The aim of this work was to prepare a foam glass (FG) decorated with nanostructured ZnO (FG/ZnO) and to evaluate its photocatalytic properties. The FG was sintered at 750°C using fluorescent lamp glass residues and white eggshell as the foaming agent (FA). The ZnO nanostructured particles were deposited on FG via microwave-assisted hydrothermal synthesis. To confirm the impregnation of the ZnO nanostructured particles onto the foam glass, images were obtained by scanning electron microscopy (SEM). The photodegradation of Rhodamine B (RB) synthetic dye was investigated over the FG/ZnO, and an interesting degradation rate of RB dye was found. The results obtained were satisfactory and demonstrate that the use of foam glass as a support for ZnO is a viable alternative and of great interest for photocatalysis, especially since it requires further treatments for ZnO powder removal.
El objetivo de este trabajo fue preparar el vidrio espumado con nanoestructurados ZnO (FG/ZnO) y evaluar sus propiedades fotocatalíticas. El vidrio de espuma (FG) se sinterizó a 750° C y utilizando residuos de vidrio de lámpara de fluorescencia (FLGR) y cáscara de huevo blanco como agente espumante (FA). Las partículas nanoestructuradas de ZnO se depositaron en FG mediante síntesis hidrotermal asistida por microondas. Para confirmar la impregnación de las partículas nanoestructuradas de ZnO sobre el vidrio de espuma, las imágenes se obtuvieron por microscopía electrónica de barrido (SEM). La fotodegradación de Rodamina B (RB). el colorante sintético se investigó sobre el FG/ZnO, que mostró una interesante tasa de degradación del colorante RB. Los resultados obtenidos fueron satisfactorios y demuestran que el uso de polvo de ZnO es una alternativa viable y de gran interés para la fotocatálisis, especialmente porque requiere tratamientos adicionales de eliminación de polvo de ZnO.
Brazil has one of the largest economies of its continent and, with its population of 207.7 million inhabitants, generates more than 208,000 tonne of urban solid waste per day [1]. In the 1191 municipalities of the three southern states of the country, 22,322 tonnes of waste is generated per day. Of these, 70.7% is destined for landfills, 18.3% for controlled landfills, and 11% for waste disposal areas. In the state of Rio Grande do Sul, 8643 tonnes of waste is generated per day [2]. Among the residues generated are those coming from fluorescent lamps, which can result in contamination of the environment with mercury [3]. This metal can remain bioavailable for several years in several environmental compartments and can cause various types of damage to living things [4]. Around 100 million fluorescent lamps are consumed annually in Brazil and 94% are disposed of in landfills without due precautions [5]. In this sense, it is of great interest to develop new technologies and/or new materials using these residues as raw material and thus minimizing the environmental impacts.
Glass waste can be used as aggregates for Portland cement and asphalt concrete [6] as well as in the production of foam glass (FG) [7], a porous material used as a thermal and acoustic insulator with thin walls of individual cells filled with gas phase [8,9]. In this way, fluorescent lamp residues can be attractive in the manufacture of FG, providing an application for this type of residue and mitigating negative environmental impacts.
Wastewater generated by industry is also of great environmental concern. Among the various industrial segments, the textile sector stands out. In the environmental scenario, it presents itself as a potential polluter due to its characteristics that are highly harmful to the environment and it is one of the largest consumers of industrial water in the world [10]. Its average consumption is 100L of water for 1kg of textile material; thus with a total processed quantity of 30 million tonnes of textiles per year, the estimated total consumption is 3000 million cubic metres of water per year [11]. In addition to the large-scale use of water, another notable characteristic of the textile industry is the large amount of dyes used, resulting in the generation of effluents with a high level of colour [12]. Approximately 100,000 types of commercial dyes are known and used in various applications, comprising an annual production of 700,000 tonnes. Of this total production, approximately 10,000 tonnes are destined for consumption by the textile industry. About 100 tonnes of dye per year is inappropriately discarded in aquatic courses by the textile industry [13].
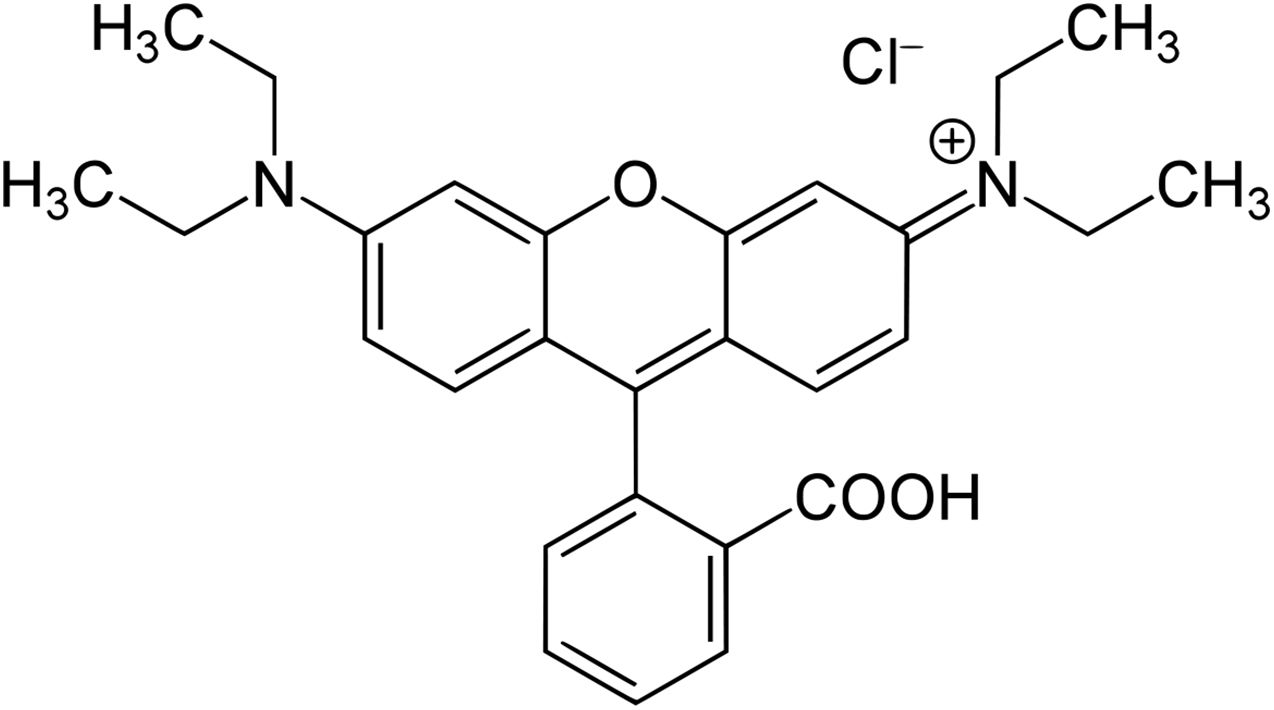
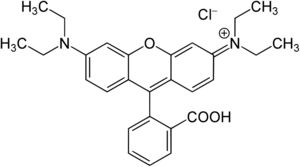
Among the different synthetic dyes, Rhodamine B (RB) stands out. This dye is recognized for its good stability and is widely used in the textile industry to dye silk, wool, leather, and cotton. Due to its widespread use, this dye is constantly found in water resources [14,15]. Some studies have reported that this organic compound may have carcinogenic and teratogenic effects on living organisms [16,17] when discarded incorrectly. The molecular structure of RB is presented in Fig. 1.
