Nanostructured calcium phosphate and biphasic calcium phosphates have been studied and stand out as biomaterials for bone regeneration. This is due to the fact that thesebiomaterials present bioactivity and morphological, chemical and crystallographic similarities to the bone apatite. The aim of the present work has been the synthesis and characterization of two calcium phosphates with Ca/P=1.5 e 1.67 as molar ratio. These were synthesized through the chemical wet process. After synthesis, the hydrated calcium phosphate powders were subsequently calcined at temperatures of 900°C/2h providing b-calcium phosphate (b-TCP) and hydroxyapatite (HA) powders. These powders were used to elaborate the biphasic powders in the wt.% ratios HA/b-TCP as follows: 80/20, 20/80, 70/30 and 30/70. The method used for the elaboration of the b-tricalcium phosphate nanostructured powder, hydroxyapatite and biphasic compositions was the attrition milling. The nanostructured powders obtained were characterized by the scanning electron microscopy technique, X-ray diffractometry. Infrared spectroscopy and Specific surface using BET model.
Biocerámicas nanoestructuradas de fosfato de calcio y composiciones bifásicas de fosfato de calcio son estudiadas como biomateriales para sustitución ósea por presentar similitud morfológica, química y cristalográfica con la apatita del hueso. Este estudio tiene como objetivo la síntesis y caracterización de los fosfatos de calcio hidratados con razones Ca/P = 1,5 y 1,67 molar, estas matrices han sido sintetizadas por solución-precipitación en vía húmeda. El polvo de fosfato de calcio hidratado se calcino a una temperatura de 900°C/2h para obtener matrices fosfato tricalcico-b (b-TCP) y hidroxiapatita (HAP). Estas matrices se utilizaron para la preparación de polvos bifásicos compuestos con en % en peso HA/b-TCP con las siguientes proporciones: 80/20, 20/80, 70/30 y 30/70. Los polvos nanoestructurados de b-fosfato tricalcico, hidroxiapatita, y las composiciones bifásicas se prepararon por molienda de atrición en húmedo. Los polvos nanoestructurados así obtenidos se caracterizaron por microscopía electrónica de barrido, difracción de rayos X análisis cuantitativo. Espectroscopia infrarroja y se ha determinado la superficie especifica utilizando el método BET.
Recent studies in vivo have demonstrated that calcium phosphates micro and nanostructurated and the biphasic compositions of hydroxyapatite/b-calcium phosphate have potential as biomaterials, in repairing defects and in the reconstruction of bone tissue.1–3 The interest in the nanoestructured ceramics of calcium phosphates is associated to their interconnected microporous microstructural characteristics, bioactivity, solubility capacity, wetability and capillarity.1,4 The nanostructured bioceramics of calcium phosphates also offer new microstructures and nanostructures with interconnected microporosity which promote bioactivity and better contact surface with the adjoining tissues. These characteristics improve the adhesive and proliferation conditions of osteoblast cells on the Surface of grains and micropores, promoting the osteointegration and the formation of a new bone tissue.1,5
This study approaches the process of synthesis and characterization of two nanostructured calcium phosphates: b-tricalcium phosphate (b-TCP) and hydroxyapatite (HA), for the subsequent elaboration of biphasic in wt.% HA/b- TCP=80/20, 20/80, 70/30, 30/70. The results presented are related to the morphological characterization of the nanostructured powders, through the use of the scanning electron microscopy (SEM) technique. The X-ray diffractometer (XRD) was used for the crystallographic characterization of the nanostructured powders. The infrared spectroscopy (FTIR) helped identifying vibrational bands of the OH– and PO43– groups. Finally the results of the superficial area analysis by the BET isotherm model will be presented.
Materials and methodsThe synthesis was carried out by the reaction of dissolution/ precipitation, method using CaO and a solution of phosphoric acid necessary for the formation of the different compositions in the Ca/P ratio molar desired, as described elsewhere.6
Calcium carbonate (CaCO3), LabMaster, was used with concentration of 99% of purity, batch number 27404. The calcium carbonate was calcined at 900°C during 3h in order to obtain CaO. The reagent used was the phosphoric acid, Nuclear, with 85% concentration. The solution of acid concentration was prepared according to the Ca/P=1.5 and 1.67 molar ratio.
The material recovered from the synthesis process after being dried in a rotary evaporator, presented itself with a white color and granular form. These were milled in a mortar and sived on a 100mm mesh, providing powders of hydrated calcium phosphates. These were calcined at a temperature of 900°C during 2h, providing the b-calcium phosphate and hydroxyapatite nanostructured powders.
