Four organic solid wastes (coffee grounds, ground nut shell, paper money sewage sludge and cork powder) have been investigated as bloating inducing agents in lightweight aggregate (LWA) manufacturing when mixed in small proportions (2.5wt.%) with three types of clays. The pellets obtained were fired in a rotary kiln at the maximum feasible temperature for 4min. A similar impact was observed both in the working conditions and in the properties of the LWAs, showing that the differences between said residues are not as decisive as those of the clays to which they are added. The organic wastes have played a crucial role in achieving reducing conditions inside the aggregate. Thus, their addition has led to substantial improvements both from an operational point of view, lowering the working temperature, and from a technological perspective, favoring bloating and the development of a highly porous and lightweight structure. Leachate studies have shown that the LWAs meet the regulatory limits. The outcomes of this investigation show that, in line with the precepts of the Circular Economy, the recovery of organic wastes of different nature can have a place in the production of environmentally friendly LWAs.
Se han investigado cuatro residuos sólidos orgánicos (posos de café, cáscara molida de frutos secos, lodos de depuradora de papel moneda y polvo de corcho) como agentes expansivos en la fabricación de áridos ligeros (LWAs) al mezclarse en pequeñas proporciones (2,5wt.%) con tres tipos de arcillas. Los pellets conformados se han cocido en un horno rotatorio a la máxima temperatura viable durante 4min. Se observó un impacto similar tanto en las condiciones de trabajo como en las propiedades de los LWAs, mostrando que las diferencias entre dichos residuos no son tan influyentes como las de las arcillas a las que se añaden. El componente orgánico ha jugado un papel crucial en la consecución de las condiciones reductoras dentro del árido. Así, su adición ha supuesto mejoras sustanciales tanto desde el punto de vista operativo, reduciendo la temperatura de trabajo, como desde una perspectiva tecnológica, favoreciendo la expansión y el desarrollo de una estructura altamente porosa y ligera. Por otro lado, los LWAs obtenidos cumplirían con los límites reglamentarios de lixiviación de metales pesados. Se demuestra que la valorización de residuos orgánicos de distinta naturaleza puede tener cabida en la producción de LWAs respetuosos con el medio ambiente.
Global environmental challenges are leading to the development of different political, economic and social strategies to reduce the negative environmental impact associated with anthropogenic activity. An example of these initiatives is the Directive 2008/98/EC [1] on waste, which includes among its objectives a transversal transformation toward a recycling society in which the concept of end-of-waste status is integrated. According to this new production paradigm, based on the principles of the Circular Economy, the milestone to be reached is the so-called zero waste, i.e., that production and consumption processes are optimized, so that waste is no longer considered as such, but is transformed into a potential raw material.
It is therefore essential to equalize as far as possible the waste input and output flows, so that the integration of such waste into products produced in large volumes could be an intelligent strategy. In this regard, for years, the manufacture of artificial lightweight aggregates (LWAs) seems to be an interesting alternative for three reasons: (i) it is a product that can be produced totally or partially from waste [2–4]; (ii) it is a product that can be applied in large volumes and in different sectors, such as agriculture, environment, urban planning, civil engineering and construction, highlighting fundamentally its application in lightweight concrete and masonry [5]; and (iii) LWA presents technological properties in terms of high porosity, lightness and low thermal conductivity, which make it, among other aspects, a potentially strategic material in the construction of the future [6], facilitating better energy efficiency of housing without sacrificing mechanical integrity, among other advantages. With these points in mind, the present work presents as a novelty the investigation of four widely spread organic wastes, of which there are no precedents or, if there are, they are very few in their application as additives in LWAs: coffee grounds, ground nut shell from almonds and hazelnuts, paper money sewage sludge and cork powder. The following are some essential aspects of each of these wastes:
Coffee is one of the most consumed products in the world, which implies that coffee producing companies generate more than 2000 million tons of waste and by-products per year worldwide [7]. Depending on whether coffee is processed wet or dry, four types of waste can be distinguished: pulp, husk, silver skin and coffee grounds. Coffee grounds are the sediment that remains as a residue after coffee brewing, and are the waste generated in the greatest quantity. Thus, for each ton of coffee, about 650kg of grounds are generated [8]. A great deal of research has been done in order to use these residues for the production of value-added compounds, such as pellets and garden fertilizers, as well as alternative fuels for energy generation, such as ethanol and biogas [9,10]. However, at present, much of these by-products are not reused and end up in landfills, resulting in high environmental pollution due to their high content of tannins and caffeine, chlorogenic acid and phenols [11,12].
In response to this, a large number of papers have been published that seek to give a second life to coffee waste. In the field of construction and aggregates, it has been shown that spent coffee grounds can be directly applied as fine-grained aggregate in concrete formulations [13]. There are also publications that have applied coffee wastes to obtain geopolymers [14] and sintered ceramics [15]. However, its use as a pore forming agent in LWAs by thermal treatment has hardly been addressed. Andreola et al. [16] carried out substitutions of red clay by spent coffee grounds in the range of 10, 15 and 20wt.% together with other additives to improve the properties of the final product. They obtained aggregates with good water absorption and nutrient release capacity, such as P and K, which could make them attractive for use in agriculture. However, there is no evidence that the aggregates expanded, and if they did, what was the effect of the addition of the coffee residues, especially considering that the red clay Andreola et al. [16] used already presented an outstanding self-expansion capacity without the need for organic additives [17]. In a recent publication by Bayoussef et al. [18], the authors used both coffee grounds and sawdust as pore-forming agents in a red clay. The percentages used ranged from 5 to 30wt.% in 5wt.% steps. The shaped pellets were fired at a relatively low temperature (1100°C) for 1h with a heating ramp of 2°C/min, which is an unconventional firing system in LWAs, where thermal shock and short firing times are usually applied. Although the addition of the two residues favored a certain decrease in bulk density (more with the sawdust than with the coffee residue), it was seen that this was due to the development of interconnected porosity (increased water absorption). This fact presupposes that volumetric expansion did not really occur, since it is known that the bloating phenomenon is associated with the formation of closed porosity to the detriment of open porosity [19].
According to the International Nut Council (INC) [20], during the 2018/2019 season the global almond crop increased by 20% over the previous 10-year average, reaching more than 1.25 million tons. The United States accounts for 80% of the world share, followed by Australia with 6% and Spain with 5% [20,21]. As for hazelnuts, the total crop for the 2018/2019 season was approximately 460,000 tons. Turkey led the world production with 63% of the share, followed by Italy with 13% [20]. Consequently, the nut industry generates a large amount of waste and by-products, mainly in the form of shells and skins (e.g., almond shells account for 35–75% of the total weight of the fruit). Although these wastes are not hazardous, the large volumes in which they are generated can make them interesting as raw material in different industrial processes. Some examples of shell recycling include milling and subsequent use as an excipient in pharmaceutical laboratories, biomass, flours as well as a base for soaps [22,23]. The review by Jannat et al. [24] shows that nut shell has been used without heat treatment as an aggregate in mortar and concrete by many researchers, or as an additive in traditional ceramics. However, there is no literature evidence of its application as a bloating agent in LWAs.
As far as paper sewage sludge is concerned, it comes from the cotton linter used in the manufacture of paper money (also called ‘banknote’ or ‘bill’), so it is a cellulose-rich waste. The paper money manufacturing figures are overwhelming, 1.45 million tons of waste from an estimated 7.5 million tons of manufactured paper, of which only 5.5 million tons are recycled into paper [25]. The waste is treated with chemical products during production, which, together with the high amount of organic matter it contains, makes it necessary to propose viable strategies for its valorization. The current destination of this waste is the landfill, with all the harmful implications this has for the environment. As was the case with the rest of the previous wastes, there are hardly any published studies showing the use of wastes similar to the one presented here. As shown by Dondi et al. [2] in their review, Pinto et al. [26] used cellulose-rich waste to manufacture lightweight aggregates, in which they observed that the quantities to be used have to be moderate. Therefore, more research is needed in this regard.
Finally, the industry producing cork stoppers, agglomerates and cork-based thermal and acoustic insulation products generates significant volumes of waste in the form of powder [27]. According to cork production data from southwestern Europe, annual production is estimated at 185,000 tons in Portugal alone, a country that represents 50% of the global industry. Of this amount, 40,000 tons are considered a residual product that, due to its reduced particle size, is insufficient for the manufacture of agglomerates, and is destined for energy production by burning [28]. The use of cork waste as an aggregate in the construction sector has very little presence in the literature. Juan Valdés et al. [29] used cork dust residues as an additive in concrete formulations, while Pereira de Oliveira et al. [30] used cork granules as a replacement aggregate in mortars. However, there are no studies in which cork residue is used to manufacture LWAs by firing.
There are several works in which other types of carbonaceous residues have been used as pore generating agents during sintering. Chiang et al. [31] used 0, 10, 15 and 20wt.% rice husk in the manufacture of lightweight bricks. High residue ratios favored an increase in open porosity and a decrease in bulk density. However, increasing temperatures worsened these properties, so that in no case was it observed that increasing high proportions of organic residue led to expansion of the sintered material. This is in line with the findings presented by Moreno-Maroto et al. [3,4] using powdered carbon fiber and ground olive pomace wastes, respectively. The authors showed that if the intended goal is bloating (phenomenon associated to the development of closed pores swollen by the gas released during the thermal decomposition of the starting material), the addition of percentages above 5wt.% of carbonaceous residue (particularly 10wt.%) prevents expansion. Conversely, low percentages of organic residue can favor bloating, with 2.5wt.% of organic residue being an adequate proportion in most cases.
Considering all of the above, previous experimental results through different investigations show that the manufacture of lightweight aggregates using organic wastes can be carried out using two fundamental methodologies, which of course may be subject to certain variations:
- 1)
Slow firing at relatively low temperatures, with high proportions of organic residue (generally above 10wt.%): the development of open and interconnected porosity is favored by combustion of the organic matter, but bloating is not produced, nor is the density obtained excessively low.
- 2)
Fast firing at high temperatures, with low proportions of organic residue (generally between 2–5wt.%): the development of a shell and a core is favored, as well as the formation of closed porosity with a low degree of interconnection due to the trapping of part of the gases generated. The density obtained is low or very low in many cases.
Considering these two mechanisms, the second one (bloating) is the archetypal of LWAs and is therefore the one on which the present study will focus, especially considering that there is hardly any information on the wastes used in this work. In addition, the investigation presented here would aim not only to study the potential recycling of these wastes to manufacture lightweight aggregates, but also to understand to what degree the differences between these wastes may affect the production process of these materials and their subsequent technological properties. To this end, it will be essential to evaluate their suitability as bloating agents using raw materials with no or very low self-expansion capacity (in this case, three selected ceramic clays).
Materials and methodsSampling, preparation and characterization of raw materialsThree clays were used as the main component of the mixtures: Spanish yellow clay (SYC), supplied by Comercial Cerámicas de Bailén (Bailén, Spain); Portuguese yellow clay (PYC), from Silveirinha (northwest of Pombal, Portugal); and Portuguese red clay (PRC), from Albergaria-dos-Doze (southeast of Pombal, Portugal). The two Portuguese clays were provided by Corbário Minerais Industriais, S.A. The clays were dried at 105°C and ground to below 200μm with a Restch® SK 100/C Spezialstahl arm mill.