Several methods for the removal of dye from textile effluents have been suggested over time [17,18]. Photocatalysis, an advanced oxidation technique, is an attractive option for the treatment of textile effluents because it is an environmentally friendly method with in situ generation of hydroxyl radicals (OH), which are strongly oxidizing, and uses stable photocatalysts and non-toxic processes leading to complete degradation of contaminants from the textile industry [19–22]. This technique uses a photocatalyst that has the potential to transform recalcitrant organic contaminants into relatively harmless end products such as CO2 and H2O without generating secondary pollutants [23].
One of the most important metal oxides with a range of applications is zinc oxide (ZnO). ZnO is a band gap semiconductor in the range of 3.2eV and is widely used in dispersed form as a photocatalyst due to the low cost of obtaining it as well as its good catalytic properties [24]. However, use in the suspended form necessitates a separation step, which may be done through filtration processes [25], nanofiltration [26], microfiltration [27], or centrifugation [28]. It is worth mentioning that such processes are expensive and time consuming. In the last decades, the use of supports for the immobilization of photocatalysts has been gaining attention. Among the proposed supports are zeolites [29], polystyrene [30], silica [31], graphite carbon nitride [32], activated charcoal [33], and glass fibre [34].
The FGs are very attractive for application as photocatalyst supports, because their high porosity gives these materials a large contact area, allowing deposition of the substrate and thus increasing the photocatalytic effect. In this context, the objective of this work is to use FG produced by fluorescent lamp glass residues (FLGR) and eggshell as a substrate for the deposition of ZnO nanostructured particles and to evaluate the photodegradation efficiency of this system in the degradation of RB dye in aqueous solution.
Materials and methodsMaterial precursors and synthetic dyePreviously decontaminated FLGR, donated by Recilux (Canoas, RS, Brazil), and eggshell without the internal film, received from local businesses, were used for the production of FG. ZnO nanostructured particles were commercially available from Synth as the analytical standard.
The phase structure of the ZnO nanostructured particles was determined by X-ray diffraction (XRD, Bruker D2 PHASER diffractometer) equipped with a Cu anode (CuKα radiation, λ=1.5406Å) operating at 30kV and 10mA. Measurements were carried out over the 2θ range of 10–80° with a scanning step width of 0.05° and time of 1s. The crystallite size of the ZnO was determined by the X-ray line broadening method using the Scherer equation [35–37]. This measurement was carried out over the 2θ range of 33–39.5° with a scanning step width of 0.01° and time of 8s. X-ray profile of standard highly crystalline silicon was used to make instrumental broadening corrections. The full-width at half maximum (FWHM) and constant of proportionality (Scherrer constant) K=0.9 were used in the measurement [35–38].
The ZnO particles were also analyzed by scanning electron microscopy (SEM) in a JEOL microscope (model JSM-6610LV).
The optical characterization of ZnO nanostructured particles was performed by diffuse reflectance spectroscopy with the Kubelka-Munk remission function [35]. The optical band gap energy of the nanostructured ZnO was determined by Ocean Optics equipment (model DH-20000) equipped with an Ocean Optics integrating sphere (model ISP-REF). The band gap measurement of the semiconductors includes the excitation of electrons from the valance band to conduction band using photons of selected frequency [35].
The RB synthetic dye (C28H31ClN2O3, MW: 479.01gmol−1, CAS Number 81-88-9) was furnished by Synth (as analytical standard). The dye was used without further purification.
FG preparationFluorescent lamp glass and eggshell were ground separately in a ball mill and sieved in a 74μm (#200, ABNT) sieve. Afterwards, the formulation was made for the production of FG with 7% foaming agent (FA) by mass [39]. Eggshell was used as FA due to its ease of production and low environmental impact [39]. The prepared blend was moulded in a uniaxial press (Ribeiro, RP0003) under a compaction pressure of 40MPa. The green bodies were dried in an oven at 100°C for 24h and then sintered in an electric oven at 750°C at a heating rate of 150°Ch−1 for 30min [39]. The chemical compositions of FLGR and eggshell were determined by the X-ray fluorescence (XRF) technique using Shimadzu equipment (XRF 1800 model). The XRD technique was used to verify the crystal structure of eggshell and glass. A Bruker D2 PHASER X-ray diffractometer equipped with a copper anode operated at 30kV and 10mA was used. To evaluate the mass loss of the eggshell during heating, thermogravimetric analysis (TGA) was performed in a Harrop STA-726 thermobalance up to 1000° C at a heating rate of 10°Cmin−1.
