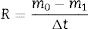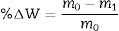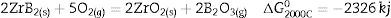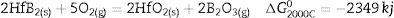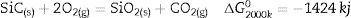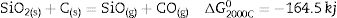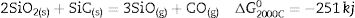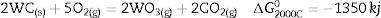ZrB2–SiC–HfB2–WC coating applied by spark plasma sintering led to the ablation resistance improvement of graphite substrate. The influence of HfB2/WC ratio was investigated on the ablation resistance produced by an oxyacetylene flame. The microstructural evolutions and phase characterization were studied using scanning electron microscopy and X-ray diffraction, respectively. It is confirmed that the ablation resistance of sample with 5% WC and 2.5% HfB2 was significantly increased with minimum ablated materials and rate of 1.1% and 2.2mgs−1 employing the results of oxyacetylene flame test. The important mechanisms of improvement of the ablation resistance were the evaporation of WO3 and SiO upon oxidation of SiC and WC in the coating.
El recubrimiento ZrB2-SiC-HfB2-WC aplicado mediante sinterización por plasma de chispa condujo a la mejora de la resistencia a la ablación del sustrato de grafito. Se investigó la influencia de la relación HfB2/WC en la resistencia a la ablación producida por una llama. Las evoluciones microestructurales y la caracterización de fase se estudiaron mediante microscopía electrónica de barrido y difracción de rayos X, respectivamente. Se confirma que la resistencia a la ablación de la muestra con el 5% de WC y el 2,5% de HfB2 se incrementó significativamente con materiales de ablación mínimos y una tasa del 1,1% y 2,2mgs−1 empleando los resultados de la prueba de llama de oxiacetileno. Los mecanismos importantes de mejora de la resistencia a la ablación fueron la evaporación de WO3 y SiO tras la oxidación de SiC y WC en el recubrimiento.
Graphite has important role in structural applications such as rocket, nozzles, turbine, supersonic spacecraft and space shuttle nose operating at high temperatures above 2500°C [1,2]. These applications are related to the various properties of graphite including high strength at high temperatures, low weight and high thermal stability [3,4]. However it oxidizes at temperatures above 400°C, therefore, its performance and properties get limited [5,6].
Compounds based on carbides and borides of the transition metals having a melting point above 3000°C are known as ultra-high temperature ceramics (UHTCs) [7–9]. These materials are favorable candidates for ablation-resistant coating on carbon substrates in harsh condition like aerospace uses [10,11]. UHTC coatings such as SiC (bond coat)/ZrB2–SiC–MoSi2[12], SiC/ZrB2–SiC–Si [13], SiC/ZrC–SiC [14,15] SiC/HfC [16] and SiC/TaC [17] were used to enhance the ablation and oxidation resistance of graphite. Fabrication of these coatings by plasma spraying and pack cementation is costly and time consuming. A porous surface and short cracks with a limited thickness of coating are the results of coatings producing using these techniques (100–500μm) [18]. UHTCs can be sintered by various fabrication methods like hot-pressing (HP) [19–22], reactive hot-pressing (RHP) [23,24], pressureless sintering (PS) [25–29], and spark plasma sintering (SPS) [30–33].
SPS is a new and fast method used for bonding of dissimilar materials and manufacturing ceramic bulks and coatings [34–36]. This is a modern technique that uses the direct current and uniaxial pressure at the same time to produce the bulk materials. It is used to obtain coating from powders on substrates in less than an hour [37,38]. One of the advantages of SPS is the high heating and cooling rate which results in the formation of dense and fine microstructure [39].
Two-layer coating containing ZrB2–SiC was used by pressure-assisted diffusion method to ameliorate the oxidation resistance of carbon-carbon composite materials [40]. The results displayed that the multilayer coating makes graphite and carbon-carbon composites resistant to oxidation under high heat flux and high temperature conditions. Passive oxidation layer is formed on the coating surface at high temperature and oxygen pressure and prevents substrate oxidation [40]. Many efforts were performed to enhance the oxidation resistance of zirconium diboride by the introduction of SiC into ZrB2[37–41]. In another study, an HfC-SiC coating was manufactured by chemical vapor deposition technique to boost the ablation resistance of carbon-carbon composites. The ablation behavior of the specimens was examined by the oxyacetylene flame. Results demonstrated that the coated specimen shows higher ablation resistance rather than uncoated specimen with a dense microstructure and no crack [16].
