Introducción. Las infecciones respiratorias superiores son la principal causa de morbilidad en niños menores de cinco años. Los objetivos de este estudio fueron los siguientes: (i) desarrollar indicadores para medir la calidad de atención a niños con infecciones respiratorias superiores (IRS) en atención primaria; (ii) evaluar la calidad de la atención para IRS que los menores de cinco años reciben en clínicas de medicina familiar.
Métodos. Se desarrollaron indicadores siguiendo el método RAND-UCLA. Estudio transversal de la calidad de atención en cuatro clínicas de medicina familiar en la Ciudad de México. Participaron 10,677 niños menores de cinco años con IRS. La fuente de la información fueron los registros médicos electrónicos de 2009. La calidad de la atención se determinó a través de 6 indicadores.
Resultados. La evaluación de la calidad de la atención identificó que el 15% de los niños tenía registros de búsqueda intencionada de signos de dificultad respiratoria; 27% recibió información sobre las señales de alarma. Más del 61% de los niños diagnosticados con IRS no estreptocócica recibió prescripción de antibióticos durante la primera consulta. En los niños con diagnóstico de faringitis estreptocócica o amigdalitis, el 57,5% recibió el antibiótico apropiado. En promedio, el 47.2% recibió la atención recomendada.
Conclusiones. Es recomendable promover el uso de los datos del expediente clínico electrónico para evaluar sistemáticamente la calidad de la atención en IRS. Es necesario considerar los problemas de calidad para diseñar las estrategias encaminadas a fortalecer la capacidad técnica del personal de salud para ejercer la práctica clínica basada en la evidencia para la atención de IRS.
Background: Upper respiratory infections are the principal cause of morbidity in children <5 years of age. The objectives of this study were (i) to develop quality-of-care indicators for evaluation of care for children with upper respiratory infections (URI) at the primary care level using data from the electronic health records and (ii) to evaluate the quality of URI care offered to children <5 years of age at family medicine clinics (FMCs).
Methods: Development of indicators following the RAND-UCLA method was used. A cross-sectional analysis of quality of care provided for children with URI in four FMCs in Mexico City where 10,677 children <5 years of age with URI participated. The source of information was data from 2009 electronic health records. Quality of care was evaluated using six indicators developed in the first stage of this study.
Results: The quality of care evaluation identified that only 15% of children had registries of intentional search of respiratory distress signs and 27% received information on warning signs. More than 61% of children diagnosed with uncomplicated and nonstreptococcal URI received antibiotic prescription during the first visit. In the case of children diagnosed with streptococcal pharyngitis or tonsillitis, 57.5% received the appropriate antibiotic. On average, the percentage of recommended care received was 47.2%.
Conclusions: It is reasonable to promote the use of electronic health records to routinely evaluate the quality of URI care. It is necessary to consider quality flaws that were found in order to endorse strategies aimed at strengthening the technical capacity of health personnel to exercise evidence-based clinical practice.
1. Introduction
Upper respiratory infections (URI) are the leading cause of morbidity among children <5 years of age. Most URI are of viral origin and resolve spontaneously; however, these conditions require providing high quality care to avoid potential complications and increase the chance of improving health outcomes1. Key points for treatment include educating the primary caretaker to provide proper homecare and seek healthcare when needed2-4. Healthcare personnel should also have clinical skills for correct diagnosis and appropriate treatment2-7.
International and national clinical guidelines for the diagnosis and treatment of URI are available to support the clinical decisions; nonetheless, the gap between actual care and the best scientifically proven care still exists. In developing countries this gap is wide due to scarcity of resources, inadequately trained health personnel and lack of standards and indicators8. Treatment for URI is far from appropriate; up to 70% of antibiotic prescriptions for URI are still unjustified5-7. The existing abuse in the prescribing of antibiotics contributes to the development of antimicrobial resistance, favors elimination of normal intestinal flora, causes adverse drug reactions and increases the costs of healthcare9,10. Evidence documenting the negative consequences of unjustified antibiotic prescription is increasing; however, the total consumption of antibiotics in Europe and Latin America is on the rise11,12.