Production of FG decorated with ZnOFor the deposition of the semiconductor onto FG, ZnO nanostructured particles were dispersed in deionized water at a concentration of 170mgL−1. Five FGs with an area of 3.8cm2 were used under magnetic stirring for 1h while submerged in the ZnO solution. After the stirring was completed, the FGs were located in a polytetrafluoroethylene reactor, sealed, placed in a microwave oven (Electrolux, MEF41, Brazil), and then heated to 160°C for 15min at a pressure of 0.49MPa [40]. The resulting material, FGs decorated with ZnO (FG/ZnO), was oven dried at 200°C for 90min. To confirm the decoration of the FGs with the ZnO nanostructured particles by microwave-assisted hydrothermal synthesis, images were obtained by a JEOL microscope (model JSM-6610LV).
Photodegradation testsFor the photodegradation tests, a 50mgL−1 solution of RB dye at pH=6 was produced. In addition, a photocatalytic reactor with a volumetric capacity of 200mL, made of transparent glass, was externally coated with PVC walls and a 160W (λ>380nm) mercury lamp was used as the source of light radiation. Three tests of the photodegradation of the RB in the solution were performed in triplicate, the first without the presence of any photocatalyst, the second using the ZnO in suspension (at a concentration of 170mgL−1), and the third using the previously prepared FG/ZnO. Two-millilitre aliquots of the RB dye solution were collected after 0, 5, 10, 20, 40, and 60min of contact with the abovementioned systems and their concentrations were determined by spectrophotometry using a UV-vis spectrophotometer (Molecular Devices, SpectraMax 190) at the maximum wavelength of the RB dye absorbance (554nm).
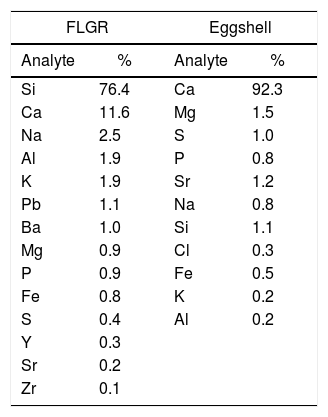
