Basalts, which cover about 70% of the Earth's surface, are igneous rocks originating from cooling and solidifying the magma on the Earth's surface. Products obtained from the melting of rocks have a wide market and can be considered the precursors of glass-ceramic technology. These materials are promising due to the possibility of converting low-cost natural raw materials into products with excellent mechanical, thermal, and chemical properties. In this article, a general overview of the basic principles for obtaining basaltic glass-ceramic materials will be made, as well as their properties, applications, and potentialities.
Los basaltos, que cubren aproximadamente el 70% de la superficie de la Tierra, son rocas ígneas que se originan al enfriar y solidificar el magma en la superficie terrestre. Los productos obtenidos de la fusión de rocas tienen un amplio mercado y pueden considerarse los precursores de la tecnología de vitrocerámica. Estos materiales son prometedores debido a la posibilidad de convertir materias primas naturales de bajo costo en productos con excelentes propiedades mecánicas, térmicas y químicas. En este artículo se realiza una descripción general de los principios básicos para obtener materiales basálticos de vitrocerámica, así como sus propiedades, aplicaciones y potencialidades.
Glass is an amorphous material that has no long-range structural order and can be defined as a condensed, nonequilibrium, and non-crystalline state of matter. However, at the limit of infinite time, the destiny of glasses is to crystallize, that is, to form ordered structures from their amorphous structure [1]. Nevertheless, with adequate chemical composition and specific heat treatment cycles, it is possible to promote the formation of crystals in the amorphous structure of the glass, obtaining glass-ceramic materials [2].
Glass-ceramics are ceramic materials in which the crystalline phases are formed by nucleation and controlled crystallization of a glass [3,4]. These materials are promising not only for their excellent mechanical properties but also for the possibility of converting natural raw materials, such as rocks, into products with mechanical, thermal, and chemical properties that are superior to the ones of the original glass [5].
Glass-ceramic products obtained from melting and heat treatment of rocks have a large market and are an excellent alternative to replace traditional materials. They can be applied as a coating due to their high resistance to abrasion wear and can be widely used in civil construction in applications such as paving and cladding panels [6,7].
In this article, a general overview of the basic principles for obtaining glass-ceramic materials will be made, as well as their properties and applications. Subsequently, the glass-ceramics obtained from basaltic rocks, their processing routes, applications, and potentialities will be presented.
Glass-ceramicsGenerally, materials can be classified, in relation to their structure, as crystalline or amorphous, depending on how the atoms are distributed. Crystalline materials are structures in which atoms are periodically distributed in space, that is, they have long-range structural order. Amorphous materials, however, have atoms randomly distributed in space and no long-range order [8,9]. A typical example of amorphous material is glasses. In 1932, W.H. Zachariasen [10] proposed a set of rules that are satisfied when a material forms a glass. The rules assume that the atomic arrangement of glasses must have a three-dimensional network with no long-rang order.
Glass-ceramic materials are formed by a combination of the two types of the structure presented (amorphous and crystalline) and can be defined as ceramic materials, in which the crystalline phases are formed by nucleation and controlled crystallization of glass. Efficient nucleation allows the formation of randomly oriented fine-grained crystals in a glass matrix [3,4]. The microstructure of a glass-ceramic is generally composed of 50–95% by volume of the crystalline phase. When the glass is heat-treated, one or more crystalline phases may be formed. Both the composition of the formed phases and the residual glass will differ from the composition of the original glass [4,5].
Nucleation and crystal growthThe basic principles for controlled crystallization of glass were determined in 1903 by Tammann, but only between the 1960s-1980s, the general theories on nucleation and crystal growth were consolidated. To transform a glass into a glass-ceramic two steps are necessary: nucleation (formation of crystalline embryos) and crystalline growth, which are processes of thermal and kinetic nature and originate an ordered structure from an amorphous system [3,11]. The term crystallization refers to the combination of these two processes (nucleation and crystalline growth) [2]. This phenomenon is also described in the literature as devitrification [11–13].
It is not possible to obtain a glass-ceramic material with special properties without controlled crystallization of the base glass, and nucleation is a decisive step in making this happen [2,3,14]. The process of nucleation of crystalline phases may happen in two ways: homogeneous nucleation and heterogeneous nucleation [2,3,14–16].
In the homogeneous nucleation process, random nuclei formation occurs at any point in the cooling point of the liquid [12,16]. Thermodynamically, with decreasing temperature, nucleation in a glass will occur when there is an orderly clustering of atoms that is sufficient for the formation of a crystalline embryo. The formation of these embryos implies a reduction in the free energy of the system. However, for their stability in the melt, the embryos must be equal to or greater than the critical radius value, which will depend on the crystallization energy and surface tension at the crystal-fused interface [14,17].
In the heterogeneous nucleation process, nuclei form from impurities or surfaces of pre-existing interfaces (molten-air, molten-crucible walls). In other words, these surfaces function as catalysts, acting to facilitate the process of nucleation and crystallization [2,3,12].
Homogeneous nucleation is rarer compared to heterogeneous nucleation. In most cases, crystallization in the glass is nucleated by surface heterogeneities. However, the production of glass-ceramics requires the formation of crystals, not only on the surface but inside the material as well. For this reason, nucleating agents are incorporated in small amounts into the glass compositions to induce and/or accelerate the crystallization process, creating discontinuities in the glass structure [4,18].
The main nucleating agents are oxides, which can be of two types, those acting by the valence change mechanism (TiO2, Cr2O3, ZrO2) and those acting by the imbalance charge mechanism (P2O5). Another nucleating agent used is metallic colloids (Pt, Ag, Cu) and can precipitate metal species by redox or photosensitive reactions [4,11,12].