SPS process was used to fabricate a dense and thick layer of ZrB2–SiC–WC coating on the graphite substrate [42]. The results showed that a uniform SiC layer formed at the interface with the main composite top coating and graphite substrate. Moreover, the SiC diffusion zone was formed with appropriate penetration depth in the graphite substrate [42]. In another investigation, the SiC-Si coatings were produced by SPS for improving the ablation resistance of graphite. The results indicated that the increasing SiC leads to the significant improvement of the ablation resistance of the coated graphite [43].
The aim of present research is improvement of the ablation resistance (under oxyacetylene flame) of graphite substrate using the ZrB2–SiC–HfB2–WC composite coating by SPS method.
ExperimentalZrB2 (3μm, 99.9wt.%), SiC (10μm, 99wt.%), Si (10μm, 99wt.%), HfB2 (5μm, 99.9wt.%) and WC (10μm, 99wt.%) powders were used for the fabrication of coatings. In all samples, the volumetric percentages of ZrB2, SiC, Si and sum of HfB2+WC were constant and only the ratio of HfB2 and WC was variable. The chemical compositions of the mixtures made to manufacture each coating are presented in Table 1. The mixture of each coating was prepared after weighing and mixing in an ultrasonic bath (Ethanol media) for 45min to homogenize it. The obtained suspension was stirred with a magnet device on the heater for 3h, and finally the suspension was dried at 100°C for 1h.
The chemical compositions of the mixtures made to manufacture each coating.
| Sample Vol.% (Wt.%) | ZrB2 | SiC | Si | HfB2 | WC |
|---|---|---|---|---|---|
| A | 64.75 (70.18) | 13.875 (7.89) | 13.875 (5.67) | 5 (9.33) | 2.5 (6.93) |
| B | 64.75 (69.4) | 13.875 (7.8) | 13.875 (5.61) | 3.75 (6.92) | 3.75 (10.28) |
| C | 64.75 (68.63) | 13.875 (7.71) | 13.875 (5.54) | 2.5 (4.56) | 5 (13.55) |