Results and discussionThe elementary chemical composition of the precursor materials used in the production of FGs is presented in Table 1. From this, it is possible to observe that the process used in the decontamination of FLGR was efficient, since there are no traces of Hg, and thus the residue can be used safely in the manufacture of ceramic bodies [41]. Further, it is possible to verify that its composition consists mainly of compounds based on silicon (Si), calcium (Ca), and sodium (Na), in addition to small concentrations of other trace elements that are present in the composition of the glass during its production.
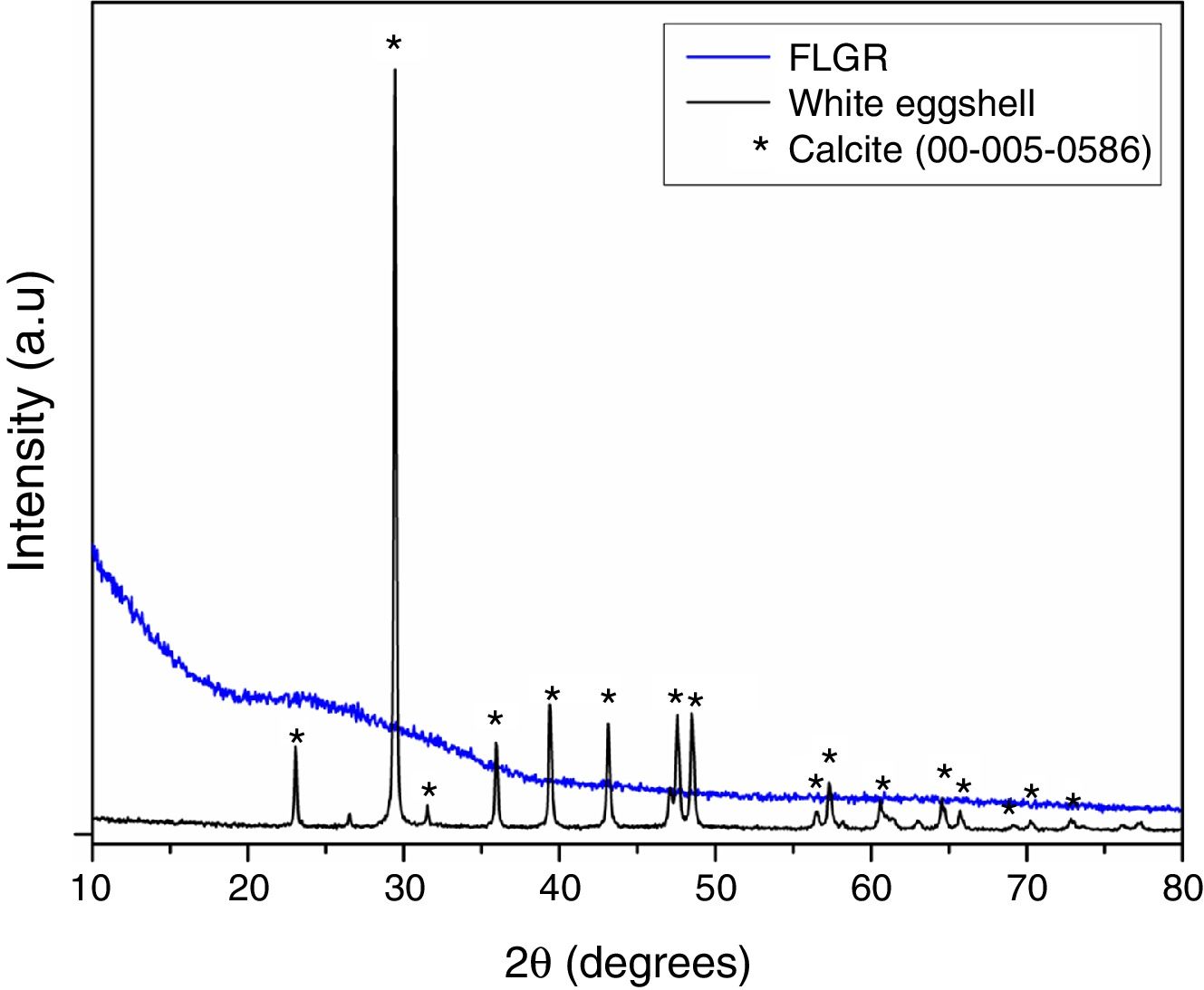
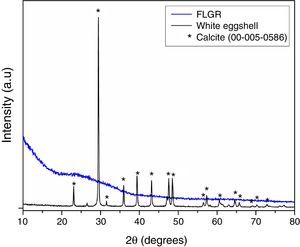
Fig. 2 shows the X-ray diffractograms of eggshell and FLGR. By means of the eggshell diffractogram, it is possible to observe maximum diffraction peaks at approximately 23°, 29°, 36°, 39°, 43°, 47°, and 48°, which are characteristic of calcium carbonate (calcite, CaCO3, JPDS Card 00-005-0586), with a rhombohedral crystalline structure. This result is in agreement with the result obtained in the X-ray fluorescence analysis, which presents Ca as the predominant element in the composition of the eggshells. The X-ray diffractogram of the fluorescent lamp residue sample has a broad band between 20° and 40°, which is typical of an amorphous material [42].
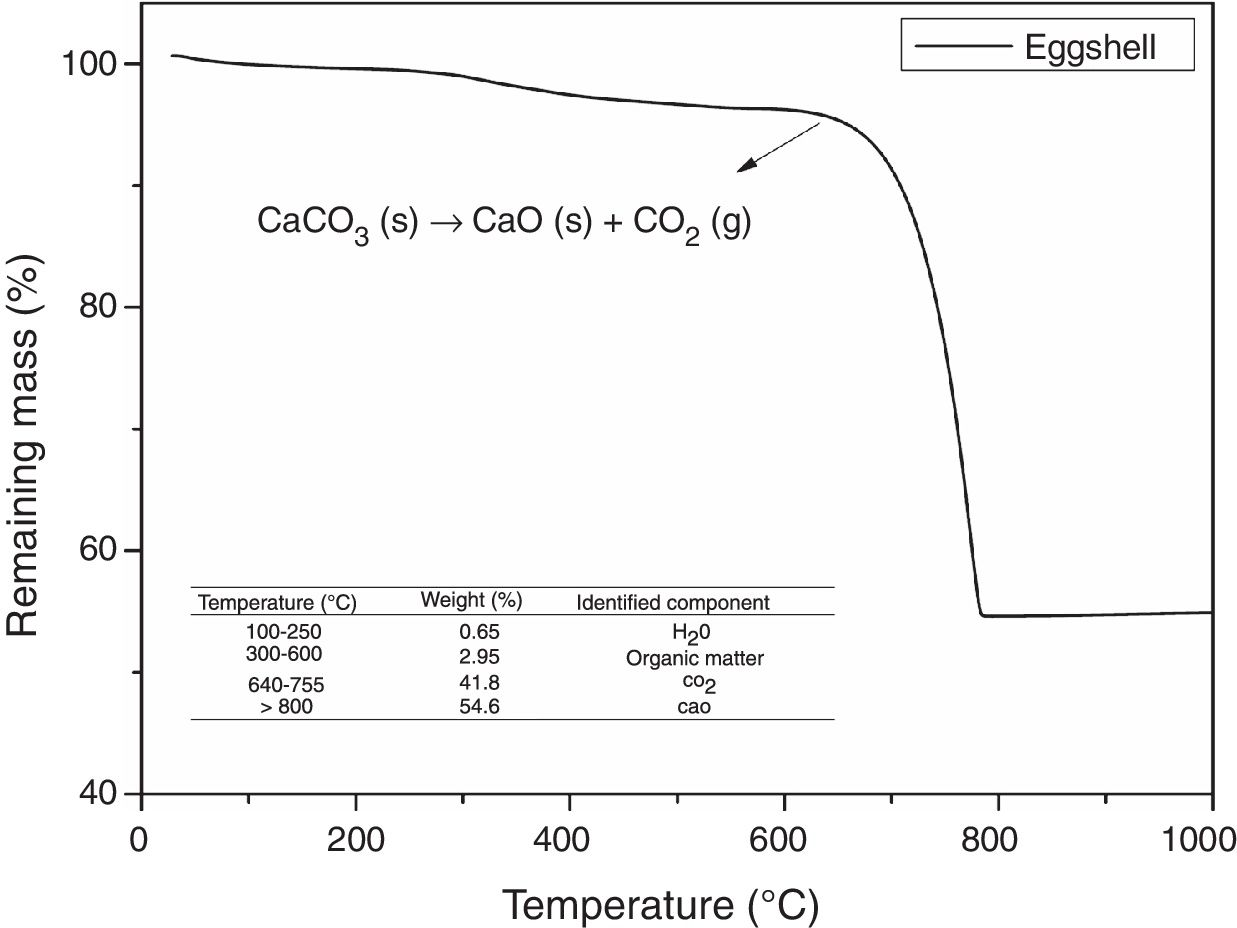
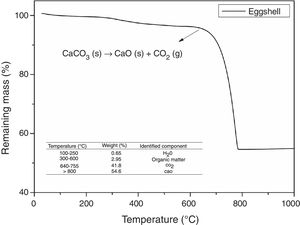
Fig. 3 shows the thermogravimetric analysis of the eggshell sample. By means of this, the presence of three zones of mass loss can be observed: the first around 250°C, attributed to the loss of the adsorbed water, the second at 300–600°C, related to the decomposition of organic matter, and the third between 640 and 755°C, attributed to the decomposition of CaCO3. For eggshell, above 800°C, predominantly CaO remains, as well as residues related to ash and inorganic compounds [43]. It is thus observed that eggshells at temperatures above 800°C release part of their mass in the form of gases, particularly CO2, which act as an FA in the molten glass. This indicates that the eggshell is a residue with potential application in the production of FGs.