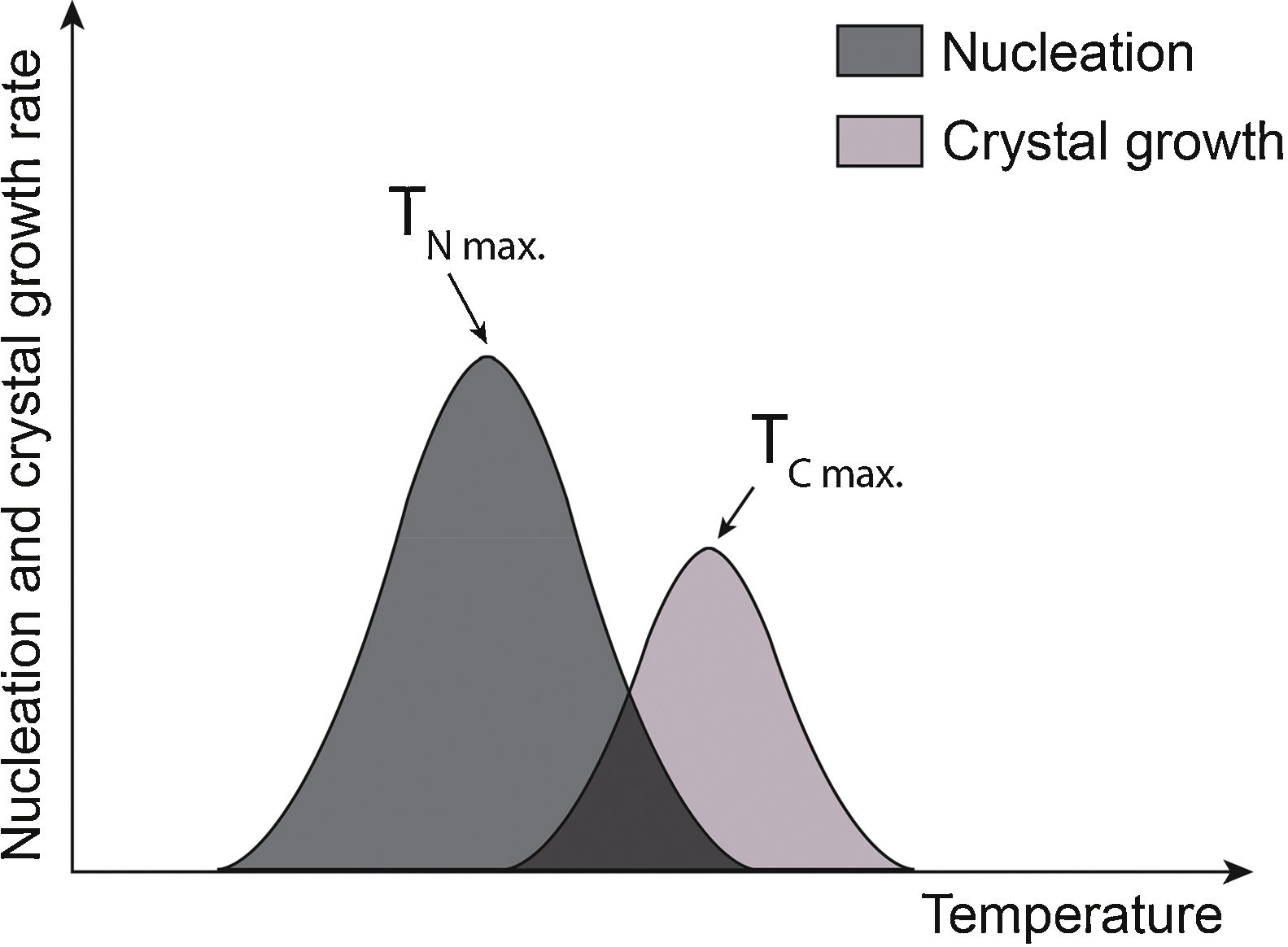
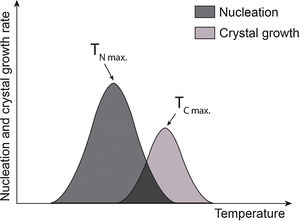
After the formation of stable nuclei inside the glass, the crystal growth stage occurs. The progress, from the kinetic point of view, is represented by two distinct curves: nucleation and crystalline growth in the function of temperature [12]. The crystalline growth rate will depend both on how easily atoms move along the structure and the temperature [7]. At very low temperatures, growth will not be favored as atoms will not have enough energy to travel along the structure. Similarly, at very high temperatures, growth will also be slow due to the difficulty of the system to dissipate the heat released by crystallization. For this reason, the nucleation and crystalline growth curves have a peak at which the rate will be maximum, and growth will be favored as heat will dissipate more easily at these temperatures [13]. In Fig. 1, TNmax, and TCmax represent the temperature at which the nucleation rate and crystalline growth are maximum. The purple area corresponds to the overlap of the curves and indicates the optimum temperature range to produce glass-ceramics. If TNmax and TCmax are distant, that is, the curves do not overlap, nucleation and crystalline growth processes must occur in two steps. Otherwise, if TNmax and TCmax are close, and the curves overlap, nucleation and crystalline growth processes occur simultaneously, and the production of a glass-ceramic material can be performed in one step [2,11,12].
Raw materials and propertiesOne of the great advantages of glass-ceramic materials is the possibility of obtaining a wide variety of microstructures with different properties, which are associated with the combination of amorphous and crystalline phase properties. Obtaining several microstructures involves both choosing the right chemical composition and an optimal heat treatment cycle to favor the process of nucleation and crystal growth [3].
The compositions of the glass-ceramic materials should be favorable for the glass formation that subsequently allows controlled crystallization. Some compositions form very stable glasses that make the crystallization process difficult, while other compositions crystallize very quickly and without control. An ideal composition allows the formation of metastable crystals that originate from glass and a thermodynamically stable mixture of crystals governed by the laws of phase equilibrium [19].
According to Holand and Beall [3], glass-ceramic can be classified, to their chemical composition, as alkaline and alkaline Earth silicates, aluminosilicates, fluorosilicates, silicophosphates, iron silicates, and phosphates. Among the iron, silicates are the basalt glass-ceramics. Products obtained from fused rocks have a large market and can be considered the precursors of glass-ceramic [6].