With respect to the organic components to be incorporated as additives in the mixtures, four solid wastes were studied: coffee grounds (CfG), ground nut shell (NuS), paper money sewage sludge (PpS) and cork powder (CkP). These were supplied by Delta Cafés (Campo Maior, Portugal), Unió Corporació Alimentària (Reus, Spain), Cotton South, S.L. (Fonelas, Spain) and Eurotapón Nuñez, S.L. (San Vicente de Alcántara, Spain), respectively. In general, these wastes presented a particle size suitable for direct application in the mixture (in powder form), but PpS, which required grinding. CfG and PpS also required oven-drying. Since these types of ceramic clays and wastes, or other similar ones, can be found in basically all countries, the results obtained from this research could be potentially “extrapolated” to other raw materials of similar characteristics.
According to the experimental protocols followed by the authors in previous publications [4,17,32–34], a complete characterization of the raw materials was carried out, determining the following parameters: particle size distribution; plasticity (liquid limit, LL; plastic limit, PL; plasticity index, PI; classification); optimum moisture content for extrusion and pelletizing (WOP); mineralogy; carbon content (total, TC; organic, OC; and inorganic, IC); chemical composition; loss on ignition (LOI); thermal behavior; and gross and net calorific values for the organic wastes (GCV and NCV, respectively). The specific methods for determining each of these parameters are shown in Table 1.
Methods and equipment used in the characterization of the raw materials.
| Parameter | Method and equipment (if applicable) |
|---|---|
| Particle size distribution | Laser Diffraction – Coulter® LS™ 230 (clays, CfG, NuS); Sieving (PpS, CkP) |
| Plasticity: liquid limit (LL) | Casagrande cup [32] |
| Plasticity: plastic limit (PL) | Thread bending method [33] |
| Plasticity: plasticity index (PI) | PI=LL-PL [32] |
| Classification | Plasticity chart [33] |
| Optimal moisture content (WOP); % | WOP=PL×1.234 [3,33] |
| Mineralogy | X-Ray Diffraction (XRD)/PANalytical® X’Pert Pro MRD diffractometer [17] |
| Total, organic and inorganic carbon (TC, OC and IC); % | Shimadzu ®TOC-VCSH analyzer |
| Carbonates; % | Carbonates (%)=inorganic carbon/0.12 [17] |
| Chemical composition, % | X-ray fluorescence/Thermo ARL ADVANT’XP Sequential XRF |
| Loss on ignition (LOI); % | 1050°C for 2h in a muffle |
| Differential Scanning Calorimetry-Thermogravimetric Analysis (DSC-TG) | Mettler Toledo® TGA/DSC 1 Thermal Analyzer equipment – Pfeiffer Vacuum ThermoStar TM GSD 320 mass spectrometer |
| Gross and net calorific values (GCV and NCV, respectively) | EN 14918 [34] |
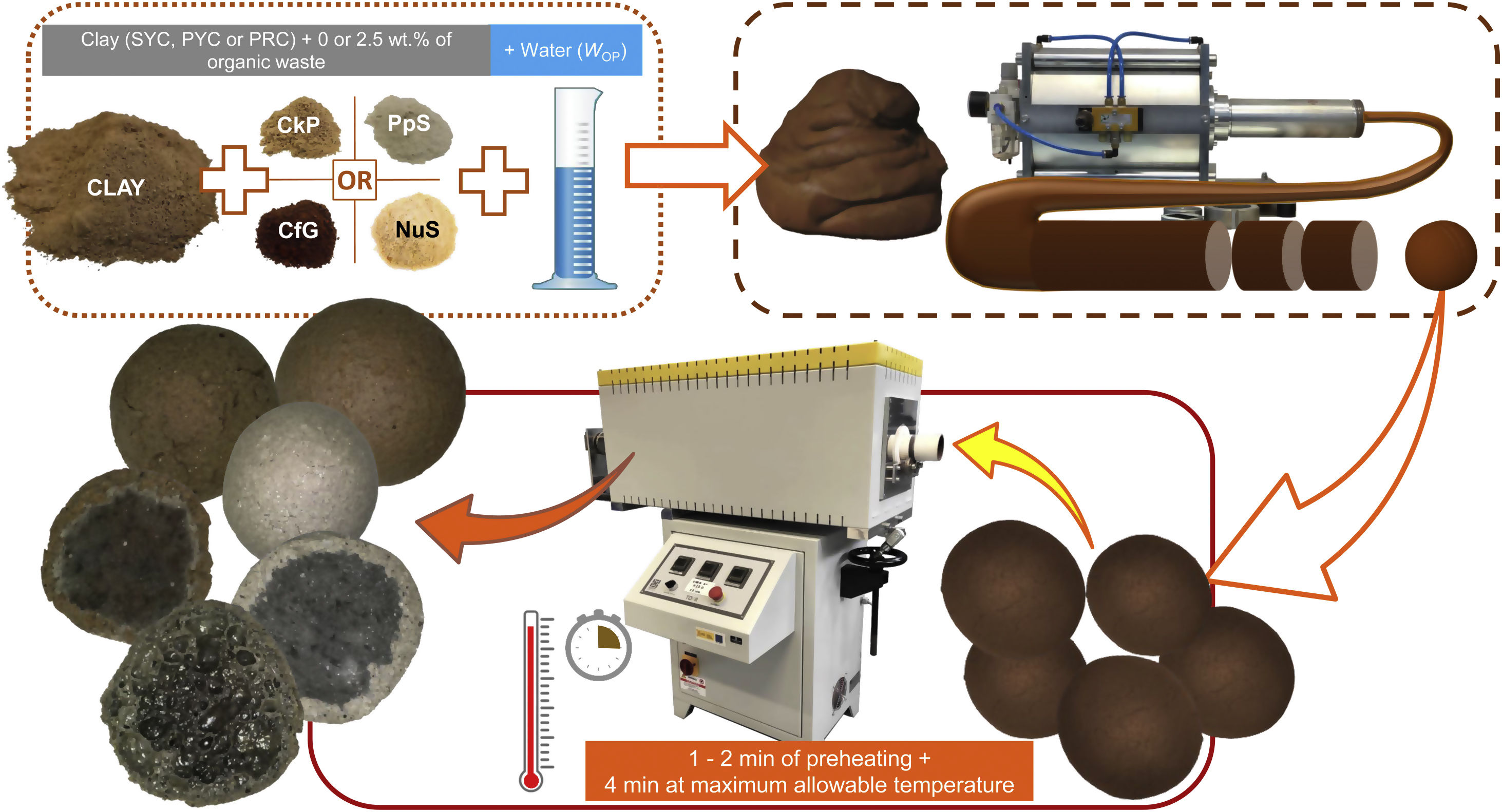
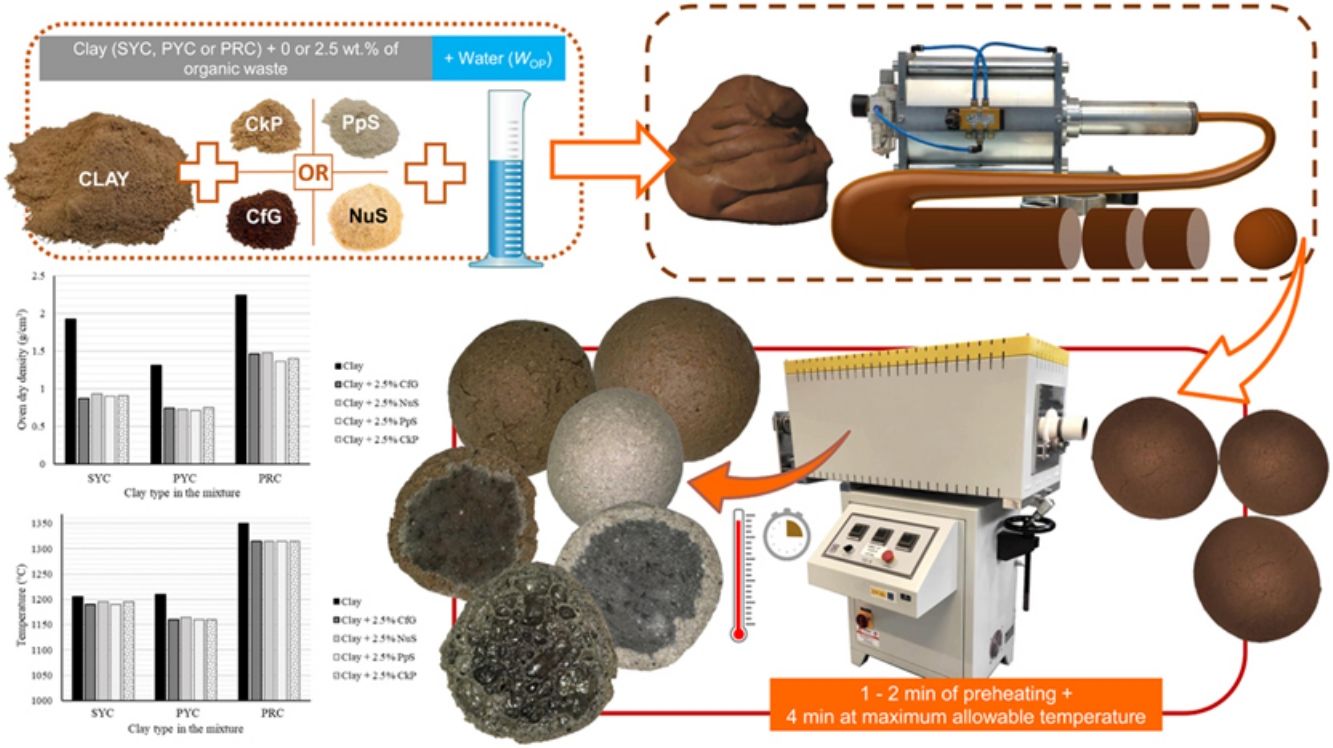
The LWA manufacturing process was carried out following the same protocol published by the authors in previous studies [4,17], which is schematically presented in Fig. 1, and can be summarized as follows:
Mixtures of clay and organic residue were prepared, the latter at a weight percentage of 2.5%. This proportion of organic additive was considered adequate considering the results published in previous works using carbon-rich wastes [3,4], because it was demonstrated that lower or higher percentages could impede bloating, as explained in more detail in the Introduction. A clay-waste dough was prepared with the moisture content corresponding to WOP, which, after 24h under sealed conditions, was extruded through a Nannetti® pneumatic extruder. The extruded material was cut and shaped into spherical pellets of approximately 10.2mm in diameter, which were dried at 105°C, after having previously been left for 24h at room temperature. The pellets were fired for 4min+1 or 2min of preheating (necessary to avoid bursting) in a Nannetti® TOR-R 120–14 rotary tubular kiln. The rotation speed of the alumina kiln tube was set at 2.5rpm. The temperature applied was the maximum one allowed for the pellets (above it they adhere to each other and to the kiln tube), in order to observe the maximum bloating that the original granular material can undergo when transformed into LWA.
Once the LWAs were manufactured, their characterization covered the study of the following technological properties: loss on ignition during the preheating (LOIpreh) and firing (LOIfiring) stages in the rotary kiln; volumetric change experienced during firing (bloating index; BI); density (loose bulk density, ρb; apparent density, ρa; and oven dry density, ρrd); porosity (total, PT; open, PO; and closed, PC, porosity); water absorption after 24h of soaking (WA24); mechanical strength (single aggregate crushing strength, S); and microstructure study. The methods [3,17,19,35–40] applied for each of these parameters are shown in Table 2. The results obtained for the LWAs manufactured with the organic wastes have been compared with those obtained without such additives in Moreno-Maroto et al. [17].