The biphasic compositions were prepared by attrition milling, which allows obtaining a better dispersion of phases during the blending process. The mixture of the nanostructured powders also was done an attrition mill, NETZSCH, with a solid/liquid concentration of 50/50wt.%in ethyl alcohol, zirconium spheres with a diameter of 2.5mm, 540rpm during 1 hour, as described by Delima et al.7 Then the colloidal suspension was dried in a rotary evaporator. The recovered material followed the same procedure of milling in a mortar and sieved as described previously.
In order to compare, the nanostructured powders of b-tricalcium phosphates and hydroyapatite also were processed through attrition milling, to evaluate the influence of this milling process on the physical and morphological characteristics of the two calcium phosphate matrices in relation to the different biphasic compositions.
The morphological characterization studies were conducted with the help of the Field Emission Scanning Electron Microscopy (FE-SEM) technique, with a JEOL equipment (JSM- 6701F), using secondary electrons image, with a 8mm working distance and electron acceleration voltage of 15kV.
The x-ray diffractometry was used to identify the present phases in the different compositions of nanostructured powders and granulated biomaterials. The studies were performed using a Shimadzu X-Ray Diffractometer Lab X XRD-6000, with an anticathode in a copper tube, wavelength l=1.54060Å, using as a parameter a diffraction angle of 2U with a 2°/min goniometer displacement, 40 kv voltage power, with a 30mA current intensity within a scanning angular range of 15° to 65°.
The Fourier Transform Infrared Spectrometer helped in the characterization of the nanostructured powders of b-TCP, HA and biphasics compositions. The equipment used was the spectrometer Perkin Elmer 100 with diminished reflectance. The test was conducted in an interval within 4000 to 300cm–1.
The BET model (Brunauer, Emmet e Teller) applied to the isotherm obtained using the volumetric method was used to determine the specific surface of the nanostructured powders of b-TCP, HA, and biphasics compositions (in wt.% HA/TCP- b): 80/20, 20/80, 70/30, 30/70. The equipment used to measure the specific surface was the ASAP 2000 from Micromeritics Instrument.
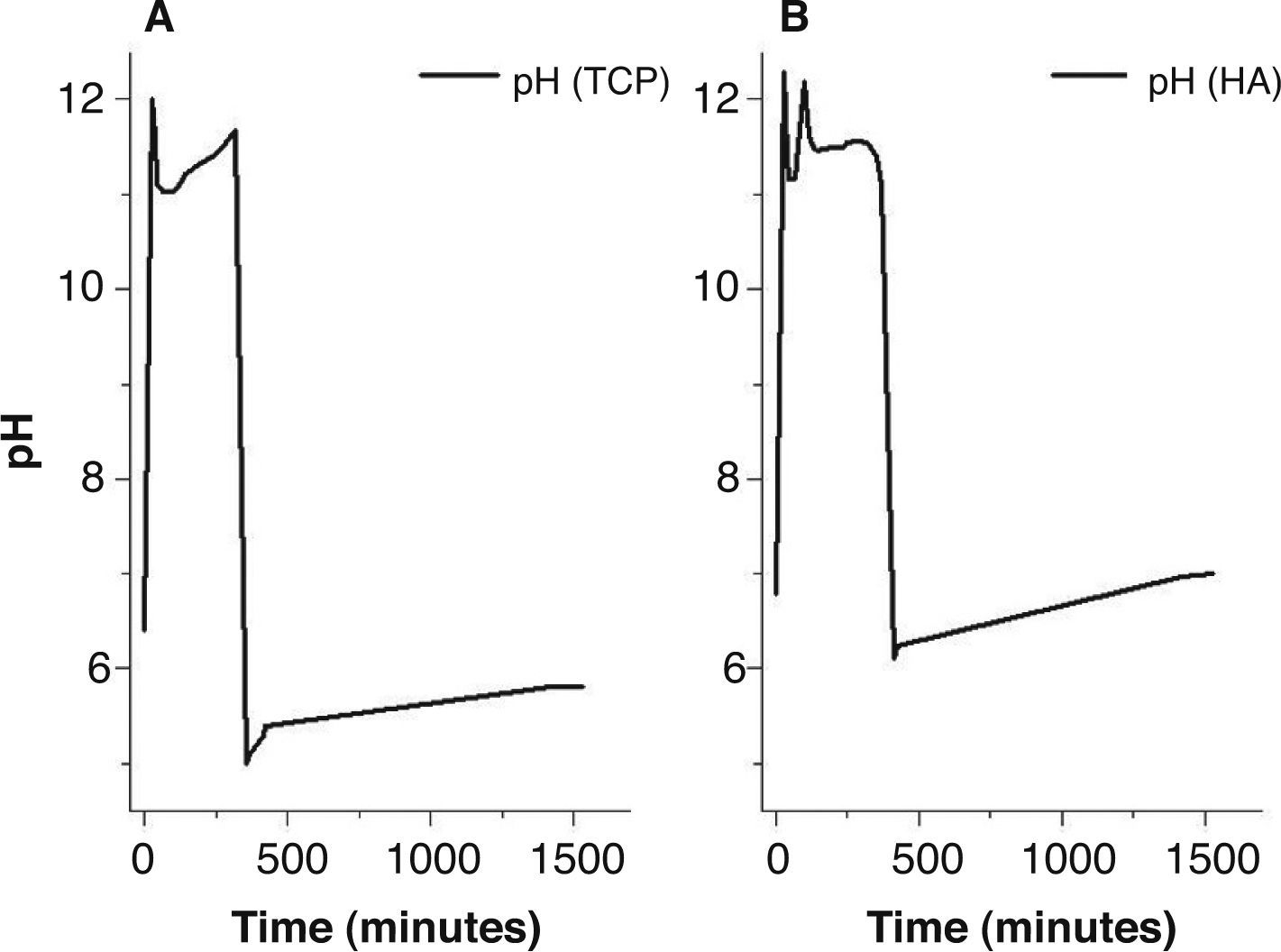
Results and discussionDuring the synthesis process, the pH of the colloidal solution was monitored for 24hours from the beginning to the end of the synthesis. Figure 1 shows the measured curves of the pH value for the composition Ca/P=1.5 molar (Fig. 1a) and for the composition Ca/P=1.67 (Fig. 1b). Based on the curves, it was observed that the pH value stabilized in about 400min, leading to a final value of pH=5.8 for the composition Ca/P=1.5 molar ratio and around 6.9 for the composition Ca/P=1.67.