Graphite disks (ø30mm×5mm) with porosity of 14% and density of 1.81gcm−3 were utilized as substrate. Before coating, these disks were grinded (Using SiC abrasive paper ANSI grit size # 320), rinsed with distilled water and dehumidified at 110°C for 2h. The inner surface of the SPS die was covered with flexible graphite sheet (Thickness of 1mm) to minimize its reaction with the coating materials. To fabricate the coatings, the SPS device (SPS-20T-10, China) with a graphite die (diameter of 3cm) under a pressure of 25MPa at 1950°C for 30min in a vacuum of 15Pa was used. More details were presented in our previous paper [44].
After soft grinding, microstructural evolutions of the coated specimens were examined using field emission scanning electron microscope (FESEM: Mira3, Tescan, Czech Republic). Energy dispersive spectroscopy (EDS) analysis was employed for the elemental analysis. In order to detect crystalline phases, the X-ray diffractometer (XRD, Bruker/ADVANCE) operating at 45kV and 40mA was used. Oxyacetylene flame test (oxidizing condition) was used to investigate the ablation properties of the coated graphite disks. The sample distance to the burner, the heat flux, test time and specimen surface temperature were 15mm, 8500W/m2, 60s, and 2000°C, respectively. Ablation rates (R) and weight changes (% ΔW) of the coatings were calculated based on the following formulas:
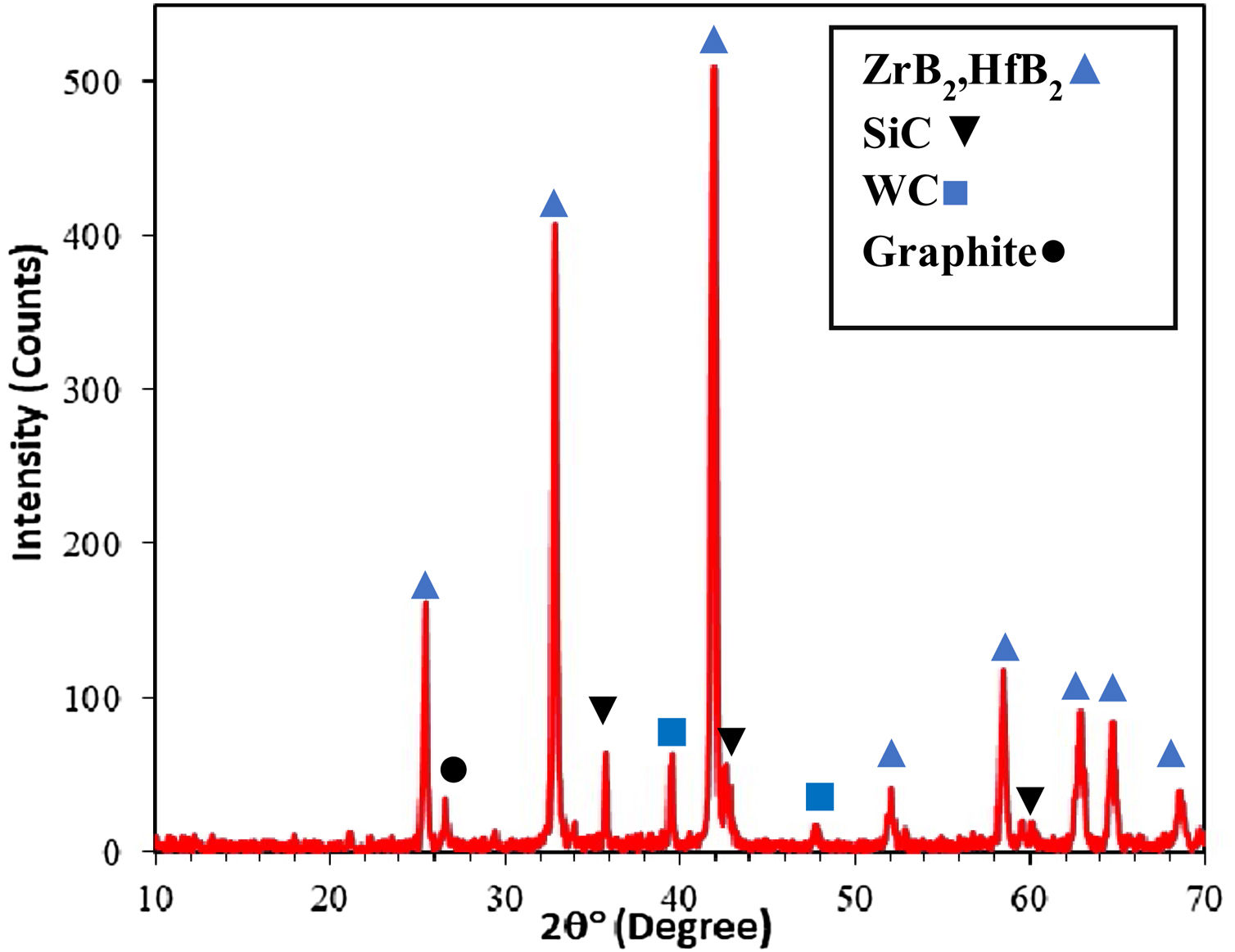
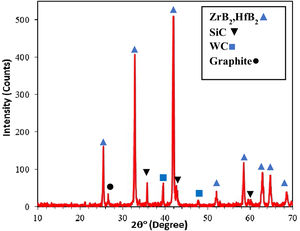
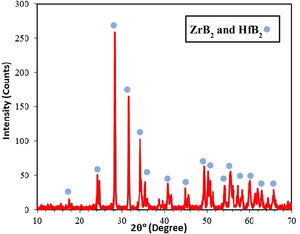
where m0 is the sample weight before and m1 is the sample weight after the ablation experiment and Δt shows the test time.Results and discussionXRD patterns of samples A, B and C were very similar to each other, therefore only one of them (sample C) is shown in Fig. 1. All peaks are related to the starting materials except for Si indicating no reaction between the composite constituents. All samples were hold at 1400°C for 15min for complete melting of Si and it's penetrating to the substrate for the formation of SiC. It is clear from the XRD patterns that the Si phase was converted to the SiC during SPS. ZrB2 and HfB2 peaks have considerable overlaps and cannot be precisely identified due to their structural similarity. A quadruple composite coating formation on the graphite substrate is confirmed owing to these observations. Adhered graphite on surface of sample is the reason of the graphite peak in the XRD pattern.