Methods and equipment used in the characterization of the lightweight aggregates obtained in this investigation.
| Parameter | Method and equipment (if applicable) |
|---|---|
| Loss on ignition (LOI) during preheating and firing; % | Weight variation before and after firing 25 specimens in a Nannetti® TOR-R 120-14 tubular rotary kiln |
| Bloating index (BI); % | Size variation before and after firing 25 specimens [35] in a Nannetti® TOR-R 120-14 tubular rotary kiln |
| Loose bulk density (ρb); g/cm3 | EN-1097-3 [36] |
| Apparent density (ρa); g/cm3 | Water pycnometry [37] |
| Oven dry density (ρrd); g/cm3 | Water pycnometry [37] |
| 24h Water absorption (WA24); % | Water pycnometry [37] |
| Porosity: Total (PT) open (PO) and closed (PC) porosity; % | PT=(1−(ρrd/2.6))×100; PO=(1−(ρrd/ρa))×100; PC=PT−PO[19,38] |
| Single aggregate crushing strength (S); MPa | Average value of 25 specimens/Nannetti®FM 96 press [3,17,39] |
| Microstructure | Scanning Electron Microscopy/Carl Zeiss Merlin™ Field Emission Scanning Electron Microscope (FE-SEM) with EDX |
| Leachate heavy metal concentrations: Cr, Ni, Cu, Zn, As, Se, Ag, Cd, Pb | Toxicity Characteristic Leaching Procedure (TCLP) – Method 1311 [40]/Agilent 7900 ICP-MS equipment |
In addition, leaching tests have been carried out on both the unfired mixtures and the LWAs obtained from them, following the Toxicity Characteristic Leaching Procedure (TCLP) elaborated in the Method 1311 of US EPA [40]. Concentrations have been determined using an Agilent 7900 ICP-MS equipment for the following heavy metals: Cr, Ni, Cu, Zn, As, Se, Ag, Cd and Pb. The objective of these leaching tests is twofold: (i) on the one hand, to corroborate that there are no high concentrations of heavy metals in the raw materials that could be potentially harmful, and (ii) on the other hand, to check whether sintering has favored the immobilization of such heavy metals, regardless of their concentration.
Results and discussionWorkability of the mixtures for pelletizingFrom the data in Table 3, it can be seen that the mixtures with SYC and PYC clays have LL values between approx. 47 and 50, PI between 20 and 27 and PI/LL between 0.4 and 0.6, while in the series with PRC the approximate values are LL between 30 and 35, PI between 11 and 18 and PI/LL of approx. 0.4 and 0.5. Analyzing the data in more detail, it can be observed that, in general, the addition of the organic wastes favors a slight decrease in plasticity. This is due to the fact that although the organic components have high water absorption capacity, they are non-plastic. This leads to the fact that while the LL values vary only slightly with respect to those of the starting clays, the PL values do tend to increase to a greater extent with the incorporation of the residues, which in turn reduces the PI value, and with it, the PI/LL ratio. This is especially noticeable in the case of the addition of CfG and NuS, whose classification becomes CL-ML type (low compressibility silty clay), while in the rest of the samples, the classification is maintained as low compressibility clays (CL). In the case of SYC-PpS, the LL is greater than 50, so the sample is classified as a high compressibility clay (CH).
Plasticity (LL=liquid limit; PL=plastic limit; PI=plasticity index), optimal moisture content (WOP) and classification (C=clay; M=silt; L=low compressibility; H=high compressibility) of the samples under study.
| Sample | Plasticity parameters | WOP (%) | Classification | |||
|---|---|---|---|---|---|---|
| LLb | PLb | PI | PI/LL | |||
| SYCa | 47.6±0.5 | 21.1±0.5 | 26.5 | 0.56 | 26.1 | CL |
| SYC-CfG | 48.2±0.4 | 28.0±1.7 | 20.2 | 0.42 | 34.5 | CL-ML |
| SYC-NuS | 46.6±0.6 | 25.8±1.8 | 20.8 | 0.45 | 31.9 | CL-ML |
| SYC-PpS | 51.1±0.9 | 25.7±0.1 | 25.4 | 0.50 | 31.7 | CH |
| SYC-CkP | 49.3±0.0 | 23.7±0.3 | 25.5 | 0.52 | 29.3 | CL |
| PYCa | 47.0±0.0 | 22.3±0.1 | 24.8 | 0.53 | 27.5 | CL |
| PYC-CfG | 48.4±1.1 | 29.4±0.3 | 19.1 | 0.39 | 36.2 | CL-ML |
| PYC-NuS | 47.2±0.3 | 26.5±0.6 | 20.7 | 0.44 | 32.7 | CL-ML |
| PYC-PpS | 49.4±0.4 | 24.0±0.9 | 25.4 | 0.51 | 29.6 | CL |
| PYC-CkP | 49.7±0.6 | 22.8±0.5 | 26.9 | 0.54 | 28.1 | CL |
| PRCa | 34.4±0.3 | 15.9±0.1 | 18.5 | 0.54 | 19.6 | CL |
| PRC-CfG | 32.8±1.1 | 19.1±0.8 | 13.7 | 0.42 | 23.6 | CL-ML |
| PRC-NuS | 29.2±1.4 | 18.2±0.4 | 10.9 | 0.37 | 22.5 | CL-ML |
| PRC-PpS | 34.6±0.9 | 16.8±0.4 | 17.8 | 0.51 | 20.7 | CL |
| PRC-CkP | 35.3±1.1 | 17.4±0.1 | 17.9 | 0.51 | 21.4 | CL |
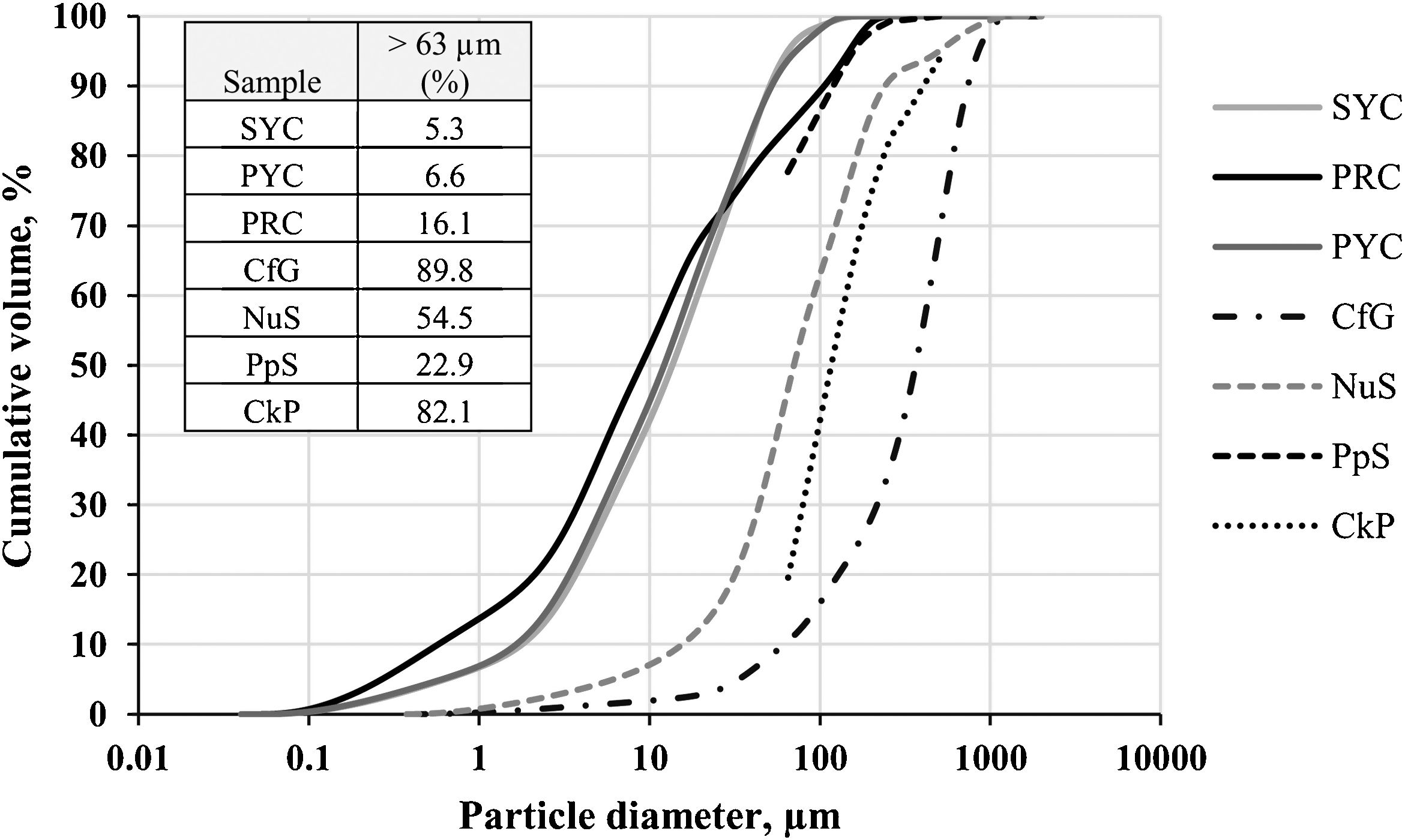
However, the decrease in plasticity has not been so high as to significantly affect the workability of the samples, and no problems have been observed when extruding and pelletizing them, with optimum molding moisture values of around 30–35% for the mixtures with SYC and PYC clays, and 20–24% for the mixtures with PRC. With respect to this last clay and its mixtures, workability has been somewhat more difficult, due to the fact that PRC has a higher sand content than the other two clays, with 16% of particles above 63μm, compared to 5–6% in SYC and PYC, whose particle size distribution is almost the same (Fig. 2). Regarding this last parameter, Fig. 2 shows that the residues have a coarser particle size distribution than the clays, which could also have contributed to the slight decrease in plasticity. Thus, it can be seen that the particle size curves of the organic wastes appear more displaced to the right than those of the clays, thus presenting relatively high percentages of coarse fraction, especially in CfG (89.8%>63μm), CkP (82.1%>63μm) and NuS (54.5%>63μm), while PpS is less coarse (only 22.9%>63μm).