Information about the quality of care for children with URI is not scarce; however, few studies have taken advantage of the clinical information stored in the electronic health record (EHR). The availability of the EHR has increased the possibility to better use the clinical information for a wide variety of purposes in addition to routine clinical care such as management of health services and quality of care evaluation13,14.
The Mexican Social Security Institute (IMSS) covers ~47 million persons and reports that URIs are among the top reasons for visits to FMCs15. Furthermore, IMSS has evidence-based clinical guidelines and uses the EHR for routine care. All clinical records are electronically stored. Nonetheless, evaluation of the quality of care for URI is not routinely performed. These circumstances prompted carrying out a study with two objectives: (i) to develop quality-of-care indicators (QCIs) for evaluation of care for children with URI at the primary care level using data from the electronic health records and (ii) to evaluate the quality of URI care that children <5 years of age receive at FMCs.
2. Methods
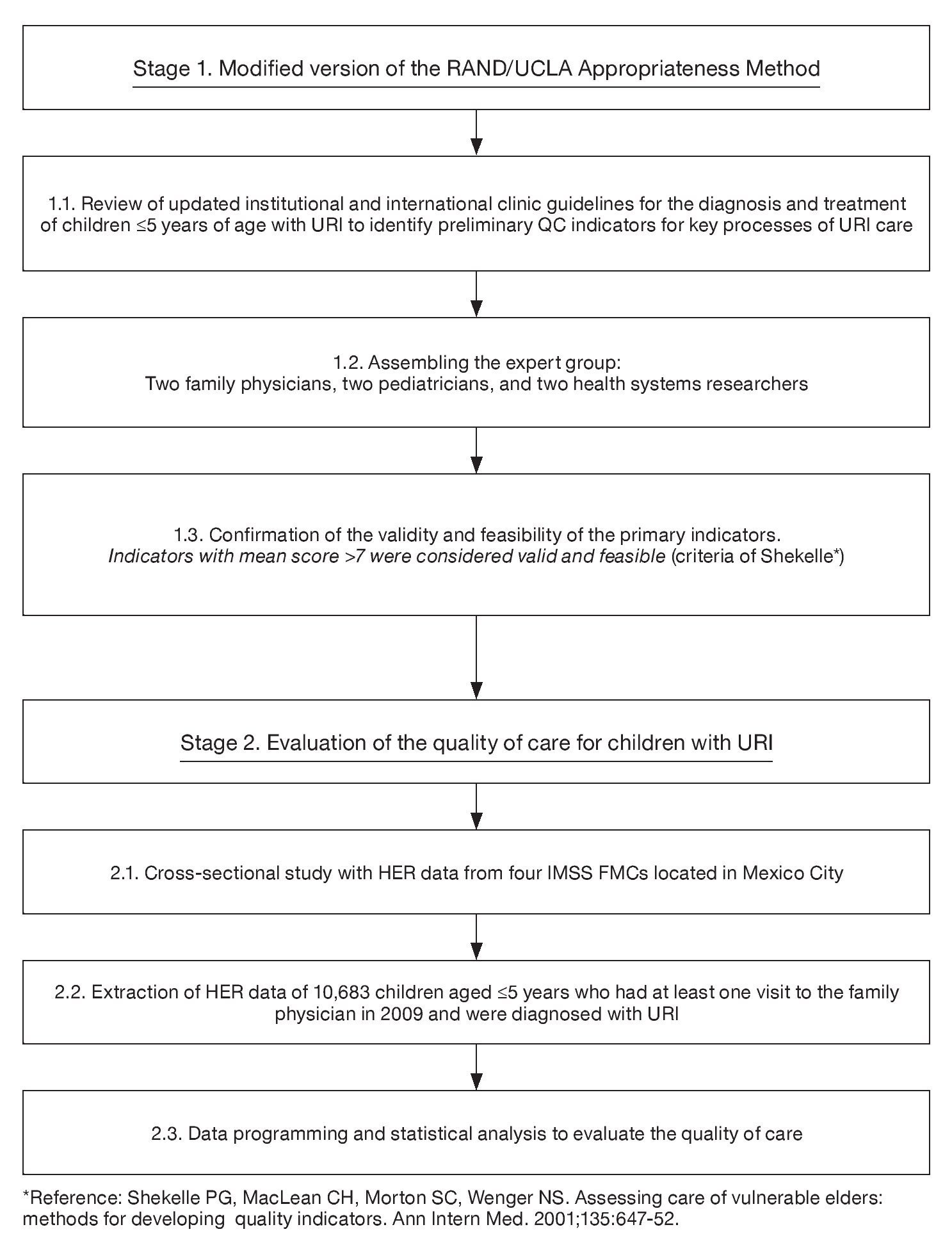
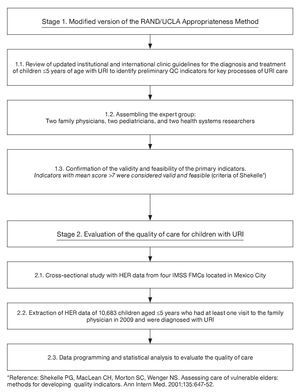
This study is part of a line of research aimed at developing a system to evaluate the healthcare quality of the five most frequent causes of visits at FMCs of the IMSS using electronic health record information. The methods to develop and validate the indicators and evaluate the quality of care for diabetes, hypertension, osteoarthritis and antenatal care were published elsewhere16-19. This study followed a similar two-stage methodology16-19 (Figure 1). First, we validated six QCIs for URI using the modified version of the RAND/UCLA Appropriateness Method20. We then used the QCIs to evaluate the quality of URI care that children <5 years received in FMCs. The study was conducted in four FMCs that covered an estimated population of ~585,535 persons. The clinics provide primary care services. The information for this study came from the practice of 206 family physicians. All four clinics had similar infrastructure.
Figure 1. Two-stage methodology to evaluate quality of healthcare using information from the electronic health record.