These materials offer a wide range of surprising combinations of characteristics due to their ability to combine the unique properties of ceramics (crystalline phase) and the specific characteristics related to the amorphous structure of glasses. The glass-ceramic properties, such as density, hardness, chemical resistance, and wear, will mainly depend on the well-dispersed crystalline phases in the glass matrix [20].
Glass-ceramic materials that have the best properties are usually those with more than 90% crystalline phase in their structure, with crystal sizes between 0.5 and 1μm [21]. As it is a ceramic material, both residual porosity and surface finish will have a great influence on the final properties of the product.
ApplicationsThe first commercially viable glass-ceramic material was developed in the late 1950s by the aerospace industry for the manufacture of radomes to protect internal radars on the noses of aircraft and rockets. The material used for this application had a very low and homogeneous dielectric constant, low thermal expansion coefficient, low dielectric loss, and high abrasion resistance [3].
Over time new applications for glass-ceramic have emerged, such as:
- •
Technical applications: Photosensitive glass-ceramic materials that could be etched. An example of this type of material is Fotoceram®, which is a lithium disilicate glass-ceramic produced by Corning. This material is used for equipment manufacturing in the micromechanical and electrical industries. Another example is Foturan®, a glass-ceramic produced by Schott AG (Germany) that is used in precision automotive engineering, integrated micro-optical, and optical systems, inkjet printheads, pressure sensor substrate, and headphone acoustic systems [3].
- •
Consumers applications: In 1959, Dr. Stookey developed the world's first glass-ceramic commercialized in the form of household dishes. This material was named Pyroceram 9608 (Corning Glass Works). Over time new consumer applications have emerged, such as the famous cooktops. One example is Eurokera® stoves that are sold worldwide today [22].
- •
Medical and dental applications: Biocompatible glass-ceramic materials for medical applications can be separated into basically two groups, that differ both in the environment in which they are applied and in the properties: implantology (prostheses that are implanted within the human body – dental implants and roots also fall into this group) and restorative dentistry (materials are used to restore natural teeth without being introduced into the human body) [3,20]. Also, recent studies present applications for biocompatible glass-ceramics in the areas of bone regeneration and tissue engineering [23,24]. Recently, Montazerian and Zanotto [25] reviewed the main properties and applications of bioactive glass-ceramics and present perspectives for the development and improvement of these materials.
- •
Waste reuse applications: The generation of by-products occurs in most industrial processes. Due to the growth of the industrial sector, the quantities of by-products become increasingly larger, which generates a constant concern with the proper disposal of these materials. The reuse of industrial waste is a viable and effective alternative for reducing environmental impacts. In this context, some studies have been done aiming at the reuse of residues to produce glass-ceramic.
Rawlings et al. [26] published a review article dealing with the production of glass-ceramic materials from wastes. In the paper, different processing methods are described for the manufacture of these materials from silicate waste, such as coal combustion ash, steel slag, fly ash, hydrometallurgical sludge, broken glass, or a mixture, among others. Karamanov et al. [27] used ashes from solid waste incinerators for the production of glass-ceramic materials by the sinter-crystallization process. Khater et al. [28], prepared different compositions using, among other components, granite quarry waste to obtain technical glass-ceramics with low production costs. They found that the glass-ceramics produced had high hardness, indicating high abrasion resistance, making them alternatives for applications under aggressive mechanical conditions.
In addition to the concern with the reuse of wastes, there is a constant search for new alternatives in materials that do not harm the environment. Basalt is abundant and an environment-friendly natural material that can be used as a raw material to produce glass-ceramics. In this context, the next topics deal with the production of glass-ceramics from basaltic rocks.
The rocksPetrology is the name given to science dedicated to the study of the origin, occurrence, and structure of rocks [29]. In general, rocks can be classified, according to the formation process, into sedimentary, metamorphic, and igneous [30–33].
Most of the Earth's solid surface is composed of sedimentary rocks. These rocks are formed from sediments produced by weathering and erosion, which over time deposit in valleys and ocean basins. As these sediments settle over the same area, the lower layers undergo an increase in pressure and temperature, that transform these sediments into rocks by physical and chemical processes. This process is called lithification (originating from the Greek word lithos, meaning stone) [31,32].
On the other hand, metamorphic rocks are formed from igneous, sedimentary, or even from other pre-existing metamorphic rocks through the process of metamorphism – which means shape change. This process occurs a transformation in mineralogy, texture, and occasionally chemical composition due to the new temperature and pressure conditions that the original rock is subjected to [31].
At last, igneous or magmatic rocks are formed from the cooling and crystallization of magma or volcanic lava (magma that flows to the Earth's surface). The composition of the magma will determine the type of igneous rock that will be formed [29,31,32,34]. Magma consists of a mixture of volatiles, fused silicates, and oxides, and its composition is predominantly oxygen, silicon, aluminum, iron, magnesium, calcium, potassium, and sodium [32]. Igneous rocks are divided into two categories: intrusive (or plutonic) rocks, that are formed from magma crystallization below the Earth's surface, and extrusive (or volcanic) rocks, that form from magmas that cool near or on the Earth's surface. The classification of igneous rocks is based on petrographic observations (texture, mineralogical composition, and color) by the quartz, alkaline feldspars, plagioclase, feldspathoids diagram (QAPF) for the plutonic rocks, and based on the chemical composition by the Total Alkali vs. Silica (TAS) for volcanic [29,34].
Igneous and metamorphic rocks represent 90 to 95% of the outer 16km of the Earth's crust [31]. Among the igneous rocks, the basaltic are the most abundant, as they occupy about 70% of the surface of the planet [35].
Basaltic rockBasaltic rocks are characterized by high hardness, dark coloration, fine grain, and silica content between 45 and 52% by mass. Due to the low silica content, its magma has a low viscosity. Common basalt minerals include olivine, pyroxene and plagioclase [29,31,35].