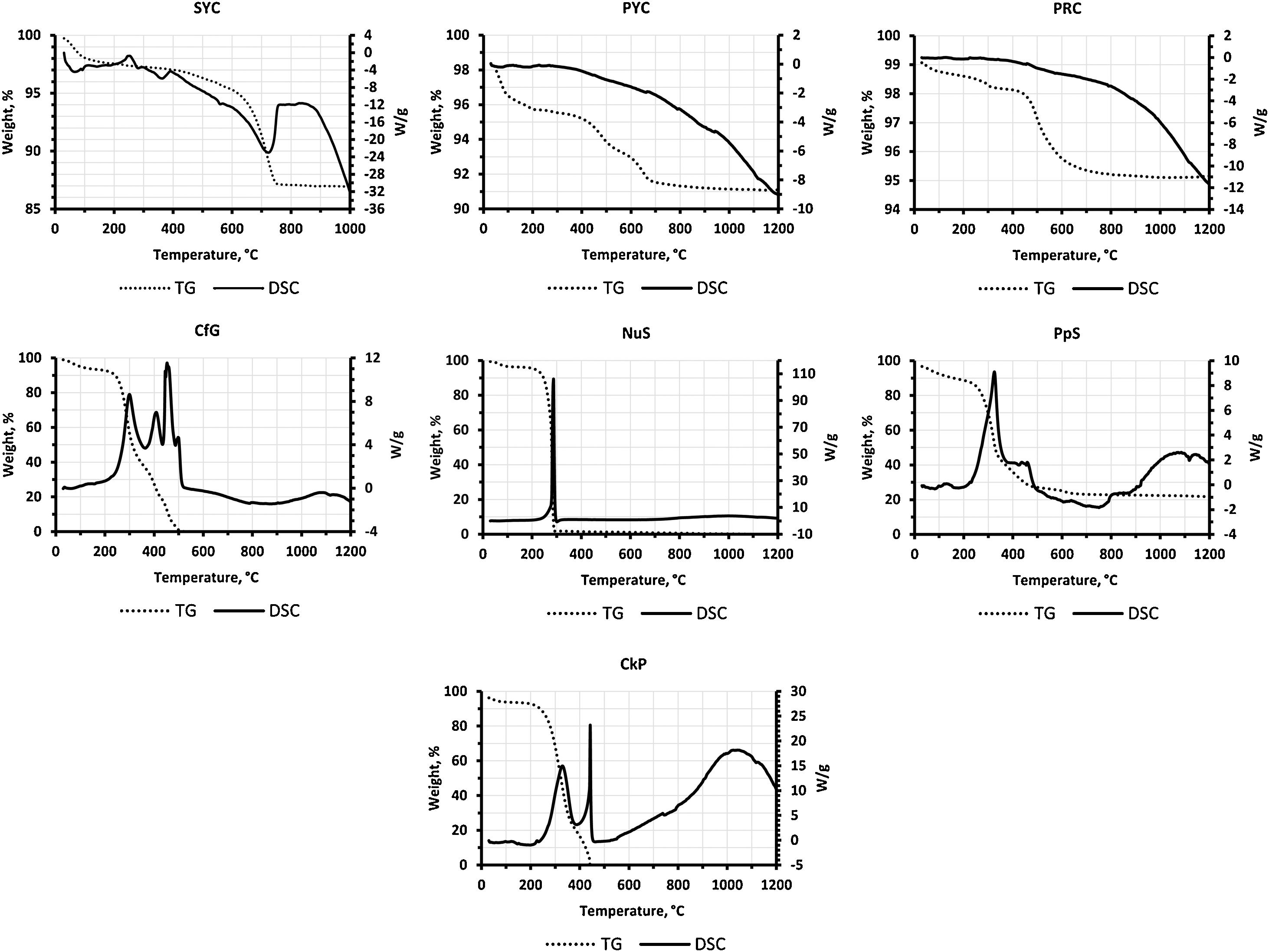
Effect of organic waste addition on the preheating timeAn essential aspect in the manufacture of LWAs is that the process must be viable from an operational point of view, which requires that the material remains intact at the time of firing. To this end, it is common to establish a minimum preheating time, which would prevent the granules from bursting when subjected to the thermal shock that occurs once they cross the first section of the kiln tube. Since the gases generated during heating can lead to pellet bursting, it is essential to know the thermal behavior of the raw materials up to this temperature. In this preheating phase, the pellet was progressively introduced into the kiln tube up to the limit with the central section, where the programmed temperature started. The temperature in the preheating zone therefore rose from the room temperature (kiln inlet) to approximately 100°C below the programmed temperature (limit with the central zone). Taking this into account, the preheating maximum temperature reached at least 1000°C in all cases. However, the fact that the kiln atmosphere presents a certain temperature does not necessarily mean that this temperature can be reached in the aggregate in such a short time due to thermal inertia. To this it must be added that there are also other variables that directly influence the kinetics of the reactions, such as the endothermic decompositions of phyllosilicates and carbonates, and the exothermic oxidation of the organic matter of the clay and that added with the residue (Fig. 3). Considering that the time the pellets were subjected to the maximum preheating temperature was relatively short (only 1, 1.5 or 2min), the average temperature applied would be approximately 600–800°C in this initial stage.
According to the information obtained by DSC-TG (Fig. 3), the thermal decomposition of the organic additives (exothermic reaction) takes place at about 500°C for CfG, 300°C for NuS and about 450°C for CkP, whose LOI results are 98.4, 96.1 and 100%, respectively (Table 4), indicating that they are wastes that decompose practically in their totality when fired. In the case of the paper-rich residue, PpS, the thermal decomposition stabilizes at approximately 600°C, although this is a residue with a lower LOI (74.5%) and therefore a higher ash content, in this case fundamentally rich in CaO (Table 4). Regarding the three clays studied, the LOI is low during the preheating stage, only 3.6% in SYC, 5% in PYC and 2.6% in PRC (Table 5), and according to the DSC-TG graphs in Fig. 3 would be due to the loss of hygroscopic water (up to ∼150°C) and interlayer water in illite and smectite (100–200°C), the loss of bound water in palygorskite (between ∼200–600°C) (only applicable to PYC), the decomposition of the little organic matter content (OC in Table 4) present in the clay (between ∼200 and 550°C) and the endothermic dehydroxylation of kaolinite (530–590°C) [41] and/or illite (∼400–550°C) [42]. At somewhat higher temperatures H2O (g) loss could also occur by dehydroxylation of palygorskite in PYC at 580–800°C [43] and of smectite in SYC and PYC at about 700°C [41]. In the case of SYC clay, the endothermic drop between 700 and 750°C due to calcite decomposition (releasing CO2) is very noticeable [41].
Carbon content (TC=total carbon; IC=inorganic carbon; OC=organic carbon), loss on ignition (LOI), chemical composition (∑Flux=K2O+Na2O+CaO+MgO+FeO+Fe2O3) of the raw materials and calorific values of the organic wastes. NA=Not applicable; GCV=Gross calorific value; NCV=Net calorific value. Mineralogy of the clays under study: Q=quartz; C=calcite; Do=dolomite; F=feldspar; Pg=plagioclase; Sm=smectite; I=illite; K=kaolinite; P=palygorskite. Major=Minerals whose proportion is in the range of approximately 20–35%; Minor=Minerals with expected proportion <20%; Traces=Minerals in which only traces have been detected (expected proportion <1%).
| Name | Carbon content (%) | LOI (%) | Chemical composition, oxides (%) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TC | IC | OC | Carbonates | SiO2 | Al2O3 | Fe2O3b | Na2Ob | K2Ob | MgOb | CaOb | TiO2 | MnO | SO3 | Cl | P2O5 | ZrO2 | BaO | SnO2 | SiO2/∑Flux | ||
| SYCa | 2.03 | 1.90 | 0.13 | 15.8 | 9.4 | 62.1 | 11.6 | 4.1 | 0.6 | 2.7 | 1.4 | 7.4 | 0.7 | – | – | – | 0.1 | 0.1 | – | – | 3.8 |
| PYCa | 0.37 | 0.29 | 0.08 | 2.4 | 6.7 | 64.4 | 14.0 | 5.5 | 0.4 | 3.2 | 2.5 | 2.0 | 1.0 | 0.1 | – | – | – | – | – | – | 4.8 |
| PRCa | 0.02 | 0.00 | 0.02 | 0.0 | 3.6 | 78.4 | 10.9 | 4.0 | 0.4 | 1.3 | 0.3 | – | 0.7 | – | – | – | – | – | – | – | 12.9 |
| CfG | 50.09 | 0.00 | 50.09 | 0.0 | 98.4 | – | – | – | – | 0.6 | 0.3 | 0.2 | – | – | – | – | 0.3 | – | – | – | NA |
| NuS | 47.63 | 0.00 | 47.63 | 0.0 | 96.1 | – | – | 0.1 | – | 2.2 | – | 0.9 | – | – | – | – | – | – | 0.2 | 0.1 | NA |
| PpS | 30.48 | 0.55 | 29.93 | 4.6 | 74.5 | 1.1 | 6.0 | – | 0.5 | – | 1.6 | 10.9 | – | – | 1.0 | 0.4 | 3.3 | – | – | – | NA |
| CkP | 64.20 | 0.00 | 64.20 | 0.0 | 100.0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | NA |
| Name | Calorific value (kJ/kg) | Mineralogy | |||
|---|---|---|---|---|---|
| GCV | NCV | Major | Minor | Traces | |
| SYCa | NA | NA | Sm, I, C | Q, Do, Pg | K |
| PYCa | NA | NA | Sm, P | I, K, Q, C | F |
| PRCa | NA | NA | Q, I, K | – | – |
| CfG | 20,961.8 | 19,463.8 | NA | NA | NA |
| NuS | 19005.9 | 17763.6 | NA | NA | NA |
| PpS | 12901.7 | 11836.9 | NA | NA | NA |
| CkP | 29,096.3 | 27,340.5 | NA | NA | NA |
Mixtures, firing conditions and characteristics of the aggregates sintered with the clays and the waste. Firing conditions (tpreh=preheating time in the kiln inlet; tfiring=firing time in the middle of the kiln; T=firing temperature), dA=average aggregate diameter; loss on ignition during preheating (LOIpreh) and firing (LOIfiring). Technological properties of the aggregates: ρb=loose bulk density; ρa=apparent density; ρrd=oven dry density; WA24=water absorption after 24h immersion; PT=total porosity; PO=open porosity; PC=closed porosity; S=single aggregate crushing strength.