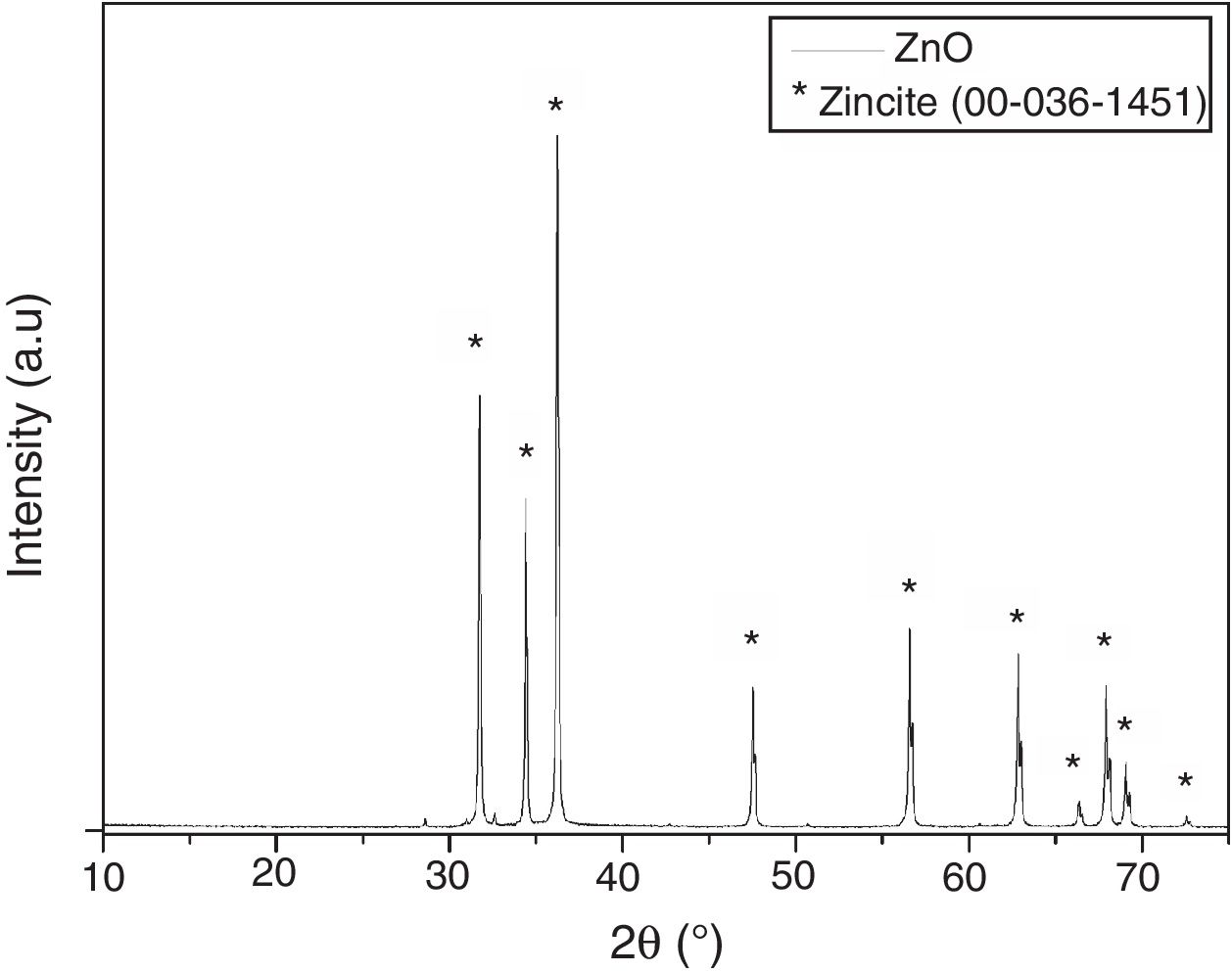
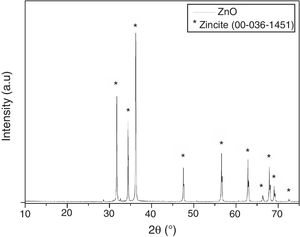
Fig. 4 shows the X-ray diffractogram of ZnO nanostructured particles. It is possible to observe the presence of diffraction patterns characteristic of the zincite crystalline phase (JPDS Card 00-036-1451), which has a hexagonal crystalline system. Furthermore, through the Scherrer equation [35–38], it is possible to verify that the crystallite size of ZnO is approximately 75nm. The crystallite size was estimated with the (101) diffraction peak (around 36.3°). This plane was chosen because it is the most prominent and well-marked [35–38].