The results obtained from the morphological characterization of the nanostructered powders of hydrated calcium on the Ca/P=1.5 e 1.67 molar ratio have revealed in their micrographs, a morphology formed by clusters of nanoparticles, smaller in size by 50nm respectively for the two compositions Ca/P=1.5 molar e 1.67 molar, as illustrated by Figures 2a and 2b.
The results obtained from the b-tricalcium phosphate nanostructured powders and hydroxyapatite (Figs. 3a and 3b) have revealed a microporous morphology formed by aggregated nanoparticles. If the micrographs obtained on the b-TCP powder are compared to the HA powder, it possible observe that the b-TCP powder shows nanoparticles bigger to those found for HA. This observation was also made by other authors that have obtained b-zr|calcium phosphates from the calcination of hydrated calcium phosphates. These explain that the slight coalescence of the crystals of b-TCP is associated to the Ca/P molar ratio and to the interfacial kinetics between nanoparticles.2,6,8–12
The results found for the b-TCP and HA nanostructured powders after the attrition milling process shows a change of the nanoparticles surface, generated by the attrition mill, where there was a reduction in size of the nanoparticles when compared to the results obtained from the calcination of nanostructured powders at 900°C/2h (Figs. 4a and 4b). This superficial change of the nanoparticles has already been identified by other authors that have used the high energy method of attrition milling in the development of ceramic powders.7,13–15
The morphological characterization obtained from the biphasics compositions are quite similar morphology as to those already found for the b-TCP and HA powders after the attrition milling. In view of this, only the micrographs obtained from the biphasics compositions 70/30 e 30/70, (Figs. 5a and 5b) are shown in this work.
The X-ray diffractometry of the hydrated nanostructured calcium phosphates (Figs. 6a and 6b), shows the presence of low crystalline apatite (Ca3(PO4)2.H2O) typical hydrated calcium phosphate phase, obtained by the wet process (Ca3(PO4)2.H2O), for the two compositions Ca/P=1.5 and 1.67 (molar ratio). This result has also been identified by other authors that have used the wet method for the synthesis of calcium phosphates.2,6,16
The diffractograms of the powders after the calcination at a temperature of 900°C/2h (Figs. 7a and 7b) shows the presence of b-TCP in the rhomboedral crystalline system with main diffraction plan [021] for the composition Ca/P=1.5 and the HA stoichiometric phase in the hexagonal system with main diffraction plan [211] for the matrix Ca/P=1.67 molar.