| Aggregate name | Firing conditions | dA (mm)c | BI (%) | LOIrotarykiln (%) | Density, ρ (g/cm3) | Porosity, P (%) | S (MPa)c | S/ρrd (Nm/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tpreh (min) | tfiring (min) | T (°C)b | LOIpreh | LOIfiring | ρbc | ρa | ρrd | WA24 (%) | PT | PO | PC | |||||
| SYC-1205a | 1 | 4 | 1205 | 9.1±0.27 | -4.8 | 3.6 | 10.7 | 1.11±0.02 | 1.99 | 1.92 | 1.7 | 22.5 | 3.3 | 19.1 | 10.7±2.9 | 5.6 |
| SYC-CfG-1190 | 1 | 4 | 1190 | 11.1±0.50 | 18.0 | 3.8 | 13.9 | 0.53±0.01 | 0.99 | 0.87 | 14.0 | 66.7 | 12.2 | 54.5 | 2.8±1.2 | 3.2 |
| SYC-NuS-1195 | 1 | 4 | 1195 | 11.0±0.50 | 16.1 | 3.5 | 13.9 | 0.55±0.01 | 1.06 | 0.93 | 12.4 | 64.1 | 11.6 | 52.5 | 2.8±1.4 | 3.0 |
| SYC-PpS-1190 | 1 | 4 | 1190 | 12.1±0.52 | 25.7 | 4.7 | 12.2 | 0.48±0.02 | 1.10 | 0.90 | 19.9 | 65.2 | 18.1 | 47.1 | 1.5±0.5 | 1.7 |
| SYC-CkP-1195 | 1 | 4 | 1195 | 11.2±0.79 | 20.1 | 4.8 | 13.2 | 0.53±0.02 | 1.31 | 0.91 | 33.6 | 65.1 | 30.6 | 34.5 | 2.6±1.4 | 2.9 |
| PYC-1210a | 1.5 | 4 | 1210 | 9.9±0.50 | 8.2 | 5.0 | 6.6 | 0.79±0.04 | 1.41 | 1.31 | 5.2 | 44.5 | 6.8 | 37.8 | 5.6±2.4 | 4.3 |
| PYC-CfG-1160 | 2 | 4 | 1160 | 11.4±0.52 | 29.4 | 4.6 | 9.8 | 0.43±0.00 | 0.88 | 0.74 | 21.4 | 71.6 | 15.8 | 55.8 | 1.2±0.6 | 1.7 |
| PYC-NuS-1165 | 2 | 4 | 1165 | 12.0±0.53 | 32.6 | 3.8 | 9.6 | 0.44±0.01 | 0.92 | 0.73 | 27.4 | 71.8 | 20.2 | 51.6 | 1.4±0.5 | 1.9 |
| PYC-PpS-1160 | 2 | 4 | 1160 | 12.9±0.76 | 36.6 | 4.8 | 10.7 | 0.40±0.01 | 0.83 | 0.71 | 19.5 | 72.6 | 13.9 | 58.7 | 1.2±0.3 | 1.7 |
| PYC-CkP-1160 | 2 | 4 | 1160 | 12.40±0.59 | 32.2 | 4.6 | 9.6 | 0.44±0.01 | 0.92 | 0.75 | 24.1 | 71.1 | 18.2 | 52.9 | 3.0±1.1 | 4.0 |
| PRC-1350a | 1 | 4 | 1350 | 9.1±0.34 | -5.1 | 2.6 | 6.1 | 1.32±0.03 | 2.38 | 2.24 | 2.7 | 11.7 | 6.1 | 5.5 | 6.1±2.3 | 2.7 |
| PRC-CfG-1315 | 1 | 4 | 1315 | 10.4±0.49 | 5.1 | 2.5 | 7.2 | 0.84±0.01 | 1.71 | 1.46 | 9.9 | 43.7 | 14.6 | 29.2 | 3.8±2.1 | 2.6 |
| PRC-NuS-1315 | 1 | 4 | 1315 | 10.4±0.19 | 6.0 | 2.0 | 6.4 | 0.87±0.01 | 1.73 | 1.48 | 9.5 | 43.0 | 14.1 | 28.9 | 4.1±1.4 | 2.8 |
| PRC-PpS-1315 | 1 | 4 | 1315 | 11.1±0.71 | 12.1 | 4.4 | 7.7 | 0.70±0.02 | 1.51 | 1.36 | 7.3 | 47.8 | 9.9 | 37.8 | 4.0±0.9 | 2.9 |
| PRC-CkP-1315 | 1 | 4 | 1315 | 10.1±0.59 | 2.3 | 5.4 | 8.1 | 0.78±0.01 | 1.64 | 1.40 | 10.3 | 46.0 | 14.4 | 31.6 | 5.2±2.8 | 3.7 |
Therefore, with regard to the preheating time required to prevent the pellets from bursting (Table 5), in the case of the mixtures with SYC and PRC, there was no change with respect to the originally established time, which was 1min. This shows that the pellets formed with these clays present an adequate (micro)structure to allow the release of most of the gases generated during the thermal decomposition of the organic wastes, avoiding internal overpressure. In the case of PYC, this clay was already initially more prone to bursting when placed into the kiln, requiring 1.5min of preheating to avoid it. This is mainly due to the fact that this clay has a mineralogical composition richer in phyllosilicates (in this case especially smectite and palygorskite) than the other two, but on the other hand, with lower proportions of degreasing minerals, which are more present in SYC and PRC (e.g., quartz, dolomite or calcite). The addition of the organic residues has led to a slight increase in this preheating time in PYC, in this case up to 2min. This would be due to the fact that the compact structure of the PYC pellets makes it very difficult for gases to escape to the outside, something that would be especially magnified by introducing the organic residues under study, which, as noted above, decompose almost completely to gas in the cases of CfG, NuS and CkP and up to 75% for PpS, according to the LOI data in Table 4.
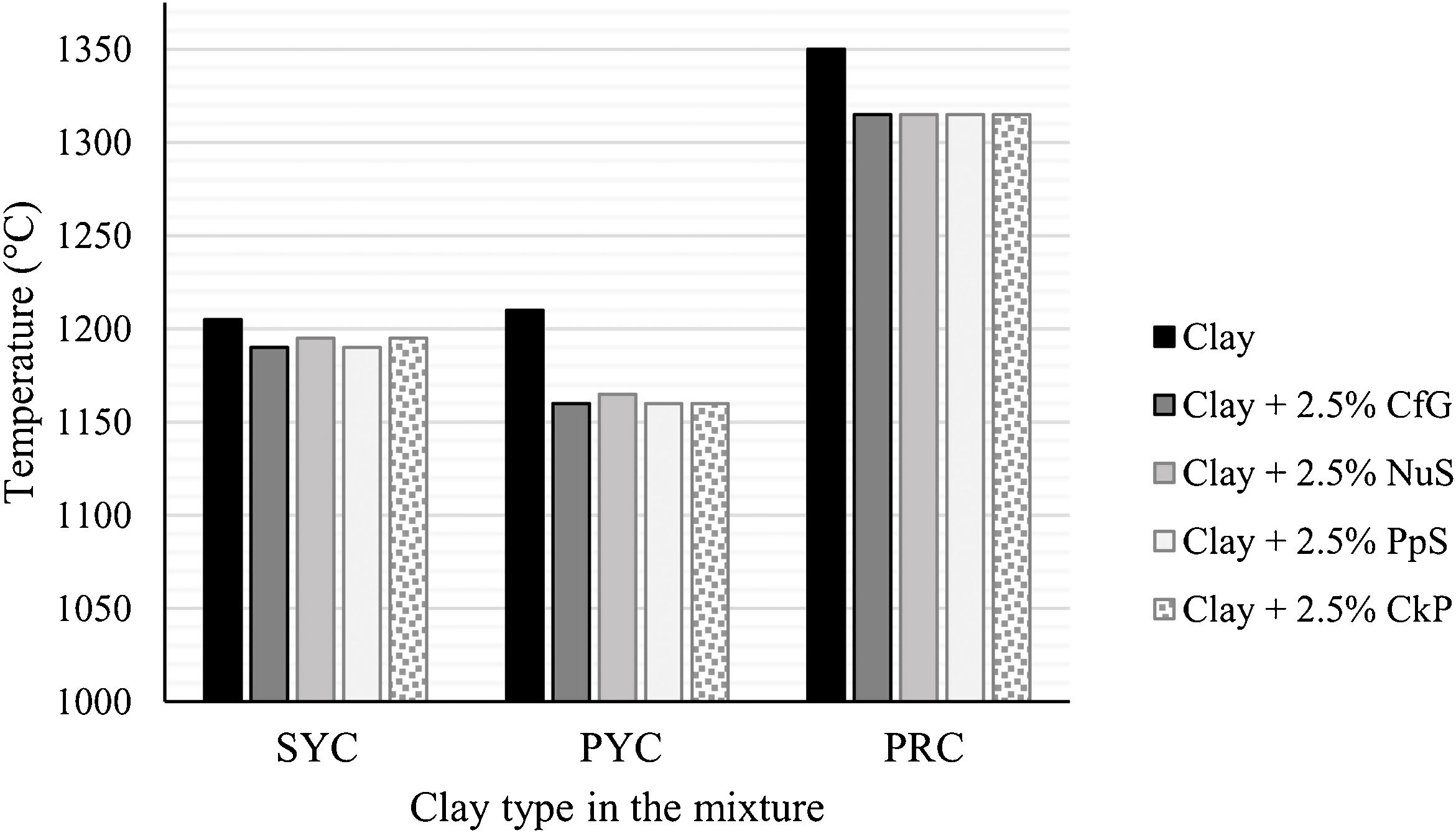
Change in firing temperature with the addition of the organic wastesRegarding the maximum working temperature allowed by the material, the data in Table 5, graphically represented in Fig. 4, show a decrease in this parameter when adding the organic wastes. This observation agrees with previous publications, such as that of Moreno-Maroto et al. [4], in which the authors studied the effect of the incorporation of olive pomace as an organic additive in mixtures with clays. It can be observed that there is really no difference between the addition of one organic residue or another, so the data can be discussed together, simply considering each type of clay. Thus, this decrease in the firing temperature is more marked in the mixtures with PYC (decrease of 45–50°C), followed by PRC (35°C decrease), while the more moderate reduction has occurred in SYC (only 10–15°C). These differences in firing temperature would therefore be directly conditioned by the particularities of each clay, and not by the type of organic residue added, so that the organic component does favor such temperature decrease, but to a different extent depending on the clay used.
According to Moreno-Maroto et al. [4], this decrease in the firing temperature upon addition of organic residues could be due to two factors:
- i)
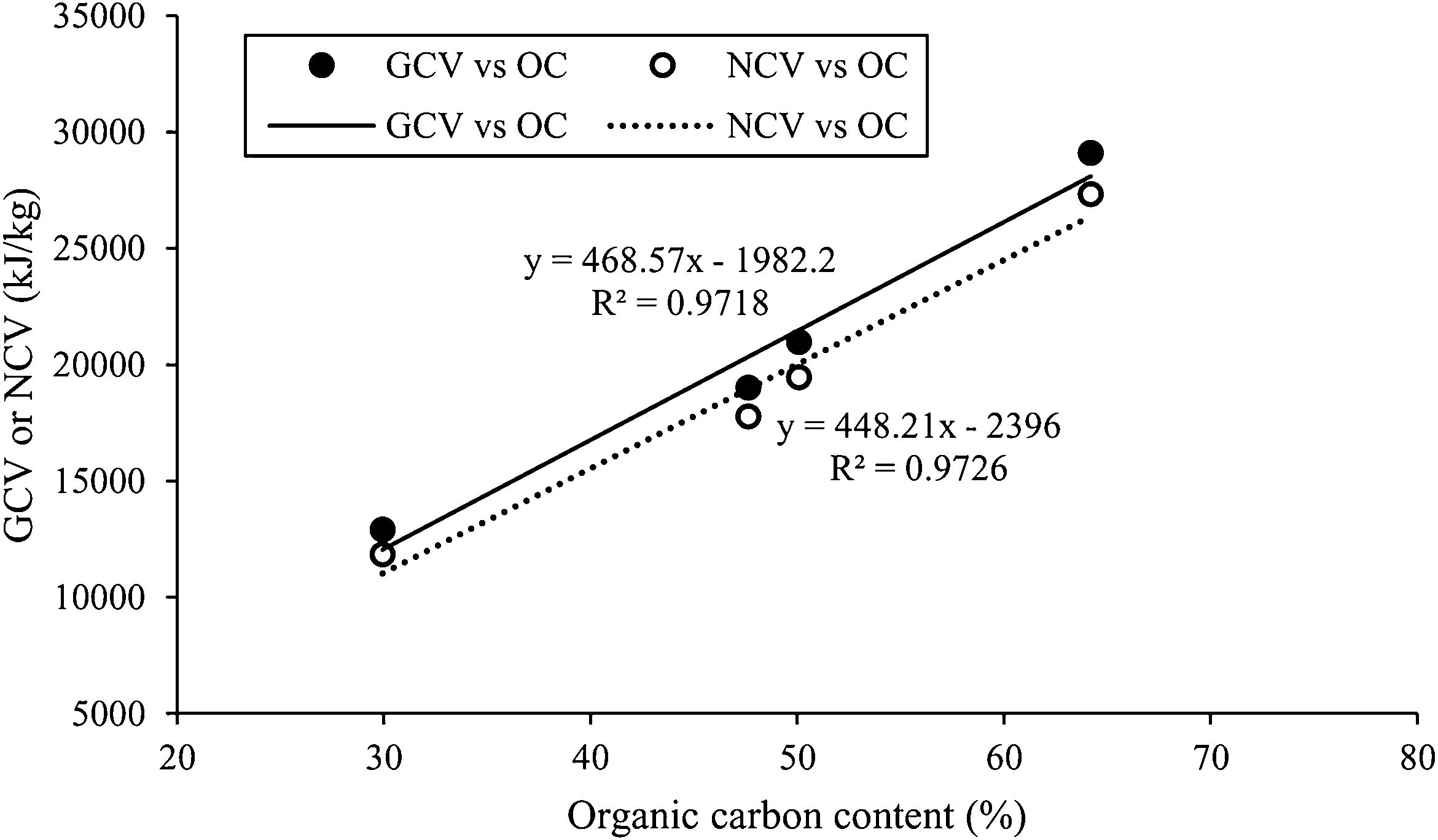
The exothermic reaction associated with the decomposition of the organic components. This is clearly noticeable both in Fig. 3 and in the calorific value data (GCV and NCV) in Table 4. Thus, the GCV values ordered from highest to lowest are around 1.3×104kJ/kg (PpS), 1.9×104kJ/kg (NuS), 2.1×104kJ/kg (CfG) and 2.9×104kJ/kg (CkP), with the NCV values being slightly lower (Table 4). Fig. 5 shows that there is a direct relationship between the calorific value of the residues and the organic carbon content, whereby the higher the OC, the higher the GCV and NCV data. Despite these differences in GCV and NCV between the organic wastes, as indicated above, the effect observed in reducing the working temperature has been similar.