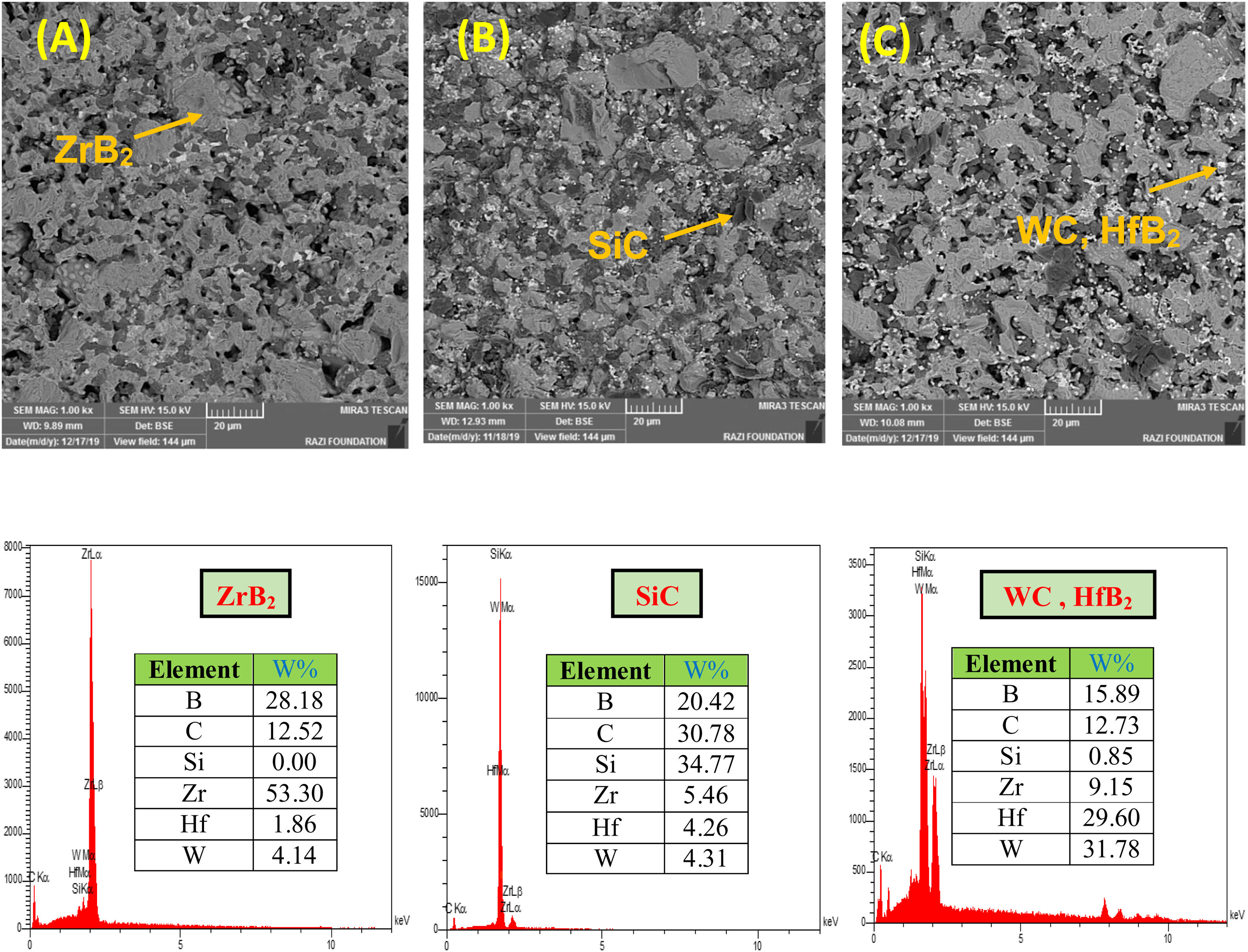
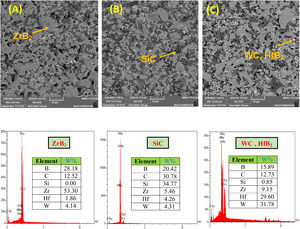
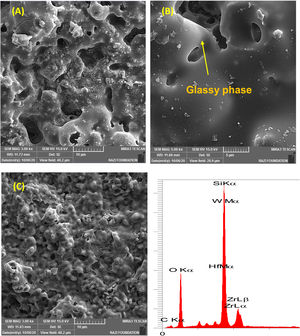
The microstructures of the coatings cross section are shown in Fig. 2. As it can be seen, it seems that there are three phases, including ZrB2 (gray zone), SiC (black zone) and WC and/or HfB2 (white zone), in the microstructure of coating according to the EDS spectrum. In contrast, WC and HfB2 phases appear brighter in the SEM images due to their higher atomic mass and density.