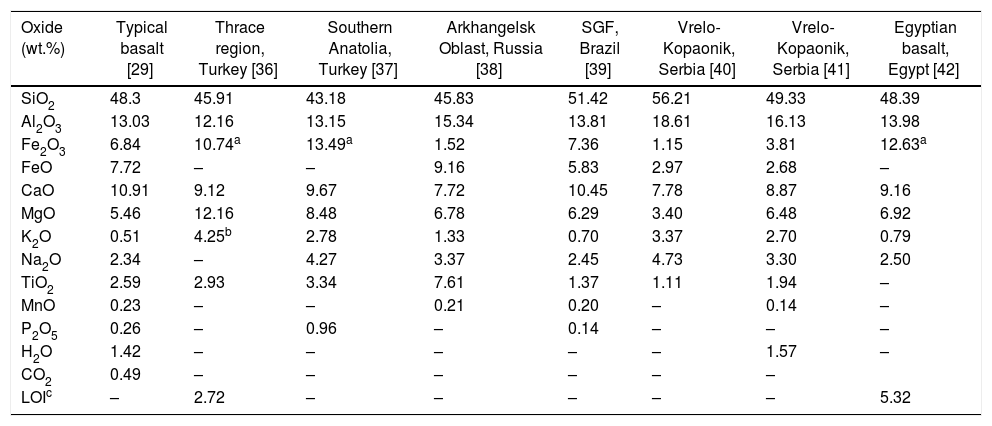
Basaltic rocks can be divided into two large groups: alkaline basaltic and sub alkaline basaltic. These comprise the subgroup of tholeiitic basalts and calcium-alkaline basalts. The vast majority of igneous rocks are formed by a class of chemical components called silicates, which are formed by structures containing cations and anions [29,35]. The composition of rocks is usually expressed as oxides as wt.% [29]. Table 1 shows the comparison of the chemical composition of basalts from different regions of the planet that were used to evaluate the possibility of obtaining glass-ceramics.
Chemical composition (wt.%) of basalts from different regions of the world.
| Oxide (wt.%) | Typical basalt [29] | Thrace region, Turkey [36] | Southern Anatolia, Turkey [37] | Arkhangelsk Oblast, Russia [38] | SGF, Brazil [39] | Vrelo-Kopaonik, Serbia [40] | Vrelo-Kopaonik, Serbia [41] | Egyptian basalt, Egypt [42] |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 48.3 | 45.91 | 43.18 | 45.83 | 51.42 | 56.21 | 49.33 | 48.39 |
| Al2O3 | 13.03 | 12.16 | 13.15 | 15.34 | 13.81 | 18.61 | 16.13 | 13.98 |
| Fe2O3 | 6.84 | 10.74a | 13.49a | 1.52 | 7.36 | 1.15 | 3.81 | 12.63a |
| FeO | 7.72 | – | – | 9.16 | 5.83 | 2.97 | 2.68 | – |
| CaO | 10.91 | 9.12 | 9.67 | 7.72 | 10.45 | 7.78 | 8.87 | 9.16 |
| MgO | 5.46 | 12.16 | 8.48 | 6.78 | 6.29 | 3.40 | 6.48 | 6.92 |
| K2O | 0.51 | 4.25b | 2.78 | 1.33 | 0.70 | 3.37 | 2.70 | 0.79 |
| Na2O | 2.34 | – | 4.27 | 3.37 | 2.45 | 4.73 | 3.30 | 2.50 |
| TiO2 | 2.59 | 2.93 | 3.34 | 7.61 | 1.37 | 1.11 | 1.94 | – |
| MnO | 0.23 | – | – | 0.21 | 0.20 | – | 0.14 | – |
| P2O5 | 0.26 | – | 0.96 | – | 0.14 | – | – | – |
| H2O | 1.42 | – | – | – | – | – | 1.57 | – |
| CO2 | 0.49 | – | – | – | – | – | – | |
| LOIc | – | 2.72 | – | – | – | – | – | 5.32 |
It is possible to observe that iron, in the basalt composition, appears in two oxidation states, Fe2+ and Fe3+, forming iron oxide II (FeO) and iron oxide III (Fe2O3), respectively. The Fe3+/Fe2+ ratio will depend on chemical composition and basaltic rock formation conditions [29]. Holand and Beall [3] argue that iron, in its two oxidation states, plays a fundamental role in obtaining glass-ceramics. According to the authors, basalt glasses, when heat treated, have the possibility of forming glass-ceramic materials if the Fe2O3/FeO ratio of the original rock composition is greater than 0.5.
Since it is an extrusive igneous rock, its mineralogical composition can be defined by the TAS diagram which relates the mass quantity (%m.) of SiO2 as a function of alkaline content (sum of mass percentages of Na2O and K2O). According to the amount of silica the igneous rocks can be classified as: ultrabasic (SiO2<45%), basic (52%<SiO2<45%), intermediate (66%<SiO2<52%) and acidic (SiO2>66%).
Due to its high chemical resistance and wear, basalt is used as a raw material in its natural form for the construction of sidewalks, pavements, and walls and applied as cladding panels [43]. Basalt is also largely used as a low-cost raw material to produce aggregates for civil construction, mainly in the form of gravel. The gravel is a rock fragment that goes through the crushing and subsequent selecting process and is classified for use according to grain size. Each type of gravel has a specific function in civil construction, ranging from concrete fabrication, paving, building construction, railways, tunnels, and even dams [44,45].
Basaltic glass-ceramicThe first patent for a glass-ceramic material, produced from a basaltic rock, was registered by George H. Beall and Hermann L. Rittler in 1971 and entitled “Process for forming a basaltic glass-ceramic product” [46]. The invention relates to the production of glass-ceramic articles by melting basalt under oxidizing conditions, followed by rapid cooling to obtain a glass which is after that heat-treated to form homogeneously dispersed crystals in a glass matrix.
The use of natural raw materials to produce glass-ceramics is of great economic, technological, and scientific importance. With an adequate chemical composition, different types of rocks can be used to obtain glass-ceramics, resulting in varied microstructures and a wide range of technological properties [47].
Temperature control and heat treatment times are fundamental to obtain the desired microstructure. The higher is the crystallization capacity of the glass and the slower is the growth rate of the crystals, the thinner the microstructure obtained. Another important factor in processing is the control of the raw material, which, because it is obtained from rocks, often does not have a good composition and mineralogy homogeneity, reflecting directly on the characteristics of the final product [7].