The source of information was the EHR. The analysis included all children <5 years of age who had at least one visit to the family physician in 2009 and were diagnosed with URI. All diagnoses should have been registered in the EHR according to the codes of the 10th revision of the International Classification of Diseases (ICD): J00 acute nasopharyngitis, J02 acute pharyngitis, J03 acute tonsillitis, J04 acute laryngitis and tracheitis, and J06 acute upper respiratory infections of multiple and unspecified sites. Children with sinusitis, acute obstructive laryngitis, epiglottitis and otitis media were not included.
The information stored in the EHR served to create a new analytic database that was built using a structured query language (SQL) and the SAS statistical package (v. 9.2). We used context sensitivity search for specific terms to identify the information from the unstructured data. The specific terms were identified through reviewing 50 individual EHR of children with URI. We then validated the extracted information by comparing a sample of 1000 records in the EHR system with the final dataset. Comparisons were made reviewing data from individual EHR with the extracted data. Finally, descriptive statistics of the study variables was performed. The unit of analysis was a child with a diagnosis of URI. For children with more than one episode of URI, the records of the first episode were analyzed.
The study variables included:
A) General characteristics of the children: sex and age
B) Medical history including health problems during 2009 such as number of URI episodes, type of the first URI episode, comorbidity and recording of body temperature at the first URI visit
C) Quality of care that was evaluated using the indicators constructed in the first stage of this study
All quality indicators were process measures. Additionally, to obtain a comprehensive evaluation of quality of care, we estimated the percentage of care that children received in relation to the recommended care21. The recommended healthcare was estimated as a proportion in which the numerator was the sum of all the indicators that a child received and the denominator was the total number of indicators that a child was eligible for.
