model guinea pig lungs with control ones with real-time RT-PCR. All these cytokines increased their expression in the asthma model guinea pig in comparison with control groups (data not shown).
One-way ANOVA followed by Tukey´s test was applied for multiple comparisons. Statistical significance was set at two-tailed p <0.05. Association between eosinophil and neutrophil counts in airway wall was assessed through Pearson correlation coefficient. Data in the text and figures represent the mean ± SE.
3. Results
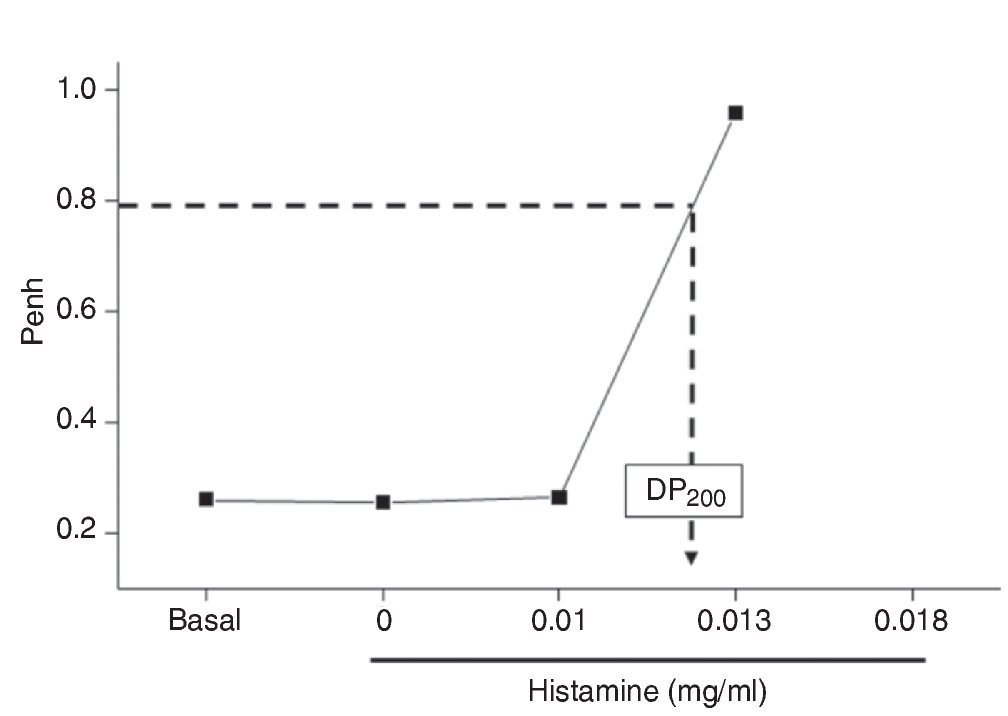
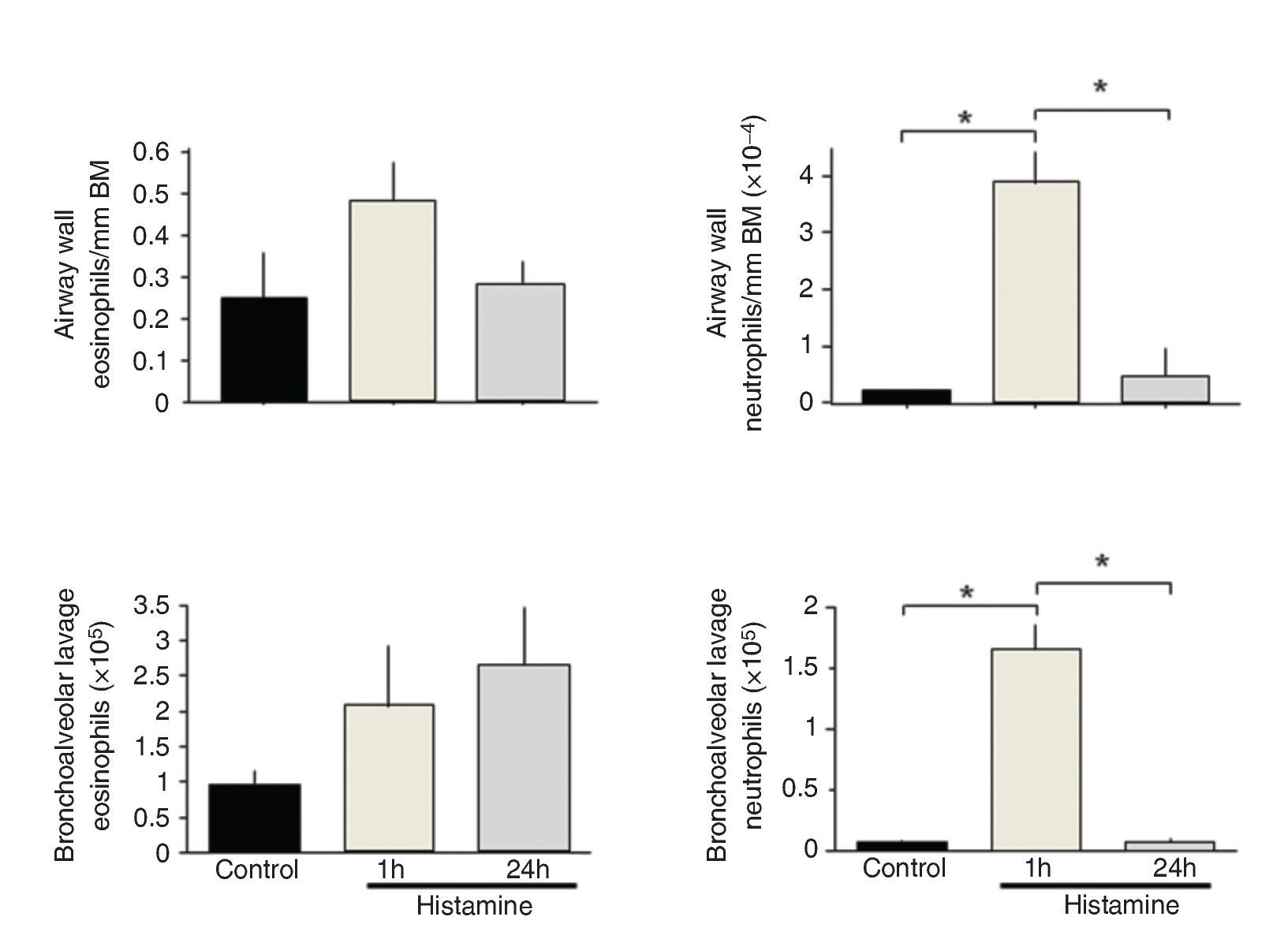
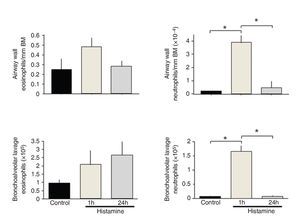
Histamine dose-response curves did not induce progressive airway obstruction, but an all-or-none response at 0.012 ± 0.002 mg/ml histamine (n = 10) was observed (Fig. 1). The numbers of eosinophils in BALF and airway wall in the control group was not significantly different from those of the immediate and 24-h groups. Neutrophil counts in BALF and airway wall from the immediate group were significantly higher than those of the control and 24-h groups (p <0.001, respectively; n = 5 each group; Fig. 2).
Figure 1 Dose-response curve to aerosolized histamine. After initial baseline acquisition, guinea pigs received noncumulative doses to histamine. The dashed arrow shows the provocative dose 200% (PD200), i.e., the interpolated histamine dose that caused a three-fold increase of basal Penh.
Figure 2 Total and differential cell counts in bronchoalveolar lavage fluid from histamine-challenged guinea pigs. The cell numbers recovered from bronchoalveolar lavage fluids are shown for each group. The bars represent the mean ± SE of n = 5 guinea pigs in each group. *p <0.001 (one-way ANOVA with Tukey's multiple comparisons test).