The preferential use of igneous rocks in the production of glass-ceramics can be justified by the easier processing due to the better consistency in chemical composition and mineralogical homogeneity when compared to other rocks. Among igneous rocks, basaltic rocks are the preferred choice because of their lower melting temperature and melt flowability, which facilitates the production process [21].
Basalts can be easily melted at temperatures around 1500°C. If cooled quickly, they form glasses. According to Holand and Beall [3], by thermally treating these glasses, it is possible to obtain a glass-ceramic material if the ratio of Fe3+ and Fe2+ oxides of the rock composition is greater than 0.5. The role of iron oxide in crystal nucleation is attributed to the Fe3+ grouping in the glass structure which, upon heating between 650 and 800°C, reacts with oxygen and forms the magnetite (FeO Fe2O3), which is the first phase to crystallize and acts as a nucleating agent to start crystallization. The ratio of iron oxidation states also affects the phase formation, crystallization rate, and final properties of glass-ceramic [6].
Since most ceramics are oxides, the partial pressure of oxygen, pO2, is an important variable in the processing of these materials as cation valence may change as temperature and pressure vary. If the cation is polyvalent, such as Fe, then its valence will depend on oxygen activity, which is directly associated with its partial pressure [48].
Glass-ceramic processThe main processing routes for glass-ceramic materials are volume crystallization, sinter-crystallization, and petrurgical [3,11]. Volume crystallization and sinter-crystallization processing routes consist of the production of glass-ceramics by the “conventional method”, in which first glass is produced and after that this glass is heat-treated to promote the formation of crystals in its structure. In the petrurgical processing route, the material is melted, and controlled cooling allows precipitation of the corresponding crystalline phases [49].
In the volume crystallization processing route, an appropriate mixture of raw materials is melted and poured into a mold to produce glass. Subsequently, this glass is subjected to a specific heat treatment to promote crystallization by the internal nucleation and crystal growth processes. In this case, nucleation occurs homogeneously from the molten glass with random nuclei formation at any point in the liquid [3,17]. In this type of processing, it is quite common to use nucleating agents to induce and/or accelerate the crystallization process [50].
On the other hand, in the sinter-crystallization processing route, the ground glass powder is used as a starting material. The powder is shaped by conventional techniques (pressing, collage, or injection) and subjected to a specific heat treatment, combining the sintering and crystallization steps. In this case, nucleation occurs heterogeneously from low surface energy sites (substrates) under which crystals grow [51]. The surface imperfections of the powder themselves act as nucleation sites [3].
Alternatively, glass-ceramics can be obtained by the petrurgical process. In this case, heat treatments similar to the natural process of mineral formation are applied, that is, the nucleation and crystallization of the molten material occur by slow and controlled cooling from high temperatures to room temperature. This process is known as primary crystallization [7,17,26]. During the slow cooling process, nucleation, and growth of certain crystalline phases may occur according to the appropriate transformation-temperature-time (TTT) diagrams [52].
In this production method, it is necessary to know the initial chemical composition of the rock and the formed glass, since it should favor the formation of melts that are capable of crystallizing phases with ease of isomorphic substitution. The crystallization in this process will depend on the ability of the structure atoms to organize with each other to form stable crystalline structures [7,21].
The petrurgical route is usually applied for the manufacture of glass-ceramic made from natural raw materials, such as rocks and minerals, and industrial waste, such as blast furnace slag and fly ash. These materials tend to melt with sudden changes in viscosity at very short temperature ranges, and this leads to a strong tendency to crystallize [7].
The petrurgical method is similar to the “Silceram” method that was developed in the 1970s at Imperial College, in London, for low-cost manufacture of glass-ceramics obtained from blast furnace slag. The “Silceram” method involves plateaus during cooling at specific temperatures for crystallization [52]. A similar method, with cooling crystallization isotherms, has already been applied for the glass-ceramic production with basaltic compositions in the studies of Cocic et al. [53] and Matovic et al. [41].
Both the petrurgical and Silceram processing methods are more economical compared to the conventional glass-ceramic processing method. The conventional method, as seen, involves reheating the base glass at two temperature stages to promote nucleation and crystalline growth, making the process costly for the energy [26,52].
Vicente-Mingarro et al. [17] obtained glass-ceramic by conventional and petrurgical methods for a basaltic composition. By the petrurgical route, the heat treatment cycle began by melting the rock at 1450°C, followed by controlled cooling at a rate of 5°C/min to a temperature of 850°C and finally air cooling. The microstructure formed presented primary crystallization of the eutectic type of the major phases, in this case, pyroxene and plagioclase. On the other hand, by the conventional route, the heat treatment cycle was applied in a glass for the secondary crystallization of a basalt glass. The glass was heated to 650°C for 2h for phase nucleation, followed by heating to 800°C for 16h for crystalline growth. Crystallization occurred homogeneously at the major pyroxene phase.
Properties and applicationsSeveral scientific studies have confirmed the possibility of using basaltic rocks for glass-ceramics production as well as the possibility of applying the different processing routes and numerous types of heat treatments.
Yilmaz et al. [36] evaluated the crystallization kinetics of a basalt glass. The samples were prepared from the fusion of basaltic rock from the Thrace region of Turkey. They concluded that the diopside phase crystallizes at 788°C, followed by the crystallization of augite at 845°C and that the glass-ceramic obtained had a very homogeneous microstructure with finely dispersed crystals.
Karamanov et al. [54] applied the sinter-crystallization method for the production of glass-ceramics from a basaltic rock from southern Anatolia. The glasses crystallization and sintering behavior were studied in the air and nitrogen atmosphere. The study authors found that densification at low temperatures is reduced by the intense crystallization process in both atmospheres, but in air, as the sintering temperature increases, FeO oxidation occurs, which causes a reduction of 15–20% in the formation of crystalline phases, compared to the nitrogen atmosphere.