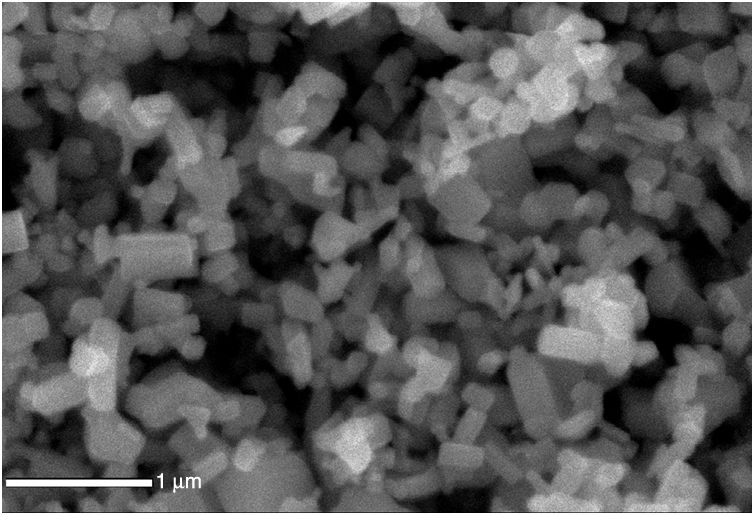
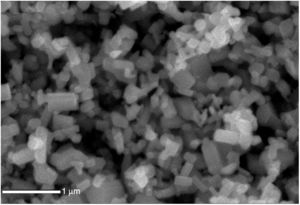
The SEM image of the ZnO nanostructured particles (Fig. 5) shows the particles with a polyhedral shape, with dimensions greater than 100nm.
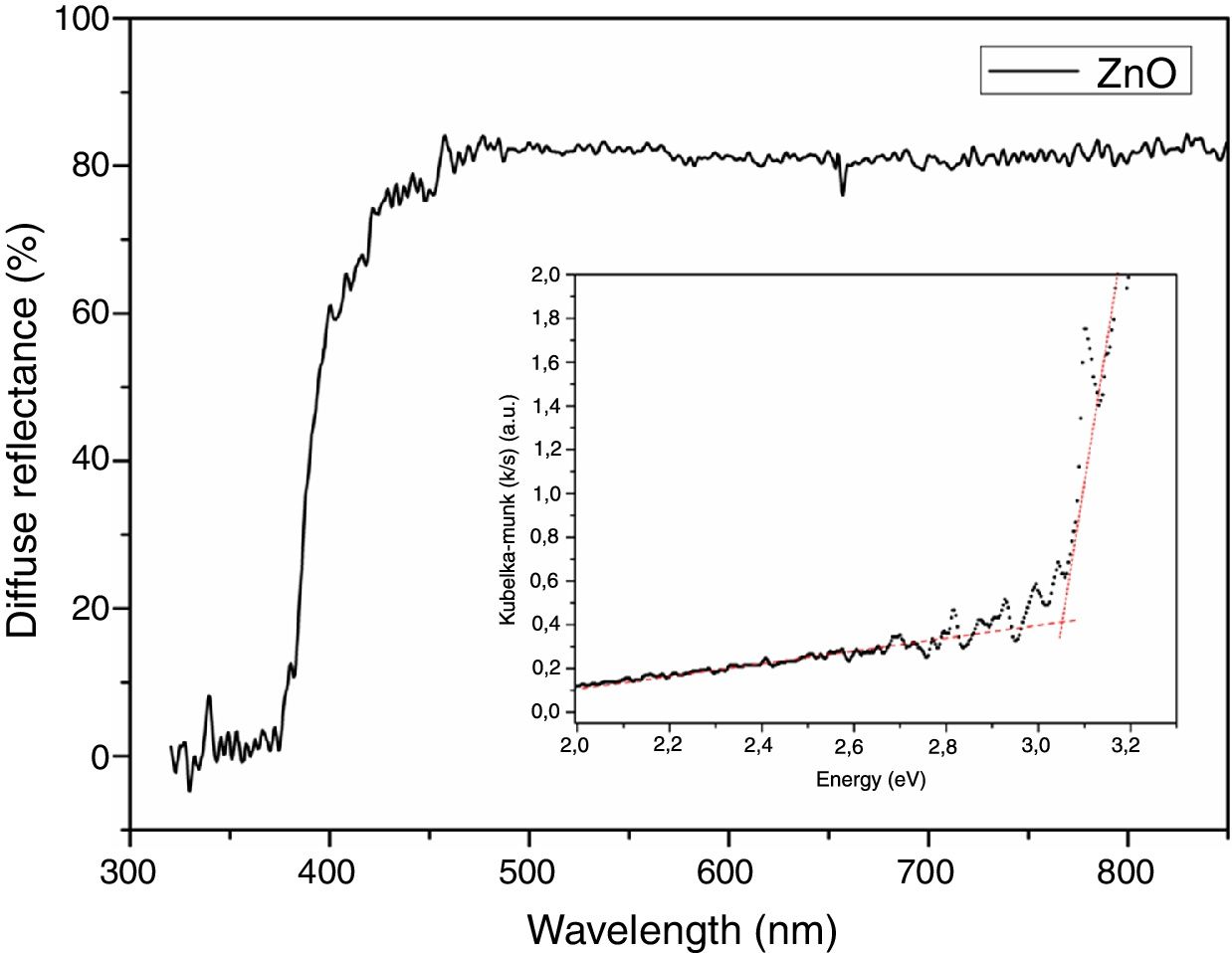
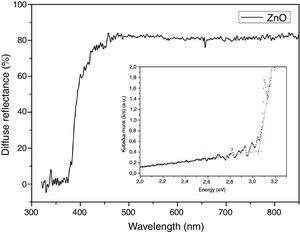
Diffuse reflectance spectroscopy was utilized to determine the band gap of the ZnO nanostructured particles and to confirm the range of UV–vis radiation absorption of oxide. Fig. 6 shows the diffuse reflectance and Kubelka-Munk plot (in detail). It could be possible to check a similar performance of the absorbance–reflectance of the semiconductor oxides: the ZnO is transparent at 400–700nm (visible region) [24,35]. In addition, at about 390nm, a sharp increase in the absorbance is observed, which is attributed to the absorption edge. The band gap energy of the oxide could also be obtained based on the onset of the Kubelka-Munk plot (shown in detail in Fig. 6) [35]. The optical band gap of ZnO particles calculated from the Kubelka-Munk remission function is 3.18eV. This result agrees with those found in the literature [24,35].