- ii)
The development of reducing conditions within the aggregate and the kiln atmosphere, a phenomenon that would be due both to the manufacturing process itself based on thermal shock, which favors a rapid decrease in the oxygen concentration in the kiln, and, on the other hand, to the incomplete combustion of the organic matter contained in the samples, which would not only favor a direct consumption of said oxygen, but would also lead to the reduction Fe2O3 present in the sample to form Fe3O4 and/or FeO, the latter being a flux capable of substantially reducing the sintering temperature [44].
Since the organic component is expected to decompose very rapidly during preheating, in theory, there would be hardly any residue left when passing to the central zone of the kiln. It would therefore be expected that the first of the above mechanisms (exothermic decomposition) would be much less relevant than the second (induction to reducing conditions) in lowering the firing temperature. Regardless of the mechanism involved in changing the firing temperature, a decrease in firing temperature could be an environmental advantage to be considered, since it could mean lower fuel consumption during firing in a potential industrial scale-up. The process of formation of the lightweight aggregate structure is explained in detail at the end of section “Internal structure and role of the organic additive in the formation of the LWA”.
Impact of organic waste addition on the technological properties of the LWAsThe information reflected in Table 5 shows that although the incorporation of organic wastes has favored substantial changes in the technological properties of the aggregates obtained, it cannot be clearly discerned that the incorporation of a specific waste has meant a greater advantage with respect to the others. In this sense, and as was done in the previous section for the study of the working temperature, an analysis can be carried out as a whole, taking into account the type of clay used in each case.
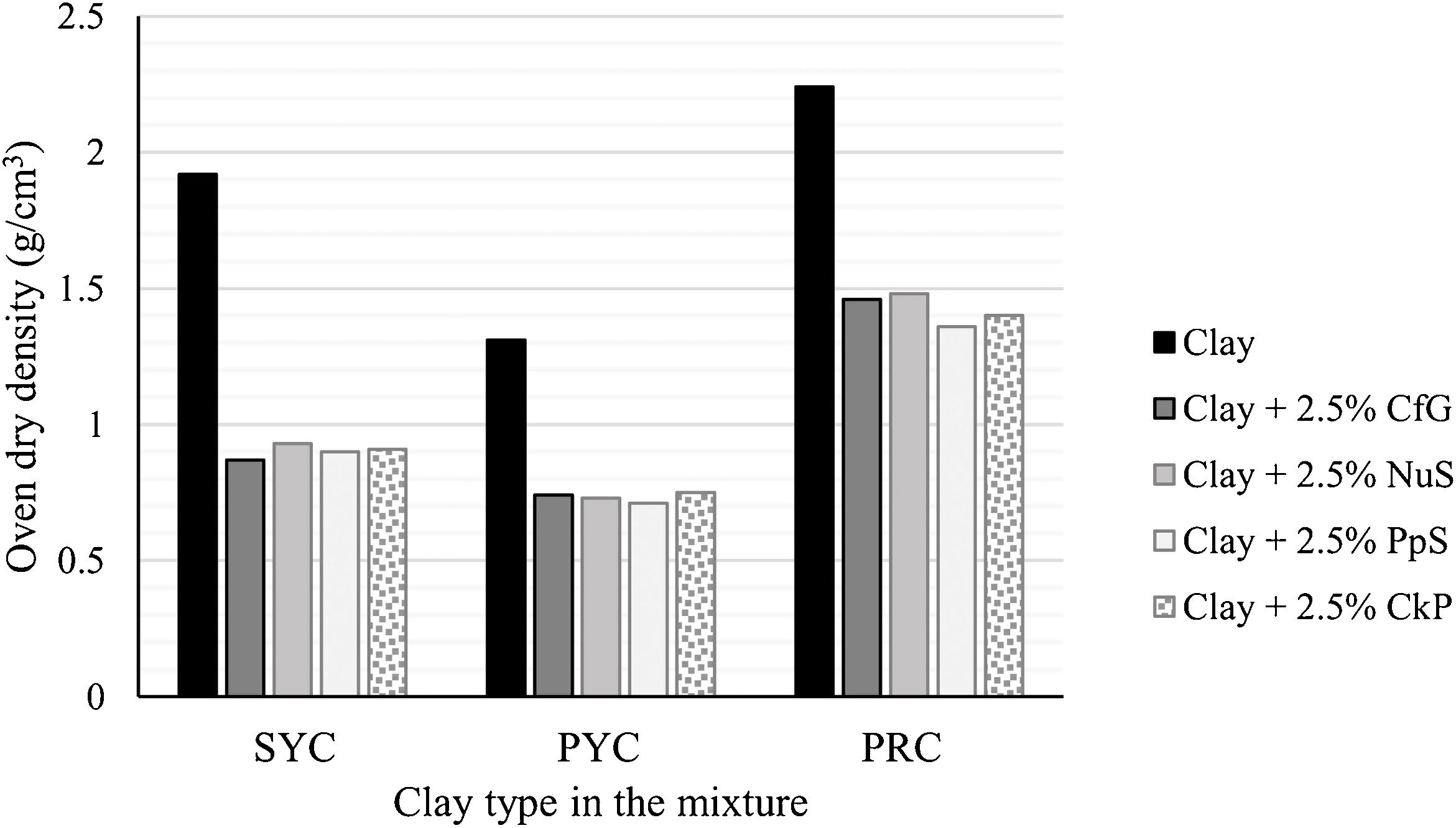
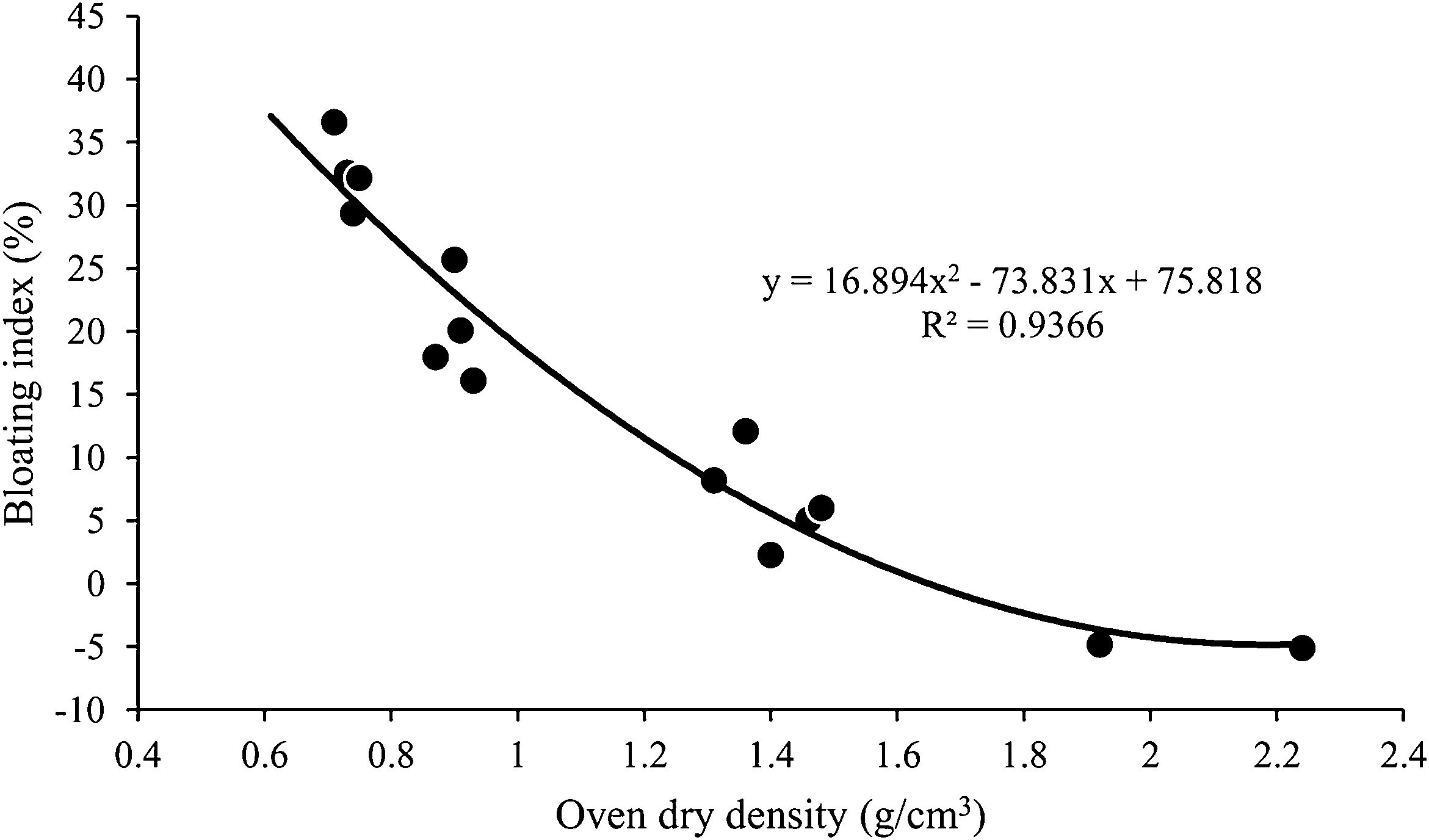
First of all, focusing on density, since it is the key parameter to determine whether an aggregate is lightweight or not, it can be observed that the addition of the organic wastes (regardless of the type) has favored a very marked decrease in the values of ρb, ρa and, ρrd, something that can be seen graphically in Fig. 6. The ρb and ρrd data would be well below the limit established by EN-13055-1 [45] (1.2g/cm3 for ρb and 2.0g/cm3 for ρrd), thus amply meeting the requirements for LWAs. Thus, in the cases of SYC and PRC, these were clays that did not expand on their own, but retracted when fired (BI around −5%), giving rise to aggregates of compact microstructure (Fig. 7) that in the case of PRC would not comply with said regulation (ρb=1.32g/cm3 and ρrd=2.24g/cm3), and for SYC (ρb=1.11g/cm3 and ρrd=1.92g/cm3) would be at the limit of compliance. However, the addition of organic residues has favored a very marked decrease in density in both cases, with ρb values around 0.7–0.8g/cm3 and ρrd around 1.4–1.5g/cm3 in PRC and, even lower in SYC, with ρb between approximately 0.5g/cm3 and ρrd around 0.9g/cm3. With respect to PYC, this was a clay that when fired at 1210°C presented a certain expansive capacity (BI=8.2%) without the need to incorporate any additives, which translated into a ρb value of 0.8g/cm3 and ρrd 1.3g/cm3. Although these results are positive and comply with the standard EN-13055-1 [45], the incorporation of 2.5wt.% of organic residue in the PYC formulation has favored an even more marked decrease in this density, which in its case, has given the lowest values of those recorded in this study, with ρb and ρrd around 0.4 and 0.7g/cm3, respectively. These results are supported by the bloating index data (BI between 30–37%), which are the highest of those recorded in this investigation. As expected, there is a direct relationship between BI and aggregate density, as can be seen in Fig. 8.