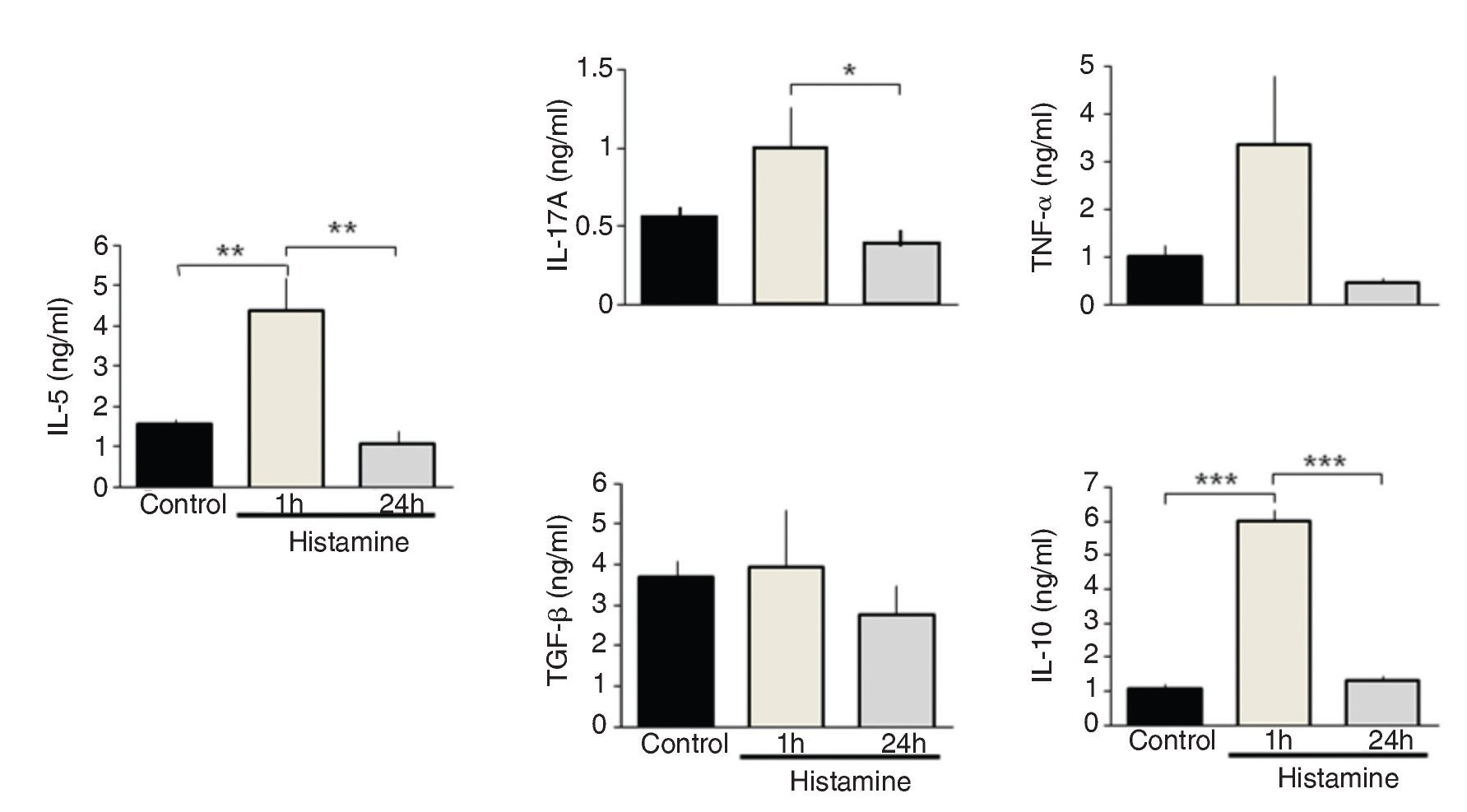
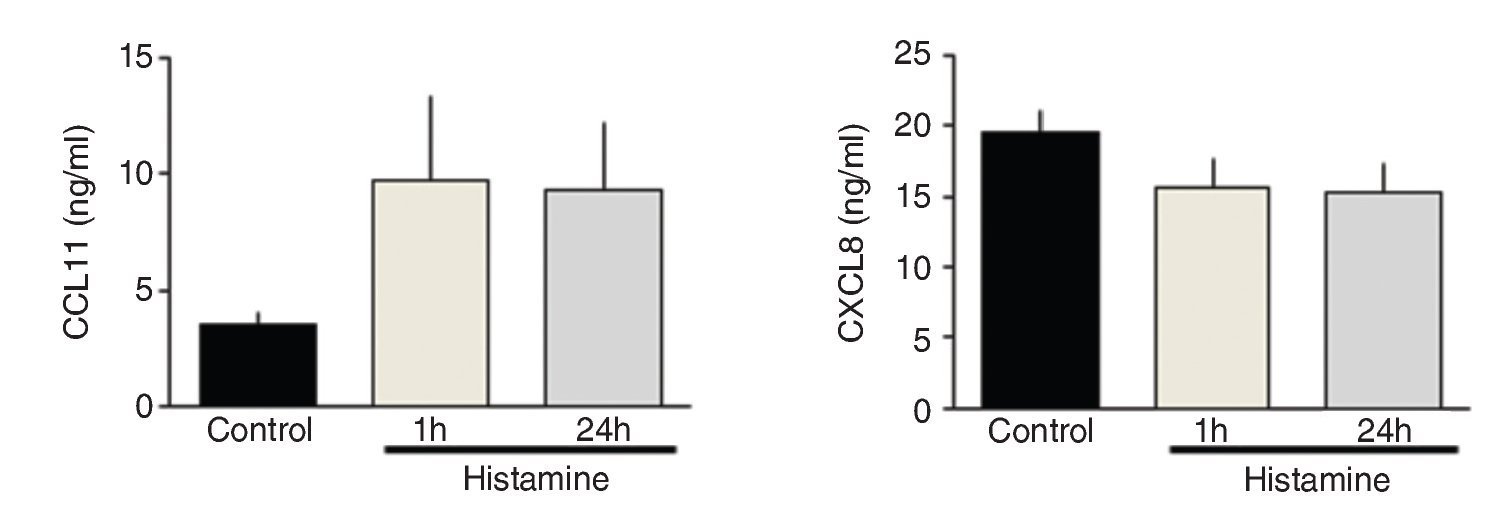
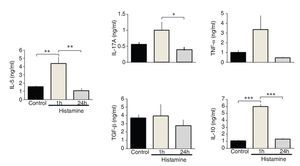
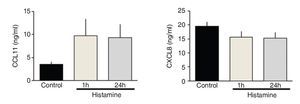
IL-5 and IL-10 levels increased significantly in the immediate group in comparison with control and 24-h groups (p <0.01 and p <0.001, respectively; n = 5 each group; Fig. 3). IL-17A levels in the immediate group were significantly different from the 24-h group but not from the control group (p <0.05; n = 5; Fig. 3). The levels of TNF-α and TGF-β1 and chemokines CCL11 and CXCL8 were not modified by histamine administration (Figs. 3 and 4).
Figure 3 Cytokine expression in lung homogenates from histamine challenged guinea pigs. The bars represent the mean ± SE of n = 5 guinea pigs in each group. *p <0.05, **p <0.01, ***p < 0.001 (one-way ANOVA with Tukey's multiple comparisons test).
Figure 4 Chemokine levels in lung homogenates from histamine challenged guinea pigs. The bars represent the mean ± SE of n = 5 guinea pigs in each group.
Pearson correlation coefficients of airway wall eosinophil counts did not reach statistical significance with cytokine or chemokine levels in lung. Airway wall neutrophil counts were associated with IL-5 (r = 0.67; p <0.01), IL-10 (r = 0.77; p<0.00001) and IL-17A (r = 0.52; p <0.05) lung levels (n = 15 in all groups).
4. Discussion
Histamine, an amine involved in a wide range of cell functions, is a valuable tool for the evaluation of airway responsiveness.1,2,6 In the current study, we found that histamine inhalation at the doses used to evaluate airway responsiveness in guinea pig increases neutrophil and lung cytokine levels.
Neutrophils are scarce cells in airway wall and BALF. CXCL8, macrophage inflammatory protein 2 and keratinocyte chemoattractant levels have been associated with neutrophil recruitment.11 Surprisingly, in our study CXCL8 levels were not associated with neutrophil recruitment. In this context, a