Karamanov et al. [37] demonstrated the possibility of obtaining ceramic materials from alkaline basaltic rocks. The rock was ground, and the resulting powder was pressed. Heat treatments in the temperature range from 1000 to 1140°C were applied to the samples. The material obtained in the treatment at 1000°C showed zero water absorption, ∼9% of closed porosity and a glass-ceramic like microstructure. The composition used showed a high tendency to crystallization with different phases formation (pyroxene, anorthite, spinel, and hematite) and crystallinity indexes of ∼60%. Even with a low-cost production cycle, it was possible to obtain a material with good properties such as bending strength of 100MPa and elasticity modulus of 90GPa. The elasticity module for common commercial soda-lime-silica glasses is typically 70–75GPa [2].
Jensen et al. [55] evaluated the hardness dependence of basalt glass-ceramics in relation to heat treatment temperatures. For the study, a basaltic composition was used to produce glasses that were treated at temperatures of 730, 850, 875, 900, 925, 937, 950, 1010, 1030, 1060, and 1084°C. Vickers hardness was measured by micro indentation with a load of 0.98N applied for 5s. Augite crystals were identified as the main phase, and it was found that the glass hardness decreases as a function of the samples’ crystallization degree. The maximum hardness, 8.5GPa, was obtained for samples with crystallization degree in the range 0.61–0.85 (corresponding to heat treatment between 900 and 937°C).
Drobot et al. [56] evaluated the effect of iron content on the sintering behavior of basalt compositions. Ground basalt was divided by magnetic separation into magnetically enriched and magnetically deficient components. The authors found that samples produced by sintering the non-magnetic component have higher hardness and acid resistance, and such materials can be used as protective coatings against acidic media. On the other hand, the magnetic component is suitable for the production of pyroxene-dominated materials, which is a fundamental requirement for the preparation of quality petrurgical raw materials.
Klein et al. [57] produced a glass from a basaltic rock of the Serra Geral Formation (Brazil) and evaluated the crystallization kinetics of magnetite without the addition of nucleating agents. The study concluded that the maximum magnetite nucleation rate is at a temperature of 650°C. With additional heating at 860°C, the second phase to crystallize is augite. Finally, they found that the glass treated at 650°C for 0.5h and then at 860°C for 1h had the best mechanical properties. The glass-ceramic obtained showed a homogeneous microstructure with finely dispersed crystals, high hardness (8.18GPa), and reasonable resistance to acid attack by NaOH.
Lima et al. [39] used a basalt from the Serra Geral Formation (Brazil) to prepare a glass frit from which glass-ceramics were produced by the sinter-crystallization process. Different heat treatments were performed in an inert atmosphere. The results show that bulk density, hardness, and crystallinity index increased with the treatment time up to the final temperature, with the highest values for the longest heat treatment (6.5h), respectively 2.39g/cm3, 74.8%, and 9.81GPa. However, a shorter heat treatment (2.5h) at the same final temperature resulted in comparable values (respectively, 2.38g/cm3, 72.3%, and 9.68GPa). As such, isothermal steps at intermediate crystallization temperatures have no relevant influence on the final microstructure. Thus, for this basalt, nucleation is a fast process, and crystal growth is likely rather limited and little improved by intermediate crystallization heat treatment steps.
Some studies have evaluated the wear properties of basalt base glass-ceramics. Basalt glass-ceramics have been used in many industrial applications, mainly as a coating for solid particle and slurry transport systems due to their high thermal and chemical resistance. For this reason, studying erosion wear behavior is important. Pavlovic et al. [40] produced and evaluated the wear of a glass-ceramic based in olivine–pyroxene basalt from Vrelo–Kopaonik Mountain (Serbia). The mass loss of the samples as a function of the cavitation time was monitored. The results showed that the material was highly resistant to cavitation wear, and can be applied in conditions in which high cavitation loads are expected. Öztürk et al. [58] assessed erosive wear resistance of commercial basalt glass-ceramic and its relationship with the impact angle of solid particles. The impact angles used were 30°, 45°, 60°, 75°, 90°. They concluded that the wear rates were strongly related to impact angles and probably the change in the impact angle changes the wear and damage mechanism. In low impact angle (30°), the erosive particles cannot cause harsh wear effects since their kinetic energies could not be completely transferred to the glass-ceramic. When impact angle increase (90°), more energy goes through the surface, and greater loss of material occurs. In medium impact angles (45°, 60°, 75°), stronger wear effects such as scraping are observed.
Also, recent studies have demonstrated the possibility of using basaltic compositions to produce machinable glass-ceramics as an alternative to replacing synthetic commercial machinable glass-ceramics. Ercenk et al. [59] added MgF2 and K2O into a basalt formulation since fluorine and potassium phases are responsible for the machinability of glass-ceramics. Compositions were prepared with 80, 85, and 90% of basalt, with K2O and MgF2. The results showed that the better machining performance was observed for the lower basalt content (80%) and proved that a machinable glass-ceramic produced from basalt is possible.