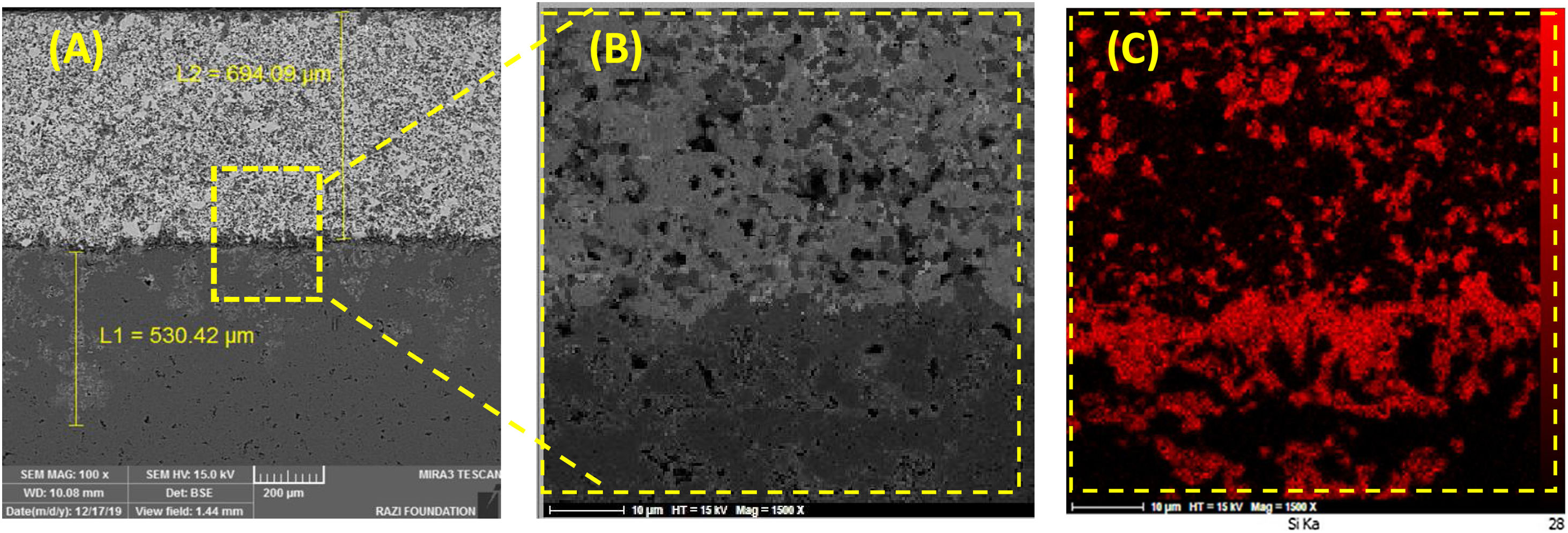
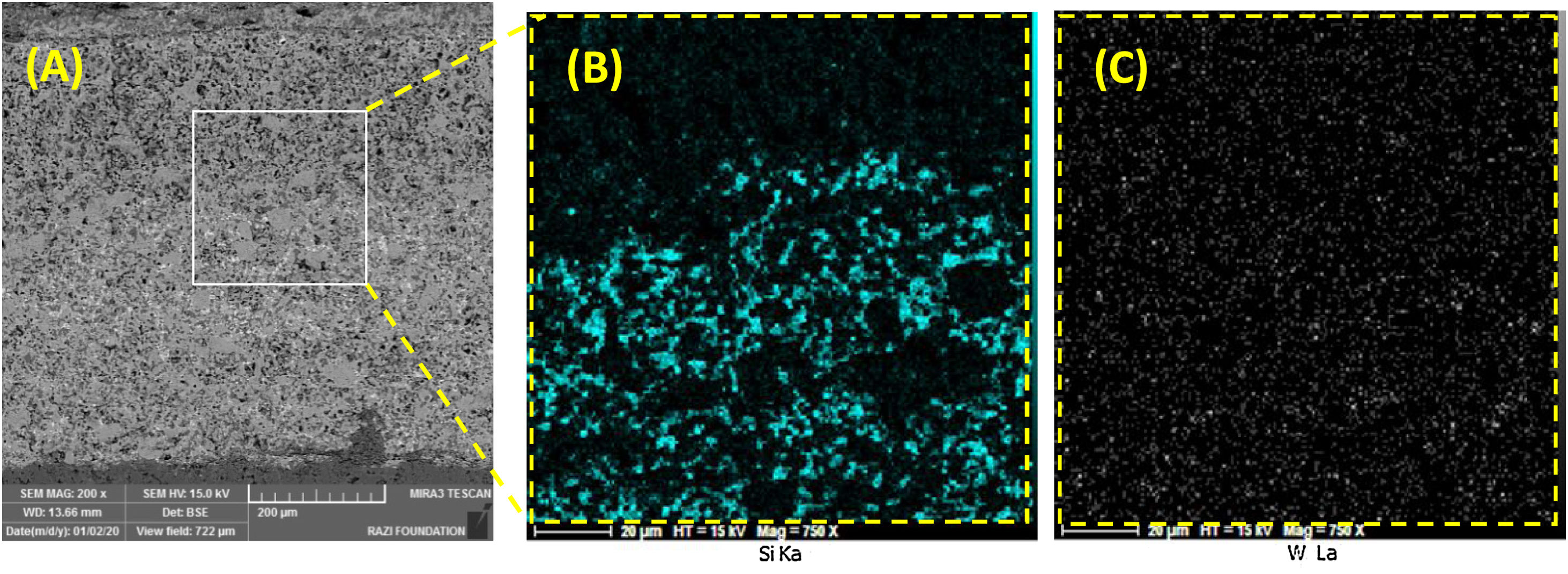
It is difficult to distinguish them because of their similar atomic mass (WC (195.85gmol−1) and HfB2 (200.11gmol−1)). Also according to the EDS analysis, it is clear that the dark grains are SiC phases, because it has low density compared to the other detected phases. The observed higher porosities in Fig. 2A and C can be attributed to the high melting point of HfB2 (3250°C) and WC (2870°C), which can prevent complete sintering of the coatings and lead to porosity formation. According to the higher porosity of sample A compared to sample C, it seems that the presence of HfB2 retards sintering process. Fig. 3 shows the cross-section of sample C spark plasma sintered at 1950°C. All coatings were calculated to have a same thickness of 1000μm before polishing. For the cleaning and removing of the adhered graphite, all sampled were polished to have a same thickness of 700μm (Fig. 3A). Sample A and B have