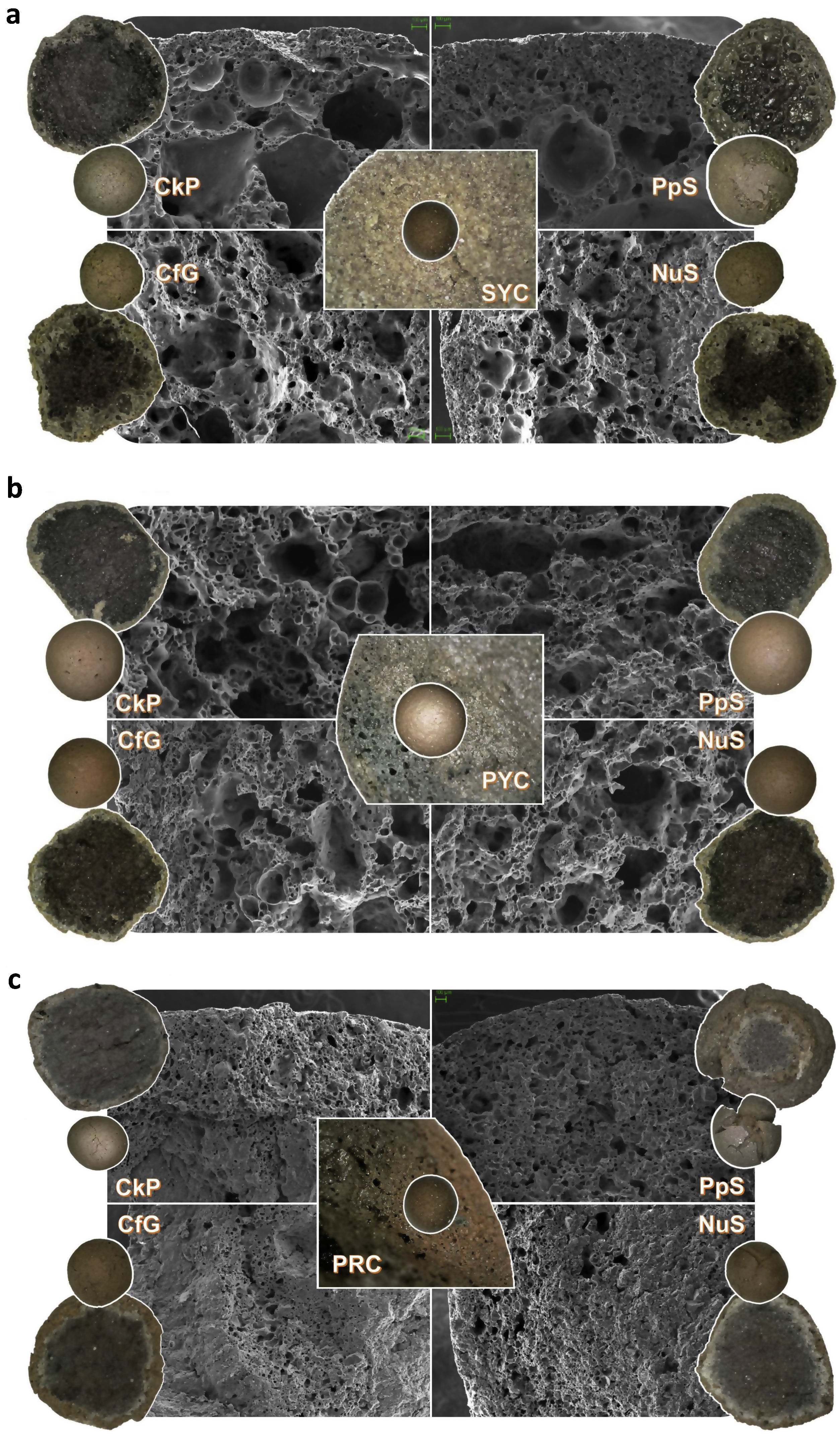
General view and (micro)structure pictures of the LWAs manufactured from clay/organic waste mixtures with SYC (a), PYC (b) and PRC (c). The pictures were taken both by SEM microscopy (grayscale images) and a Dino-Lite AM7915MZT digital microscope (color images) for each of the clays both without additive (center of each sub-figure) and with the addition of 2.5wt.% cork residue (top left), paper money sludge (top right), coffee grounds (bottom left) and ground nut shell (bottom right).
The density and bloating index data are explained by the development of a highly porous internal structure, formed by the combustion of the organic residues in the clay mixtures, a mechanism that will be discussed in section “Internal structure and role of the organic additive in the formation of the LWA”. This is more than evident both in the images in Fig. 7 and in the porosity measurement data in Table 5. Therefore, the most porous structures have been generated in the LWAs obtained from the mixtures of organic residue with PYC clay (PT∼72%), followed by the mixtures with SYC (PT∼65%), with the least porous structure in the LWAs fired with PRC mixtures (PT∼43–48%), being dominant a closed type porosity, PC, which can exceed 50% in SYC and PYC formulations, and 30% in PRC. This closed porosity is reflected in the formation of more or less rounded pores, as can be seen in Fig. 7, which is particularly noticeable in the LWAs developed from the mixtures with SYC and PYC (Fig. 7a, b), which have much larger pores than those of the LWAs obtained from the formulations with PRC (Fig. 7c). The remaining open porosity, PO, generally less than 20%, favored water absorption results of 10–20%, 20–27% and 7–10% in the LWAs produced from the waste mixtures with SYC, PYC and PRC, respectively. This type of interconnected porosity, which promotes hydraulic conductivity is positive in different applications, such as in case of applying the LWA in agricultural or horticultural crops, green roofs, water treatments, as well as even when applied in lightweight concrete, where after pre-wetting, the LWA can favor a better internal curing and the development of an improved interfacial transition zone, ITZ, between the aggregate and the cement paste [5].
The development of a more porous structure has led to a decrease in the crushing strength, which would now range between 1.5–2.8, 1.2–3.0 and 3.8–5.2MPa, for the LWAs obtained with the addition of the organic components in SYC, PYC and PRC, respectively, whose original S, without additive, was 10.7, 5.6 and 6.1MPa. Despite this decrease in mechanical strength, the results are in the same order, or even higher, than those of other commercial LWAs used in structural concrete, such as Argex AR 4/10–550, whose S value is 1.3MPa for specimens with dimensions similar to those of this investigation [4].
Internal structure and role of the organic additive in the formation of the LWAAccording to the results of plasticity (Table 3), particle size distribution (Fig. 2) and chemical composition (Table 4), although SYC and PYC are very similar clays, the addition of the organic components has resulted in different firing temperatures and technological properties in the LWAs. This finding seems to point to the fact that the main difference is that SYC has a much higher carbonate content (mainly calcite) than PYC (15.8% vs. 2.4%) and a slightly higher iron content than PYC (5.5% vs. 4.1%), aspects that may have led to different sintering conditions and technological characteristics, more favorable in the case of the yellow Portuguese clay. On the contrary, in the case of the residues, it has already been discussed that they present certain differences with regard to particle size, carbon content, LOI and calorific value, and yet their impact on both the firing temperature, as in the properties of the LWAs obtained, has been very similar to each other. These results are indicative that although the addition of the organic component significantly affects the manufacturing process and the properties of the sintered material, the possible variations in the characteristics of said organic residue do not seem to be really decisive, something that, on the contrary, does occur with the type of clay.
Fig. 7 shows that a common factor to all the LWAs developed in this research is the formation of a clearly differentiated shell-core structure, the latter presenting a darker color (from gray to black) and a porosity much more remarkable than the former. This last aspect is especially noticeable in the LWAs obtained with the SYC and PYC mixtures (Fig. 7a, b), whose porosity, as indicated in the previous section, is much higher than that of the PRC series (Fig. 7c), whose microstructure is much more compact, containing smaller porous than the others. Similarly, the core color tends to be darker in the SYC- and PYC-based LWAs than in the PRC ones. It can also be observed a narrow, light gray region that surrounds the darkest area of the core (especially in the PRC-based varieties), and that represents a transition area between the shell and the core of the aggregate. This change in the tonality of the aggregate section, from the brown of the shell to the blackish core, passing through the narrow light gray transition zone, are indicators of different redox conditions during firing.
The color variations and porosity development can be understood according to the mechanism of lightweight aggregate formation. During the few minutes that the pellets remain inside the kiln, the temperature must rise to achieve the right conditions for developing the lightweight aggregate. In order to do so, thermal inertia and endothermic reactions, such as dehydroxylation of phyllosilicates and decomposition of carbonates (Fig. 3), must be overcome. In this sense, the exothermic oxidation of organic compounds (Fig. 3) could help to counteract endothermic reactions, affecting also other factors that will be explained below. The gases generated by the decomposition of minerals and organic compounds have to flow through the very fine and tortuous porosity to escape from the aggregate. It is also important to note that sintering starts from the surface, so a priori the gas permeability should be rapidly reduced. Furthermore, in theory, the formation of a viscous liquid phase near the LWA surface could hinder the penetration of oxygen into the inner core of the LWA, which may result in a reduction of the atmospheric pressure in the outer zone, which could alter the kinetics of phase formation phenomena [46].