The study protocol was approved by the IMSS National Research and Ethics Committees (CNIC: 2008-785-008). The study used only existing EHR information. All personal data of the children were de-identified; therefore, informed consent was not requested16-19.
3. Results
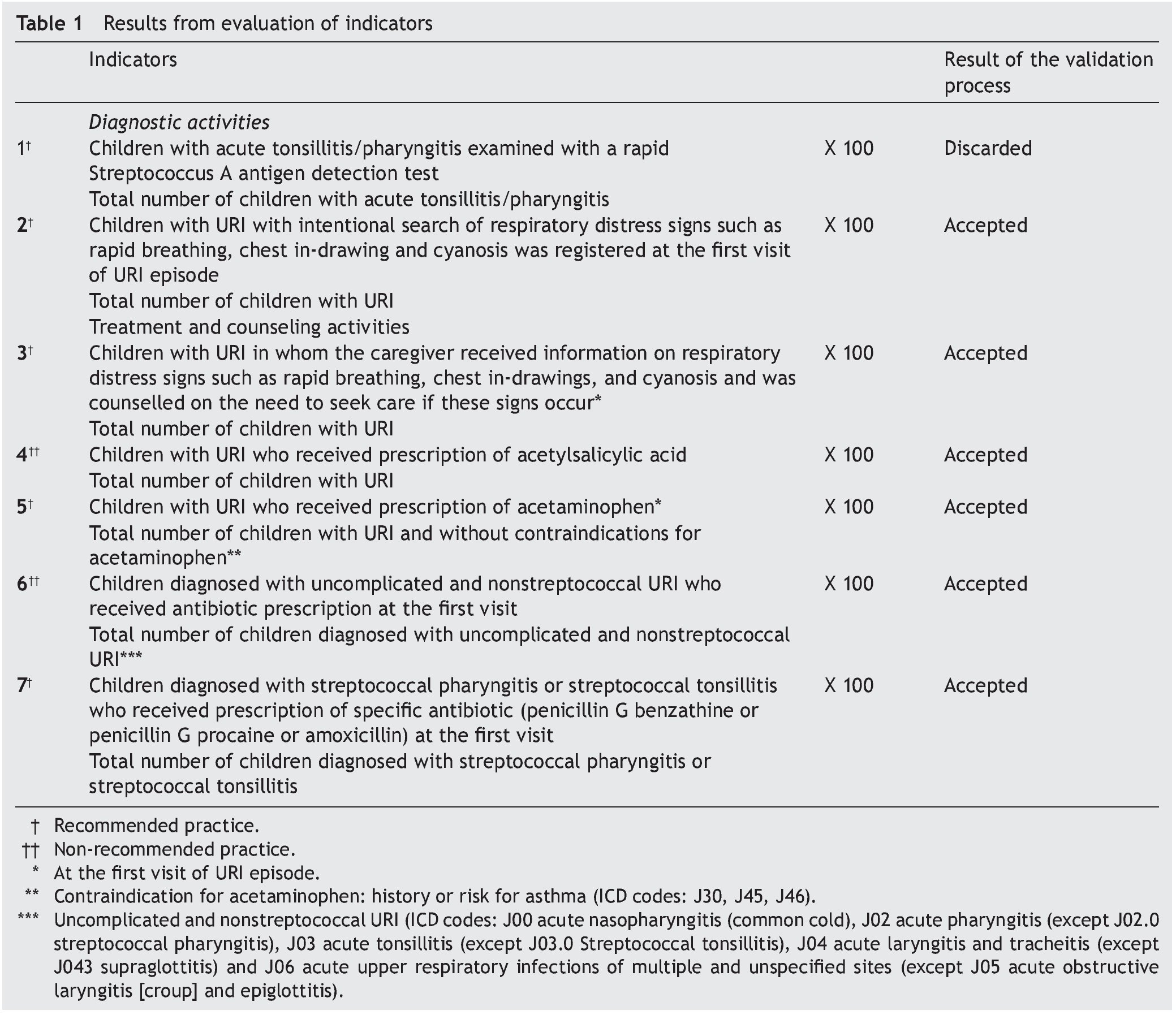
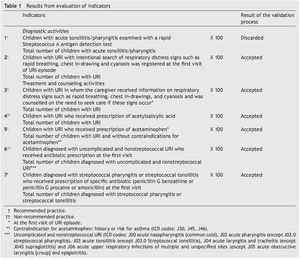
Stage 1: The panel of experts agreed that six of seven QCIs for URI met the criteria for validity and feasibility. Only one indicator related to the diagnostic process based on rapid Streptococcus A antigen detection test was considered not to be feasible because of its unavailability in FMCs. The final set of indicators comprised four indicators that reflected recommended practices and two that were not recommended practices (Table 1).