similar SEM about the penetration depth of Si; therefore their images were not introduced here. It is also observed that the penetration depth of Si into the substrate is up to 500μm (Fig. 3A) which leads to enhance the adhesion strength. Graphite (substrate) and coating are well interconnected with no crack, porosity, delaminating and other microstructural defects. The formation of SiC phase is due to the diffusion of molten Si into graphite cavities and the chemical reaction between carbon and silicon under the high temperature and pressure of SPS. This secondary SiC phase not only can increase the coating density and strength because of its reaction bonded nature and second, but also it can promote the adhesive strength of coating to the graphite substrate [45]. This dense intermediated layer prevents oxygen diffusion increasing the oxidation resistance. As seen in Fig. 3C, the Si concentration increases from the surface of the coating toward the substrate. In fact, most of the ZrB2, HfB2 and WC grains were concentrated on the outside of the coating. On the other hand, SiC was mainly concentrated in the interface between the substrate and the coating.
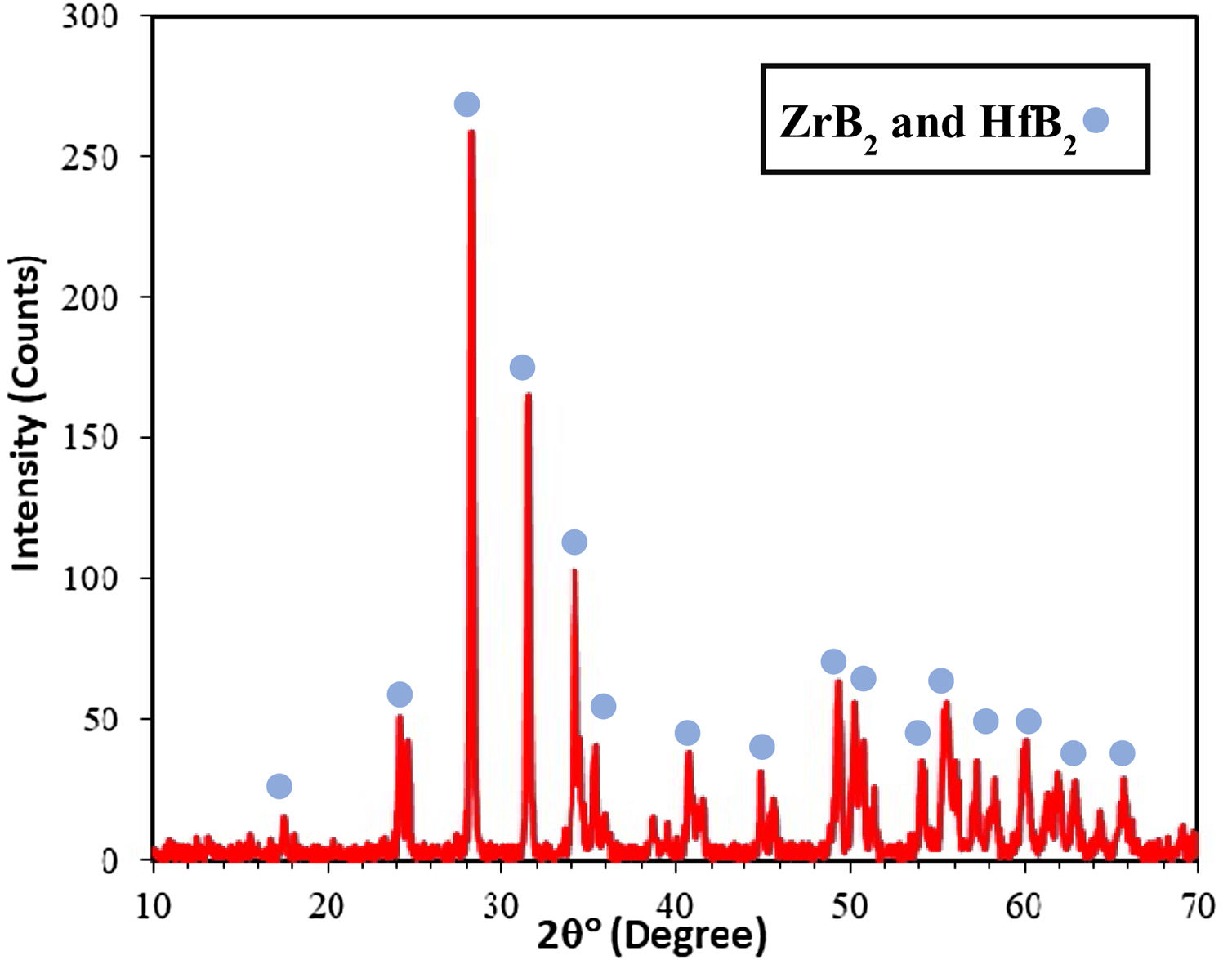
The high temperature oxyacetylene flame was used to evaluate the ablation resistance of the coatings exposing at 2000°C for 15s. Weight changes percentages (ΔW) and ablation rate (R) of the samples are calculated and presented in Table 2. As seen, ablation resistance of all coated specimens is better than that of uncoated specimens. Sample C had lower ablation rate and higher ablation resistance than samples A and B. XRD analysis related to the surface of sample C after the oxyacetylene test is shown in Fig. 4. As can be observed, the main phases of the coating are ZrO2 and HfO2. Disappearance of the primary phases of composite is related to their oxidation and evaporation according to the following chemical reactions. All thermodynamic data are from reference [46].