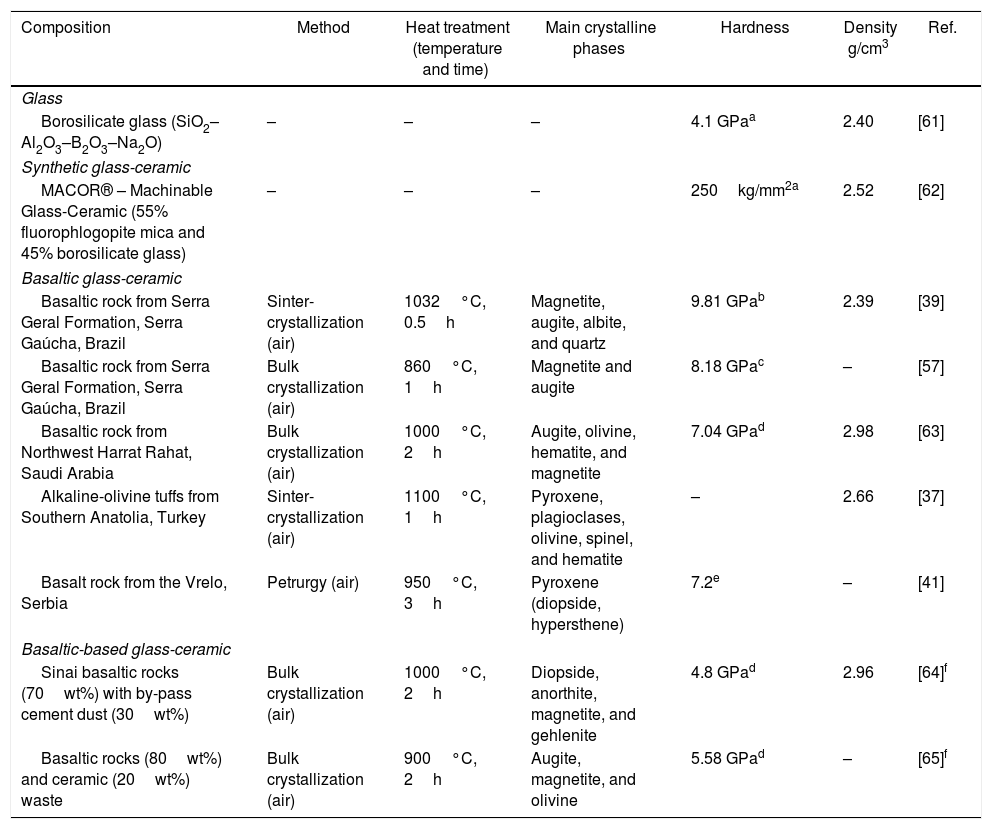
A very recent study has evaluated the thermal, electrical, and physical properties of glasses and glass-ceramics of basalt compositions. Khater et al. [60], prepared six glass compositions based on basaltic rocks and successive additions of cement waste with different proportions from 10 to 50wt.%. The results showed that the coefficient of thermal expansion lies between 48.78 and 59.76×10−7°C−1, and that the glass transition temperature started at 668.79°C and ranging between 668.79 and 632.34°C, and softening temperature (Ts) between 737 and 711.6°C. Bending strength and Vickers microhardness show a gradual decrease from 118 to 56MPa and 6120 to 4020MPa, respectively, with increasing cement dust content. Contrary to the mechanical performance, the density increases from 2.79 to 2.96g/cm3 by increasing cement dust content. The authors concluded that specimen with 90% basalt and 10% cement dust was the best and may be used for the industry of electronics covering. Table 2 shows a summary of properties of some basaltic and basaltic-based glass-ceramics comparing to a glass and a synthetic glass-ceramic.
Summary of properties of some basaltic and basaltic-based glass-ceramics comparing to a glass and synthetic glass-ceramic.
| Composition | Method | Heat treatment (temperature and time) | Main crystalline phases | Hardness | Density g/cm3 | Ref. |
|---|---|---|---|---|---|---|
| Glass | ||||||
| Borosilicate glass (SiO2–Al2O3–B2O3–Na2O) | – | – | – | 4.1 GPaa | 2.40 | [61] |
| Synthetic glass-ceramic | ||||||
| MACOR® – Machinable Glass-Ceramic (55% fluorophlogopite mica and 45% borosilicate glass) | – | – | – | 250kg/mm2a | 2.52 | [62] |
| Basaltic glass-ceramic | ||||||
| Basaltic rock from Serra Geral Formation, Serra Gaúcha, Brazil | Sinter-crystallization (air) | 1032°C, 0.5h | Magnetite, augite, albite, and quartz | 9.81 GPab | 2.39 | [39] |
| Basaltic rock from Serra Geral Formation, Serra Gaúcha, Brazil | Bulk crystallization (air) | 860°C, 1h | Magnetite and augite | 8.18 GPac | – | [57] |
| Basaltic rock from Northwest Harrat Rahat, Saudi Arabia | Bulk crystallization (air) | 1000°C, 2h | Augite, olivine, hematite, and magnetite | 7.04 GPad | 2.98 | [63] |
| Alkaline-olivine tuffs from Southern Anatolia, Turkey | Sinter-crystallization (air) | 1100°C, 1h | Pyroxene, plagioclases, olivine, spinel, and hematite | – | 2.66 | [37] |
| Basalt rock from the Vrelo, Serbia | Petrurgy (air) | 950°C, 3h | Pyroxene (diopside, hypersthene) | 7.2e | – | [41] |
| Basaltic-based glass-ceramic | ||||||
| Sinai basaltic rocks (70wt%) with by-pass cement dust (30wt%) | Bulk crystallization (air) | 1000°C, 2h | Diopside, anorthite, magnetite, and gehlenite | 4.8 GPad | 2.96 | [64]f |
| Basaltic rocks (80wt%) and ceramic (20wt%) waste | Bulk crystallization (air) | 900°C, 2h | Augite, magnetite, and olivine | 5.58 GPad | – | [65]f |
Basalt glass and glass-ceramic have numerous industrial applications, especially when abrasion and corrosion resistance is required [36]. Due to the possibility of obtaining materials with excellent properties from a low-cost raw material, basalt glass-ceramics are an alternative for replacing widely used traditional materials, such as metals, used for coating. In addition, due to their long-term chemical stability characteristics, they are excellent cast iron pipe coating materials used in the chemical industry to transport corrosive fluids [7,21]. The first glass-ceramics produced from basaltic rocks were used to encapsulate hazardous wastes due to their high chemical resistance [47,66].
Currently, in Asian and European countries, basaltic rocks have been widely used to produce various products, including fibers, fabrics, rods, yarns, and coatings. The Demech Company [67], located in Pune, India, has been working with basalt products since 1987. Its main products are coated pipes. According to them, basalt has excellent chemical resistance properties combined with high resistance to corrosion and abrasion. The processing route used by the company consists of selective extraction of the rock, subsequently melted at 1500°C and formed into molds or by centrifugation in cylindrical tubes. Finally, the parts undergo heat treatment cycles to promote basalt crystallization.