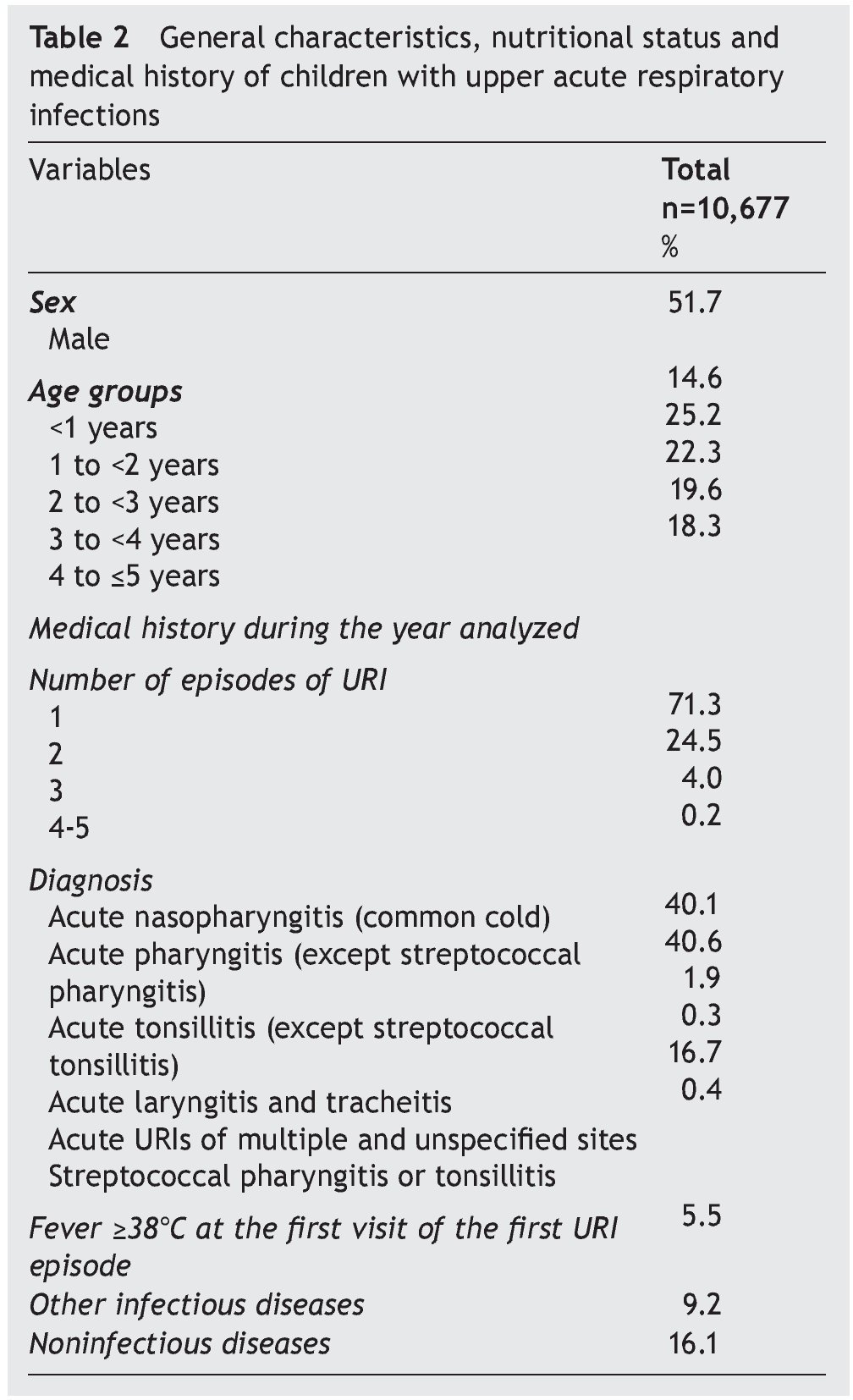
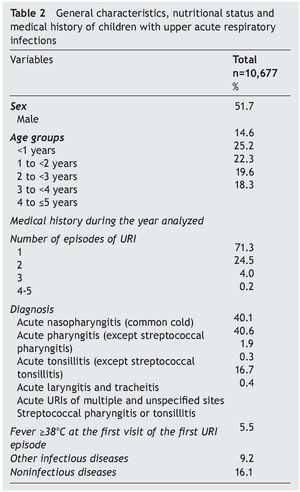
Stage 2: In 2009 there were 40,678 children ≤5 years of age registered in the FMCs; 16,257 children had at least one visit to the family physician and 10,683 were diagnosed with URI. Six children with diagnosis of sinusitis were excluded from the analysis. The final analysis included 10,677 children. There were 71.3% of children who had one URI episode during the analyzed year and 4.2% experienced ≥3 episodes. The most frequent diagnoses were common cold (40.1%) and acute nonstreptococcal pharyngitis (40.6%). Only 0.4% had a diagnosis of streptococcal pharyngitis or tonsillitis; 5.5% had fever ≥38°C at the first visit (Table 2).
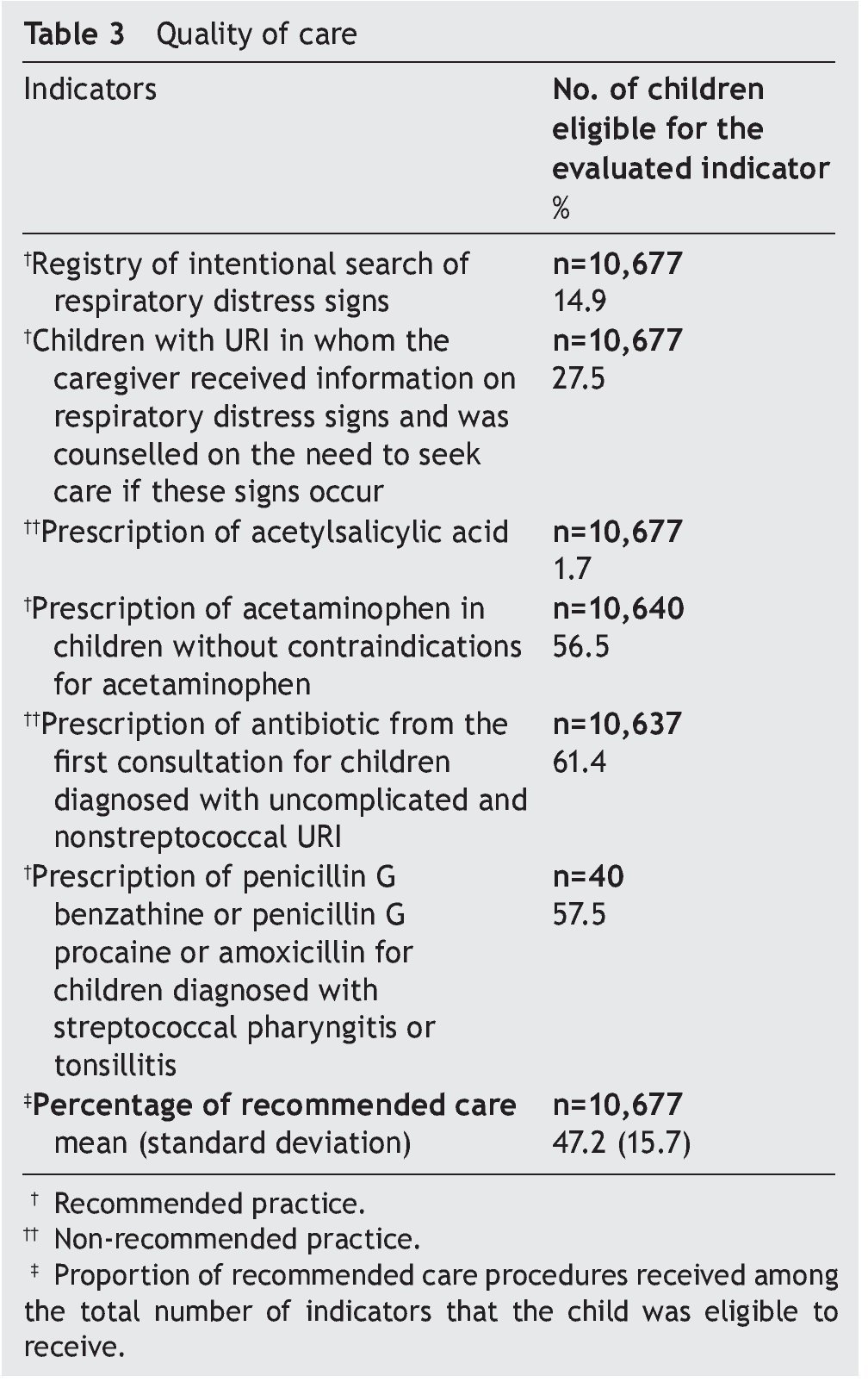
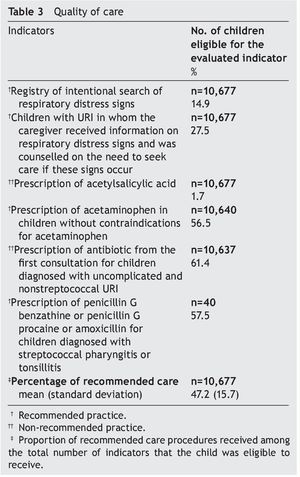