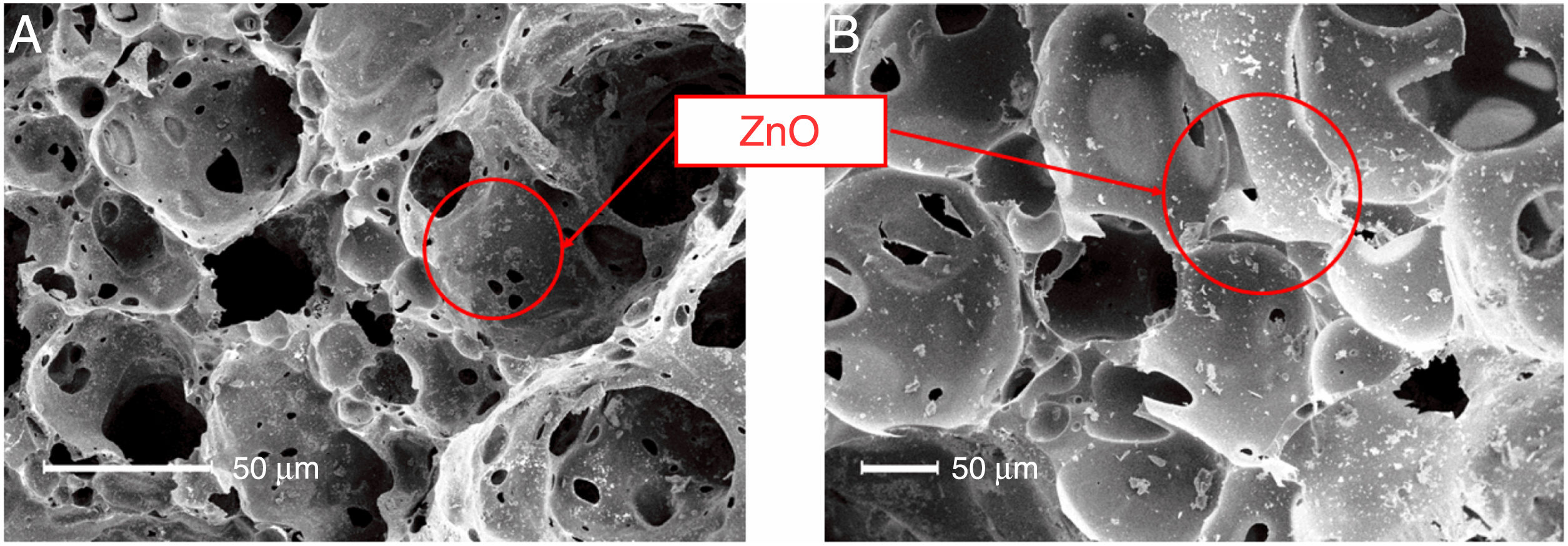
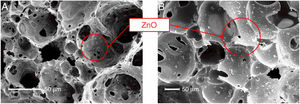
SEM micrographs (Fig. 7) confirmed the presence of the ZnO powders on the surface (Fig. 7A) and inside (Fig. 7B) the FGs. As can be seen, the ZnO powders (white particles) decorated the entire porous surface of the sample (homogeneous recoating) and penetrated the internal pores of the FG structure. This result demonstrates that the methodology adopted here using microwaves as a decoration method was efficient for the production of the FG/ZnO system.
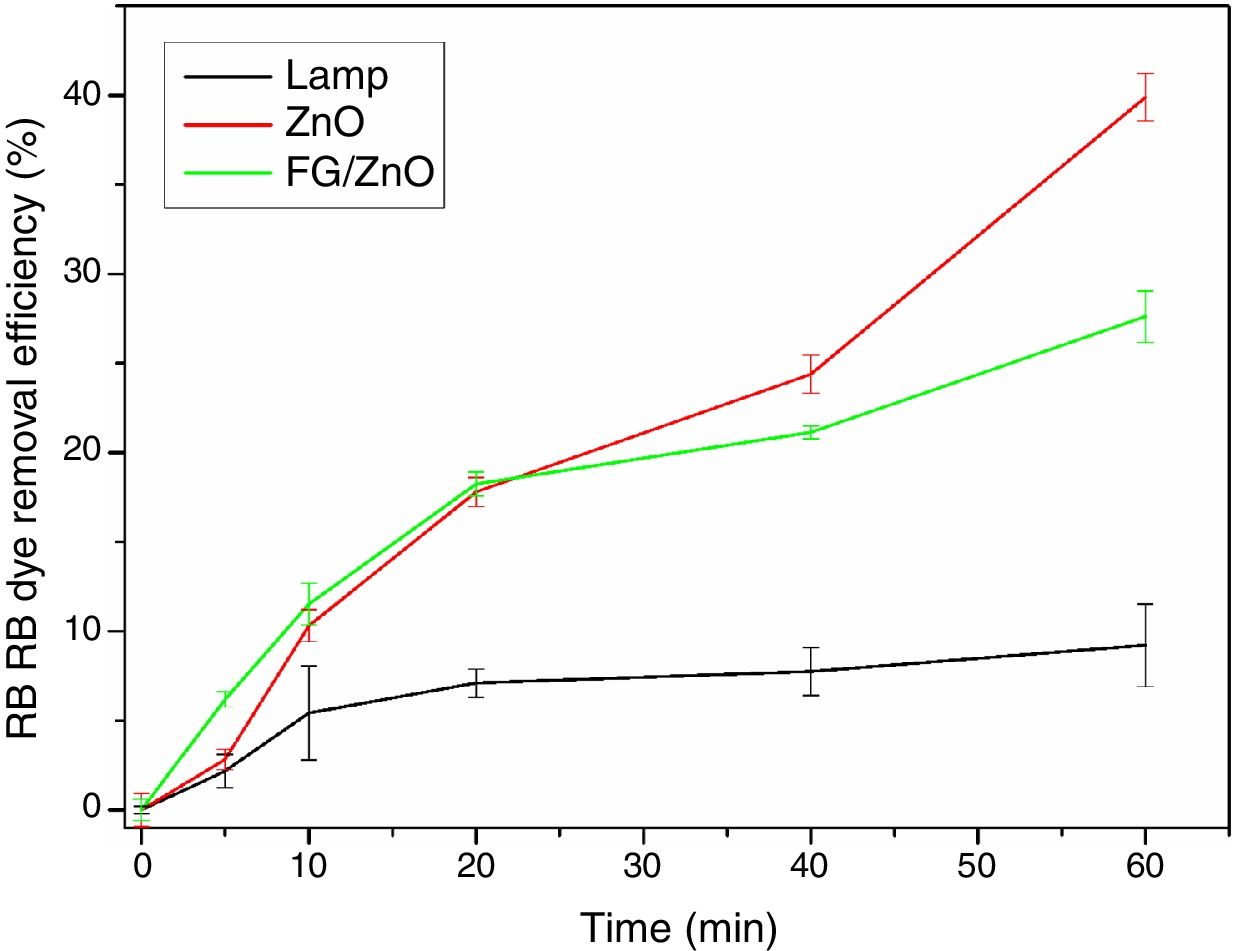
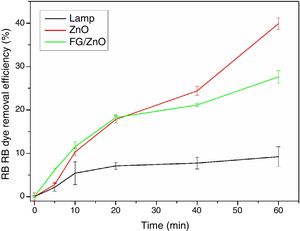
The results of photodegradation of the RB dye are shown in Fig. 8. This result shows that ZnO in suspension presented higher photocatalysis of RB dye, reaching a photodegradation rate of 39.9%, followed by the FG/ZnO system, which showed a degradation efficiency of RB dye of 27.6%. The lamp without the photocatalyst had a degradation efficiency of 9.2%. It is observed, therefore, that the suspended ZnO showed a degradation efficiency higher than that presented by the FG/ZnO system, as has already been reported in the literature [44]. However, for practical purposes, the use of particulate matter is problematic due to the additional step of filtration which must be added to the process, thus generating higher costs for its use. Thus, the FG/ZnO system is attractive because it presents similar photocatalytic activity for the studied system and also because it does not require an additional stage of filtration to remove the photocatalytic agent.
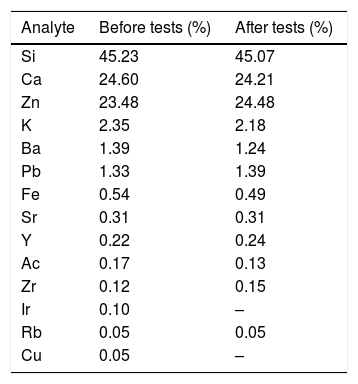
In order to verify whether there was loss of ZnO to the solution after the photocatalytic tests, FG/ZnO samples were analyzed by XRF before and after the photocatalysis tests. Table 2 shows the presence of glass-forming elements [Si, Ca, and potassium (K), among others], Ca (present in eggshell), and zinc (from the decoration with ZnO). Other elements were detected in smaller quantities and are traces that are present in the composition of the glass during its production.
Results of XRF for the FG/ZnO system before and after the photocatalysis.
| Analyte | Before tests (%) | After tests (%) |
|---|---|---|
| Si | 45.23 | 45.07 |
| Ca | 24.60 | 24.21 |
| Zn | 23.48 | 24.48 |
| K | 2.35 | 2.18 |
| Ba | 1.39 | 1.24 |
| Pb | 1.33 | 1.39 |