However, as has been experimentally demonstrated in a previous investigation [19], almost all of the gases generated actually escape out of the aggregate. In fact, according to the work published by Moreno-Maroto et al. [19], contrary to what was thought until then, the amount of gas directly involved in the expansion process of the lightweight aggregate is negligible, representing less than 0.1wt.% of the original sample. This means that actually the development of a viscous matrix is much more determinant than gas release in developing the typical porous structure of LWAs. In this sense, it can be stated that the gases generated during the decomposition of the organic wastes used in this research would not participate directly in the formation of the pores, especially considering that they are components that are rapidly calcined in the kiln, long before the internal porosity begins to be generated. However, it is true that the combustion of the organic components could lead to changes in the redox conditions in the kiln atmosphere [19] and especially in the interior of the aggregate itself, as mentioned in Section previous sections. Thus, while the shell of the aggregate sinters rapidly under predominantly oxidizing conditions (resulting in a lighter color and lower porosity in the shell), inside it the oxygen concentration would be clearly reduced by the combustion of the organic components, which could be incomplete, leading to the formation of certain reducing gases, such as CO, which in turn would facilitate the reduction of the iron to form fluxing forms, such as FeO. Although in principle it could be thought that this process occurs mainly at the temperatures programmed in the center of the kiln, the formation of the “black core” typical of iron reduction begins to be clearly observed during preheating, as can be seen in Fig. 9 for two specimens of PYC-PpS as an example. To this should be added the difficulty of air entry from the outside due to the formation of the low porous shell when sintering becomes more intense. From this, it follows that the development of this FeO would facilitate not only the formation of a gray to black core (which could also be due to remnants of not fully oxidized organic components) [44], but also the destruction of most of the initial crystalline phases, giving rise to amorphous phase with the appropriate viscosity to encapsulate part of the gases generated not only during iron reduction, but also possibly from the thermal decomposition/dehydroxylation of certain mineral phases (such as phyllosilicates or carbonates), to form in this way the typical closed porosity-rich structure of LWA.
Black core formed during PYC-PpS preheating, reaching maximum temperatures of 700°C (left) and 960°C (right). While the specimen preheated to 960°C matches the preheating actually applied, the one preheated to 700°C is shown to demonstrate that iron reduction reactions are very likely to start even at relatively low temperatures.
The results of the leaching study are shown in Table 6. The concentrations of the elements measured are in all cases very low, regardless of the clay used or the residue employed. Thus, for those elements for which the US EPA [47] sets limits (Cr, As, Se, Ag, Cd and Pb), even without taking into account those concentrations that have remained below the detection limit (which, as can be seen, were quite a few), the concentrations are between 2 and 4 orders of magnitude lower than the maximum threshold. Thus, for example, considering the data above the detection limit, in general, concentrations for these metals have been measured in the unfired mixtures below 10ppb, and in many cases, even below 1ppb. The concentrations of Se and Ag are negligible in practice. In the fired aggregates, on the other hand, with a few exceptions that will be explained later, a slightly higher concentration for these metals is observed, something that is most noticeable in As, which can increase its concentration by 1 or 2 orders of magnitude with respect to the unfired material (see for example the mixtures with SYC, in which As increases from less than 5ppb to about 40ppb). Despite this slight increase in concentration in the LWAs, the values are still well below the regulatory limits, so in this sense, it can be categorically stated that both the unfired mixtures and the aggregates obtained from them do not pose any threat to the environment or public health. This is of course something that meets expectations, considering that the clays used are of an inert nature (in fact they are clays used in the ceramic industry) and that the organic residues do not have an origin that would make them potentially dangerous.
Results of heavy metal concentration (ppb) in the unfired mixtures and the lightweight aggregates (firing temperature indicated) extracted by the leaching TCLP test and comparison with the threshold allowed for each element according to the limits established by US EPA [47]; bdl=below detection limit.
| Sample | Cr | Ni | Cu | Zn | As | Se | Ag | Cd | Pb |
|---|---|---|---|---|---|---|---|---|---|
| US EPA TCLP threshold (ppb) | 5000 | Not given | Not given | Not given | 5000 | 1000 | 5000 | 1000 | 5000 |
| SYC-CfG | 0.2 | 3.0 | 16.3 | 60.6 | 3.8 | bdl | 0.0 | 2.2 | 1.2 |
| SYC-CfG-1190 | 3.6 | 168.9 | 86.0 | 94.5 | 39.5 | bdl | bdl | 0.1 | 12.2 |
| SYC-NuS | bdl | 6.1 | bdl | bdl | 0.5 | 2.0 | bdl | 0.2 | bdl |
| SYC-NuS-1195 | 6.4 | 177.1 | 92.9 | 107.6 | 40.2 | bdl | bdl | 0.0 | 8.2 |
| SYC-PpS | bdl | 4.1 | 0.8 | bdl | 1.9 | 1.3 | bdl | 0.2 | bdl |
| SYC-PpS-1190 | 5.9 | 34.0 | 35.5 | 97.6 | 15.4 | bdl | 0.0 | 0.0 | 9.2 |
| SYC-CkP | 0.8 | 2.9 | bdl | bdl | 1.2 | 1.6 | 0.1 | 0.7 | 13.3 |
| SYC-CkP-1195 | 2.9 | 278.5 | 184.9 | 230.6 | 41.3 | bdl | 0.0 | 0.2 | 9.6 |
| PYC-CfG | 0.1 | 4.3 | bdl | bdl | 0.8 | 1.3 | bdl | 0.3 | 15.1 |
| PYC-CfG-1160 | 1.6 | 43.2 | 40.4 | 69.5 | 7.7 | bdl | bdl | 0.1 | 2.2 |
| PYC-NuS | 1.6 | 6.7 | 8.2 | 57.7 | 2.5 | bdl | 0.1 | 3.0 | 15.8 |
| PYC-NuS-1165 | 1.5 | 80.7 | 63.2 | 116.0 | 7.0 | bdl | bdl | 0.3 | 5.8 |
| PYC-PpS | bdl | 8.3 | 8.2 | 53.1 | 4.1 | bdl | 0.1 | 1.8 | 9.7 |
| PYC-PpS-1160 | 1.5 | 40.7 | 39.7 | 74.0 | 38.9 | bdl | 0.0 | 0.1 | 3.8 |
| PYC-CkP | 0.5 | 6.7 | 15.8 | 76.0 | 1.6 | bdl | 0.0 | 2.7 | 50.5 |
| PYC-CkP-1160 | 0.9 | 90.4 | 72.1 | 68.6 | 13.3 | bdl | 0.0 | 1.1 | 7.0 |
| PRC-CfG | 1.8 | 13.7 | 52.8 | 212.3 | 1.2 | bdl | 0.0 | 0.2 | 12.3 |
| PRC-CfG-1315 | 1.3 | 105.6 | 59.7 | 112.8 | 21.3 | bdl | bdl | 0.1 | 11.8 |
| PRC-NuS | 2.7 | 10.7 | 82.4 | 162.9 | 0.6 | bdl | 0.0 | 0.3 | 12.6 |
| PRC-NuS-1315 | 0.9 | 98.0 | 73.1 | 344.7 | 11.9 | bdl | bdl | 0.1 | 8.4 |
| PRC-PpS | 3.8 | 15.7 | 42.9 | 161.9 | 1.3 | bdl | 0.1 | 0.5 | 9.9 |
| PRC-PpS-1315 | 0.9 | 18.7 | 26.7 | 96.4 | 7.2 | bdl | bdl | 0.0 | 7.6 |
| PRC-CkP | 2.2 | 11.5 | 92.1 | 332.8 | 0.7 | 0.0 | 0.1 | 0.5 | 9.1 |
| PRC-CkP-1315 | 1.1 | 100.4 | 120.4 | 188.2 | 19.7 | bdl | bdl | 0.2 | 12.7 |
With respect to the three elements for which the US EPA [47] does not establish maximum permissible values, Ni, Cu and Zn, these are in turn the ones with the highest concentrations. Taking as a reference first the unfired samples, Ni shows concentrations below 5–10ppb in the mixtures with SYC and PYC and slightly above 10ppb in those with PRC. Cu would be below 20ppb in the mixtures with SYC and PYC, and between 50–90ppb in those with PRC. For its part, Zn is very low in the mixtures with SYC, but generally exceeding 50ppb in the formulations with PYC and 150ppb, even reaching more than 300ppb, in PRC. In line with what was previously discussed for the other elements, in general the concentrations of Ni, Cu and Zn are lower in the unfired samples than in the sintered LWAs, whose concentrations, as shown in Table 6, are greater than 50–100ppb in many cases, exceeding in some cases 200–300ppb for these elements.
Although many studies have shown that sintering usually favors the immobilization of heavy metals, and with it a lower leachability of these elements [48,49], the results obtained in this work would point to the opposite for the samples studied, something in accordance with previous publications [4]. It is true that there are some data in Table 6 that would show a certain reduction of the element concentration when sintering the material in the form of LWA (for example, Cr in mixtures with PRC, Cd in general, or Pb in the mixtures with PYC), but these cases are exceptions. Therefore, although the general increase in leachability during sintering does not pose any risk to the environment or public health for the samples of this study (the heavy metal concentrations are very low), it is an aspect that should be addressed in those future investigations using raw materials potentially contaminated with heavy metals.
ConclusionsThe present investigation has covered the study of four organic solid wastes (coffee grounds, CfG; ground nut shell, NuS; paper money sewage sludge, PpS; and cork powder, CkP) to check their effectiveness in acting as bloating inducing agents when mixed in small proportions (2.5wt.%) with three types of clays, two of them without self-expansive capacity and the remaining one capable of developing LWAs, although not too lightweight. The main conclusions that can be drawn are as follows:
- -
Although the organic wastes had distinct characteristics, the main differences observed in the manufacture of LWA and the final technological properties have been detected when changing from one clay to another, and not so much from one residue to another. Therefore, LWA production seems to be more sensitive to the change of mineral raw material than to the change of organic component.
- -
The addition of the organic wastes has favored the firing temperature to positively decrease in the range of 10–50°C depending on the clay used in each case, which could lead to energy savings and reduction of gas emissions during the production of LWA.
- -
Regarding the technological properties of the LWAs obtained, in all cases the incorporation of organic wastes has favored a volumetric increase over the starting material, giving rise to more porous and, therefore, significantly lighter structures than those of the clay without organic additive.
- -
In line with the findings of previous studies [19], the thermal decomposition of the organic wastes and the consequent released gases, would not directly intervene in the formation of pores, but would lead to a change in the redox conditions. This phenomenon can promote the reduction of the iron present in the sample and the development of a suitable viscosity to trap part of the gases coming from such a reductive process and/or from the thermal alteration of other mineral phases (e.g., carbonates or phyllosilicates).
- -
The leachable heavy metal concentrations measured both in the raw materials and in the LWAs have been in any case very low, not posing any risk to the environment or public health.
Therefore, the outcomes of this investigation show that, in line with the precepts of the Circular Economy, the recovery of organic wastes of different nature can have a place in the manufacture of lightweight aggregates, materials that due to their technological properties could be key in future sustainable strategies in construction, civil engineering, agriculture and the environment.
CRediT authorship contribution statementCarlos Javier Cobo-Ceacero: Data curation, Investigation, Writing – review & editing. José Manuel Moreno-Maroto: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Marta Guerrero-Martínez: Data curation, Investigation. Manuel Uceda-Rodríguez: Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing. Ana B. López: Writing – original draft, Writing – review & editing. Carmen Martínez García: Funding acquisition, Project administration, Resources, Writing – review & editing. Teresa Cotes-Palomino: Conceptualization; Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This research was conducted as a part of the ECO-MET-AL Project (PID2019-109520RB-I00), “Can industrial and mining metalliferous wastes produce green lightweight aggregates? Applying the Circular Economy” funded by the Spanish Ministry of Science, Innovation and Universities, framed in the “Ayudas a “Proyectos I+D+i” en el marco de los Programas Estatales de Generación de Conocimiento y Fortalecimiento Científico y Tecnológico del Sistema de I+D+i y de I+D+i orientada a los Retos de la Sociedad, Convocatoria 2019”.