Table 3 shows the results of the quality of care evaluation; 15% were registered as an intentional search for respiratory distress signs. During the first URI visit, 27.5% of caregivers were informed about alarm signs and counseled to seek care promptly if such signs appeared. This percentage increased to 41% when all URI visits were analyzed. Acetaminophen was prescribed to 56.5% of children in whom this medication was not contraindicated; 1.7% of children were prescribed acetylsalicylic acid and 61% of children diagnosed with uncomplicated and nonstreptococcal URI received antibiotic prescription at the first visit. The most prescribed drugs were penicillin (75.9%), sulfonamides and trimethoprim-sulfamethoxazole (16.3%), and macrolides (7.0%). Children diagnosed with acute tonsillitis and acute URIs of multiple and unspecified sites received antibiotics more often than children with other URI diagnoses (information not presented in the table). There were 40 cases with diagnosis of streptococcal pharyngitis or tonsillitis; 57.5% received appropriately prescribed antibiotics. The percentage of recommended care that these children received was on average 47.2% (Table 3).
4. Discussion
Results of this study ascertain the feasibility to evaluate quality of care for URIs using routine EHR data and allow identifying the areas of opportunity for improvement. One such area is the intentional search and registry of respiratory distress signs, which allows evaluating the severity of URI. The lack of intentional search jeopardizes proper diagnosis and timely treatment. Previous studies that evaluated quality of care for patients with chronic diseases using EHR noted the importance of having complete, clear and reliable information22 which, in turn, can reduce medical errors and increase treatment effectiveness23.