The diffractograms obtained from the powders of the composition b-TCP and HA after attrition milling (Figs. 8a and 8b), show the same crystalline structures as previously observed for the composition b-TCP e HA before the attrition milling process (Figs. 7a and 7b).
A slight reduction in the intensity of the phases of the most intense peaks was observed if compared to the diffractograms for the composition b-TCP and HA, before and after the attrition milling process, as shown in Figures 7a-7b, 8a-8b and previously explained.
The results obtained from the x-ray diffractometry on the biphasics powders show the presence of representative peaks from the b-TCP and HA phases for all the biphasic powder compositions, where only a slight variation in the intensities of the peaks between the compositions, which is related to the presence of the concentration of phases in % in each two phase powder composition.
The results obtained by the infrared spectrometry (FTIR) over the nanostructured powders b-TCP and HA are shown in Figures 9a and 9b. The spectra shown the vibrational bands corresponding to PO43– around 1090cm–1, 1020cm–1, 940cm–1, 600cm–1, 560cm–1, 420cm–1, referring to the OH– groups, over the spectrogram obtained from HA in around 3560cm–1, 1740cm–1 and 630cm–1, indicating that the nanostructured powder is formed by HA as observed in the DFR charts represented and identified by other authors.17–20
The results obtained over the two phase nanostructured powders have revealed in their FTIR spectra the same vibrational bands of the PO43– e OH– (Fig. 9c) groups. These have already been observed for the b-TCP e HA powders. These results indicate that the two phase powders are really composed by b-TCP e HA, as also observed in the results obtained by the X-ray diffractometry (Figs. 7a and 7b).
Table 1 shows the results of the specific surface on the calcium phosphates nanostructured powders after attrition milling b-TCP, HA and bihasic compositions: in wt.% HA/ b-TCP=80/20, 20/80, 70/30, 30/70. The results show higher values for the HA matrix, getting to 11.6 m2/g. The presence of a larger concentration of the matrix b-TCP in the biphasic compositions was observed, which lead to a slight reduction of the specific surface for these compositions, if these results are associated to the matrix HA. This small variation in the specific surface between the compositions can be explained by the slight variation of the morphological characteristics, previously observed throught the micrographs represented by Figures 3a and 3b, where there is a thin morphology of nanoparticles for the HA matrix.
ConclusionThe development of nanostrutured calcium phosphates powders is a current research topic and has generated new perspectives in the development of biomaterials for bone tissue replacements. These new biomaterials can be used in orthopedics, in traumatology and dentistry, as a matrix element in filling defects and regeneration of bone tissue.
The literature shows that the developments of b-tricalcium phosphates hydroxyapatite nanostructured powders and biphasics compositions have two basic targets: improve physical characteristics as open porosity, superficial area of grains and microporosity, and biomaterial solubility.
Morphological characterization has shown that the matrix HA has a morphology that is more refined than the matrix b-TCP. It has been observed that this fine morphology of the HA matrix has also influenced the values of the superficial area obtained by BET, showing slightly superior superficial area, for the compositions with a larger concentration of the HA. These fine morphologies found can be a potential in the development of biomaterials for bone replacement and leads to innovative results in the microstructural level of microporosity and of surface grains and microporos.
The physical characterization has revealed that the nanostructured powders are formed basically by crystalline HA and b-TCP phases. This result has shown that the nanostructured powders are formed by the constituent parts of the bone tissue. This will allow the development of microporos biomaterials with differentiated characteristics of superficial area of grain and microporous in relation to conventional biomaterials, which could generate an innovation in the near future in surgical procedures for bone tissue repairing and reconstitution. The results obtained by BET show that the nanostructured powders offer nanometric morphologies with a superficial area that promises wettability, adherence and cellular proliferation in these surfaces.
The results found in this work are encouraging and show that the calcium phosphates nanostructured powders can offer biomaterials with close architectures as to those of the bone structure, which can be a differential among the biomaterials of bone tissue replacement and repairing in a near future for biomedical applications.
The financial support of the Higher Education Personnel Training Coordination (CAPES-BRASIL) and Institute Ceramic and Glass (MAT2013-48426-C2-1R).