| Fe | 0.54 | 0.49 |
| Sr | 0.31 | 0.31 |
| Y | 0.22 | 0.24 |
| Ac | 0.17 | 0.13 |
| Zr | 0.12 | 0.15 |
| Ir | 0.10 | – |
| Rb | 0.05 | 0.05 |
| Cu | 0.05 | – |
It is possible to observe that the percentage of Zn remained practically the same after the application of the FG decorated in the photocatalytic tests (variation within the error range of the detector of the equipment). This indicates that the decoration process was successfully performed and eliminates concern about possible contamination of ZnO in the aqueous medium after the application of FGs for the treatment of aqueous effluents. Thus, from this result, we can conclude that the FG/ZnO constitutes a single step in the photocatalysis process, requiring no further filtration, because the adhered ZnO remains in the FG structure after the treatment.
ConclusionsThe results show that it was possible to produce FG decorated with ZnO nanostructured powder with photocatalytic properties using a microwave-assisted route. The micrographs obtained by SEM show that the decoration of ZnO powder under the support material was superficial and internal. The results obtained in degradation of the RB dye using FG/ZnO were satisfactory by the proximity to the results of nanostructured ZnO suspension, since by making use of a support for the semiconductor it is possible to eliminate the filtration step in the process. It was also possible to conclude that the material adhered completely to the FG, thus avoiding the need for a second process to separate the ZnO from the solution. Making use of recyclable waste to manufacture the carrier and eliminating the filtration step in heterogeneous photoprocesses eliminates additional costs while minimizing the environmental impacts generated by the incorrect disposal of such wastes. This makes the application of FG/ZnO in dye treatment systems in solution very attractive.
The authors thank the Coordination of Improvement of Higher Level Personnel – CAPES for the financial support to this project, the Recilux Company for the donation of the glass residues used in this work and the Laboratory of Ceramic Materials – LACER of the Federal University of Rio Grande do Sul/RS – UFRGS for the support in the analysis. We are also grateful to Centro de Microscopia Eletrônica da Zona Sul (CEME SUL – FURG) for the use of the SEM microscope.