Teaching caregivers about alarm signs and counseling to seek prompt care is crucial for URI management. Delay in seeking healthcare can contribute to up to 50% of deaths due to URI24. WHO estimates that timely healthcare can reduce by 20% the risk of deaths due to URI in children25. In the present study only 27% had documented recommendations regarding alarm signs at the first visit for an episode of URI. This finding indicates the need to include this component in a quality of care improvement program.
The appropriate and responsible use of antibiotics by health personnel is key to decreasing antimicrobial resistance, adverse drug reactions and waste of resources. In Mexico26,27 and other countries11,28,29, this is an unresolved problem despite multiple efforts. In 1998, a study conducted in Mexico reported that up to 66% of public and private medical physicians prescribed antibiotics inappropriately to children ≤5 years of age with URI26. This study was carried out 15 years ago and a variety of educational interventions and programs have been conducted to improve quality of care for URI; however, the inappropriate use of antibiotics remains. As mentioned previously, in the present study 61% of patients received inappropriate prescriptions for antibiotics. This happened despite the growing evidence that antibiotic use to treat uncomplicated URI has minimal or no benefit on the clinical outcome and that the overall number of courses of antibiotics needed to prevent one serious complication of URI is >4,00030.
Current IMSS3,4 and international2,31 clinical guidelines recommend delaying or not prescribing antimicrobials for treatment of nonstreptococcal or uncomplicated URI. Acetaminophen is the medication of choice due to its anti-pyretic and analgesic properties; thus, it is recommended not only for fever but also for pain relief. However, in the present study the prescription of acetaminophen was infrequent; only 50% of children without history or risk for asthma were prescribed acetaminophen. Furthermore, 1.7% received acetylsalicylic acid despite the fact that this drug is contraindicated in children due to the risk of Reye’s syndrome.
The above-mentioned care process flaws help to understand why less than half of recommended care practices (47.2%) was provided. In the U.S., children with URI receive 92% of indicated care21. These findings reveal the need to update family physicians on the use of the current guidelines that constitute a high quality of care for URI.
The study has several limitations. First, the possibility exists that the EHR has incomplete information, which can lead to underestimate the quality of care. For example, it is possible that physicians may have been provided information on respiratory distress signs, but without registering such information on the EHR. However, we still consider that the EHR information can be a reliable proxy of the action taken by the family physicians18,19. Second, at FMCs most of the diagnoses are being made on clinical grounds without microbiological confirmation. Third, the generalizability of this study is limited to four FMCs and possibly does not reflect the quality of URI care in Mexico.
In conclusion, it is reasonable to promote the use of EHR to routinely evaluate the quality of URI care. It is necessary to consider quality flaws that were found in order to promote strategies aimed at strengthening the technical capacity of health personnel to exercise evidence-based clinical practice.
Acknowledgments
We appreciate the participation of the panel of experts in the development of quality of care indicators. We also thank the medical directors of the participating clinics for their collaboration in the study. We thank David Gómez Trigo for technical assistance in SAS programming of the study variables.
Authors’ contributions
SVD conceptualized the research, conducted the literature review, coordinated the development, definitions, and programming of the indicators, interpreted the data and wrote the article.
RPC conceptualized the study, participated in the development of the indicators and contributed in drafting the article.
DABD participated in the interpretation of data and contributed in drafting the article.
MERM participated in the development of the indicators and critically reviewed the manuscript for significant intellectual content.
All authors approved the final manuscript.
Ethical disclosure
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of Data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Funding
This study was funded by Consejo Nacional de Ciencia y Tecnología (CONACYT) SALUD-2005-02-14455.
Conflict of interests
The authors declare they have no conflict of interests.
Acknowledgments
We appreciate the participation of the panel of experts in the development of quality of care indicators. We also thank the medical directors of the participating clinics for their collaboration in the study. We thank David Gómez Trigo for technical assistance in SAS programming of the study variables.
Received 9 July 2015;
accepted 13 July 2015
* Corresponding author.
E-mail:rperez@iabd.org (R. Perez-Cuevas).