direct association was observed between neutrophil counts and the levels of Th2 cytokines such as IL-5, IL-10 and the Th17 cytokine IL-17A. High IL-5 levels have also been observed in plasma from guinea pigs after histamine inhalation.12 Likewise, IL-5, an important cytokine in eosinophil activation, has also been involved in neutrophil recruitment in vivo.13 IL-10 is released by various cells including neutrophils, and it has potent anti-inflammatory properties.14 Other cytokines such as TNF-α and TGF-β were not modified by histamine inhalation in guinea pigs; however, the IL-17A levels increased immediately after histamine inhalation compared to the 24 h levels. IL-17A is mainly produced by Th17 cells and neutrophils and has strong pro-inflammatory effects as well as recruitment of neutrophils into the airways through CXCL8.15 Thus, it is likely that the increase of IL-5, IL-10 and IL-17A levels could be the result of the recruitment and activation of neutrophils into the airways.
Histamine can induce eosinophilic migration by promoting CCL11 expression during an allergic human skin reaction.16 However, we did not observe eosinophil recruitment in BALF or airway wall. Thus, the effects of histamine in chemokine production are variable and may depend on the histamine dose.
In conclusion, histamine inhalation induced rapid neutrophil lung infiltration not associated with CXCL8 expression. Th2 and Th17 cytokines are likely involved in the recruitment and activation of neutrophils.
Acknowledgments
Patricia Ramos-Ramírez was the recipient of a Ph.D. scholarship from CONACyT (#256077) and is a doctoral student from the Posgrado en Ciencias Biomédicas, Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México (UNAM).
Conflict of interest
The authors declare no conflict of interest of any nature.
Received 13 January 2014;
accepted 21 February 2014
* Corresponding author.
E-mail: perkins@unam.mx (B. Bazán-Perkins).