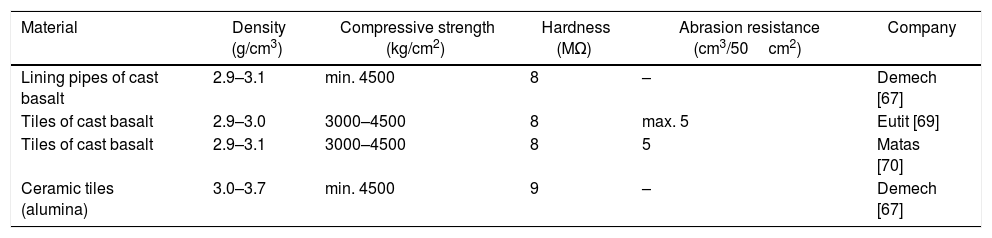
The company Kalenborn [68] produces cast basalt items used worldwide under the ABRESIST brand, offering reliable protection against frictional wear in components of the steel industry installations and pipelines in thermal power plants. According to the company, cast basalt products have a low friction coefficient and therefore offer excellent protection against abrasive wear. The Eutit company [69], located in the Czech Republic, produces basalt products in the form of tiles, sewage systems, and lining pipes. Table 3 shows some physical properties of commercial basalt glass-ceramics comparing to ceramic tiles.
Physical properties of commercial basalt glass-ceramics comparing to ceramic (alumina).
| Material | Density (g/cm3) | Compressive strength (kg/cm2) | Hardness (MΩ) | Abrasion resistance (cm3/50cm2) | Company |
|---|---|---|---|---|---|
| Lining pipes of cast basalt | 2.9–3.1 | min. 4500 | 8 | – | Demech [67] |
| Tiles of cast basalt | 2.9–3.0 | 3000–4500 | 8 | max. 5 | Eutit [69] |
| Tiles of cast basalt | 2.9–3.1 | 3000–4500 | 8 | 5 | Matas [70] |
| Ceramic tiles (alumina) | 3.0–3.7 | min. 4500 | 9 | – | Demech [67] |
More recent studies have pointed to the possibility of using basalt-based glass-ceramics for application as a sealant for fuel cells. The solid oxide fuel cells (SOFC) are devices able to convert chemical energy into electrical energy by redox reactions between a fuel and an oxidant. It is a “clean” way to produce energy [71]. Ercenk et al. [72], used a basalt-based composition to produce glass-ceramic as a sealant material for SOFC. They added SiO2, Na2O, CaO, MgO and B2O3 to the basalt. A sealant material should have thermal and mechanical properties to resist against harsh environmental effects at high operating temperatures. Important parameters for these materials are glass transition temperature (Tg) and glass softening temperature (Ts) that are used to determinate flow properties of glass. So that, without any damage to the interface at operating temperature, high Tg temperature and low Ts are needed. The results of the study showed that the basalt base glass-ceramic sealant material exhibited promising properties to use for SOFC. Softening temperature of the basalt decreased from 1184°C to 956°C thanks to the additives. However, it is not enough for sufficient bonding and wetting, probably. Therefore, complementary studies need to be carried out.
Another study showed the possibility of using basaltic glass-ceramic materials for application as insulators and semiconductors due to their dielectric properties. The results show that the basalt glass-ceramic can be used in low dielectric materials in the electronics covering industry [73].
Some studies have presented the possibility of using basalt glass-ceramics coated on steel to improve the resistance to wear, corrosion, oxidation, erosion, and heat of these materials. Basalt powder is deposited on the metal by the plasma spraying process, and then a heat treatment process is applied for crystallization. The studies showed satisfactory and promising results (see, for instance [74–76], and references therein).
The use of basaltic rocks for fiber production has also caused great technological interest because of its interesting properties. They are inorganic fibers with high mechanical strength, high-temperature resistance, excellent chemical stability, and easy processing, and it is not toxic, it is natural, ecological, and low cost [77,78]. They generally have an equal to or greater than that of fiber glass. Elasticity modulus (GPa) for some fibers: basalt fiber, 95–115; E glass fiber, 73–78; S glass fiber, 83; aramid fiber (1414), 124–130 and carbon fiber (T300), 230–240 [79].
For fiber production, the basaltic rock is melted at 1400°C. The melt is extruded under hydrostatic pressure through a platinum-rhodium mold to produce continuous basalt fibers. Cutting machines are used to cut the fibers to the desired length according to the application. The fiber dimensions are in the range of 10–20μm in diameter and 3–130mm in length. A major advantage of using basalt fibers is that it uses less energy and is cheaper to manufacture than carbon and glass fibers [77,80].
The main applications for basalt fibers are in the field of composite materials. Fibers are mainly used in civil engineering as reinforcement for concrete [77,78,80–83]. Also, basalt fibers are also used for the fabrication of lighter, high-quality hybrid composite materials (materials that combine two or more fiber types in the same matrix) for infrastructure applications [84]. One limitation for the use of basalt fibers in structural polymer matrix composites is due its tendency to oxidize and crystallize for temperatures higher than ∼300°C. These surface and microstructural modifications make the fiber more fragile, which limits its tensile strength [85].
Final commentsGlass-ceramic products obtained from rocks have a large market and are an excellent alternative to replace traditional materials. Basaltic rocks are characterized by high hardness, dark coloration, and fine grain. Due to its high chemical resistance and wear, basalt is used as a raw material in its natural form for the construction of sidewalks, pavements, and walls and is also largely used as a low-cost raw material to produce aggregates for civil construction.
However, through cycles of heat treatments and variations in chemical composition, basalt can be used for the production of glass-ceramics, with very varied microstructures with a wide range of properties, allowing the application of these materials in the most diverse areas, ranging from coatings and paving resistant to corrosion and wear to fibers and fabrics. In addition, more specific applications, such as the production of fuel cells, insulators, and semiconductors, are also being explored.
Thus, this review presented the possibility of transforming basalt, a natural, abundant, and low-cost raw material, into a glass-ceramic material, with high added value, excellent properties, and numerous applications.
Conflict of interestThe authors declare that they have no conflict of interest.
Authors gratefully acknowledge the financial support from the Brazilian Research Agency Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) (L.F. Lima, M.Sc. grant – 88887.177761/2018-00) and the Brazilian Government Agency Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) – grant 305528/2018-1.