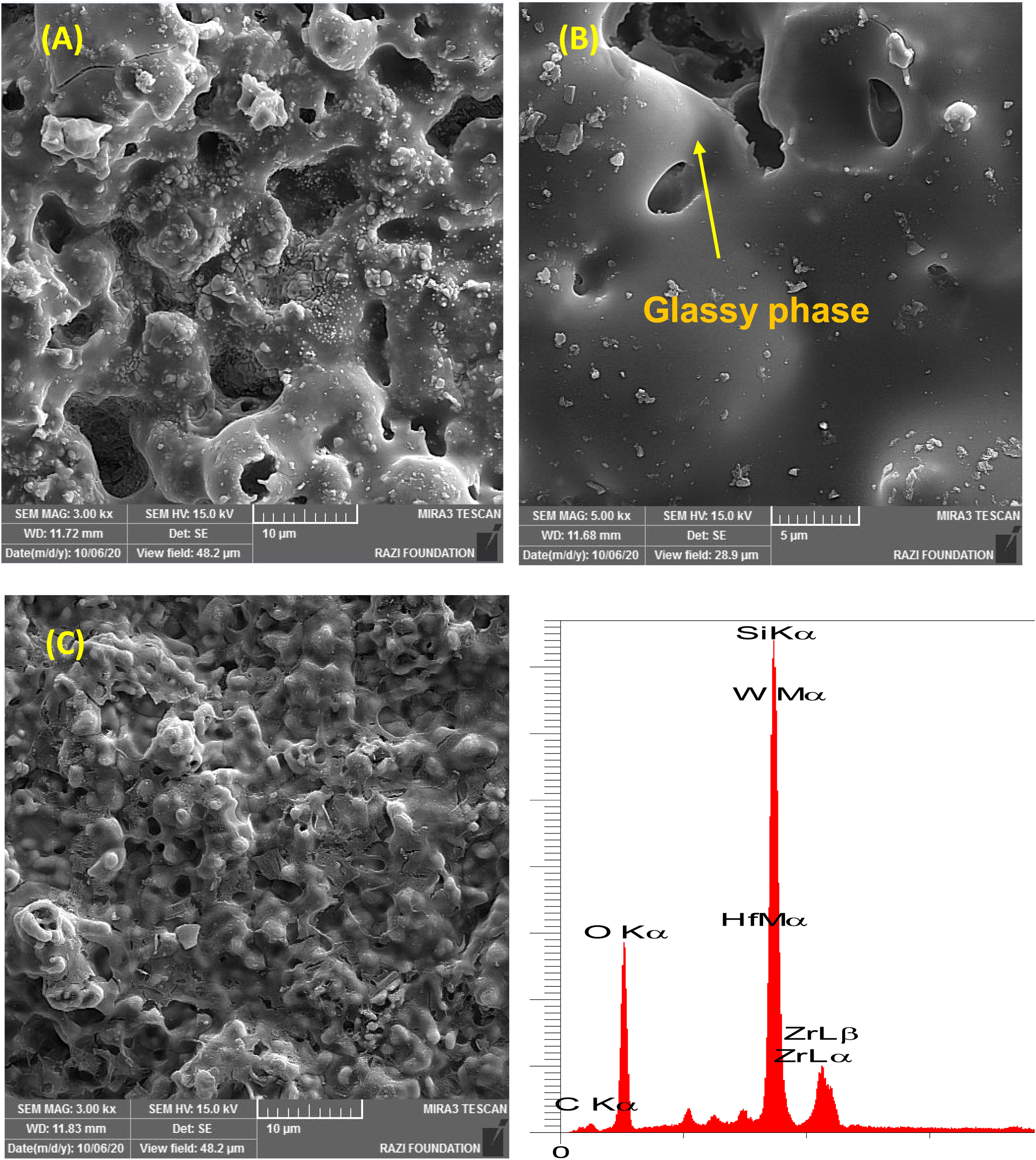
As seen all reactions have negative Gibbs free energy; therefore all of them can be performed during ablation test. Fig. 5 shows SEM photographs of the sample B after oxyacetylene test. As discussed before, the SEM images of other samples are very similar together; therefore their images were not introduced here. Due to the emergence of SiC phase in the composite and its oxidation during ablation test (Reaction 3), a glassy high viscous SiO2 phase is formed. This phase with other oxides (caused by the above reactions) forms a low viscous glassy phase on the surface of coating that prevents oxygen penetration and oxidizing of underlying layer. This glassy phase is shown in Fig. 6. Reaction 4 and 5 leads to the release of SiO and CO gases and consequently creates porosity at the top of the coating. In the next stage, WC oxidation to WO3 and its evaporation (Reaction 6) absorb the main thermal energy of the flame which prevent the oxidizing and destruction of the main coating structure (ZrB2 and SiC). Thermal conductivity of HfB2 (104w/mk) is higher than ZrB2 (23–25w/mk) [47]; therefore it spreads the flame heat throughout the coating and the substrate by increasing the thermal conductivity of the coating which results in the decreasing the overall temperature of the coating. These phenomena promote the ablation resistance of coatings. Owing to the higher ablation resistance of sample C and its higher WC content, it can be concluded that the WC has a greater effect on increasing the composite ablation resistance in comparison to HfB2.
ZrB2–SiC–HfB2–WC composite coatings on the graphite substrate were ideally manufactured by spark plasma sintering. The ablation resistance of the graphite was significantly increased by coatings. However, the composition of the sample C with 5% WC and 2.5% HfB2 showed the best ablation resistance. Formation of silicate glassy phase in the surface of coating increased the oxidation resistance. The main mechanism of the ablation resistance in these coatings was the formation of WO3 and SiO gases and their evaporation during heating.