This work aims at developing an ideal glass that can harness the properties of both chemically tempered glass (CTG) and fully tempered glass (FTG) by combining chemical and thermal tempering process. Glass samples with dimension 120×50×4mm were prepared and first immersed in potassium nitrate salt (KNO3) inside a salt bath and subjected to heating at temperatures of 450°C and 500°C with immersion time of 4h and 5h for each temperature respectively in a muffle furnace to produce CTGs. The CTGs were then later subjected to thermal tempering at a fixed temperature of 450°C to produced bi-treated glasses (BTGs). Scanning electron microscope (SEM) was used to investigate the microstructure of the glasses while tests such as hardness, flexural strength and impact strength were used to evaluate the mechanical behavior of all the glasses. The results showed that the BTGs displayed high flexural strength which increases as temperature and time increases over the CTGs and FTG respectively while the hardness values of the BTGs also improved considerably as temperature and time increases over the CTGs and FTG respectively. However, the BTGs displayed decrease in impact strength as compared to CTGs and FTG. In term of microstructure, the BTGs displayed a compacted nodule-like structure evenly distributed throughout the glass matrix which accounts for the good mechanical behavior of the developed BTGs.
El objetivo de este trabajo es desarrollar un vidrio ideal que pueda aprovechar las propiedades del vidrio templado químicamente (VTQ) y del vidrio totalmente templado (VTT) al combinar el proceso de templado químico y térmico. Se prepararon muestras de vidrio con una dimensión de 120 × 50 × 4mm y se sumergieron por primera vez en sal de nitrato de potasio (KNO3) dentro de un baño de sal y se sometieron a calentamiento a temperaturas de 4.500 y 5.000°C con un tiempo de inmersión de 4 horas y 5 horas en cada temperatura, respectivamente, en un horno de mufla para producir VTQ. Posteriormente, los VTQ se sometieron a templado térmico a una temperatura fija de 4.500°C a los vidrios bitratados producidos (VBP). El microscopio electrónico de barrido (MEB) se utilizó para investigar la microestructura de los vidrios mientras que pruebas como la dureza, la resistencia a la flexión y la resistencia al impacto se utilizaron para evaluar el comportamiento mecánico de todos los vidrios. Los resultados mostraron que los VBP contaban con una elevada resistencia a la flexión que aumentaba a medida que aumentaban la temperatura y el tiempo en los VTQ y VTT mientras que los valores de dureza de los VBP también mejoraron considerablemente a medida que aumentaron la temperatura y el tiempo en los VTQ y VTT, respectivamente. Sin embargo, los VBP mostrados disminuyen la resistencia al impacto en comparación con los VTQ y VTT. En términos de microestructura, los VBP mostraron una estructura compacta similar a un nódulo, distribuida uniformemente a través de la matriz de vidrio, lo que explica el buen comportamiento mecánico de los VBP desarrollados.
In its broadest sense, glass is a collective term used for an unlimited number of materials of varying composition in a glassy state that exhibits glass transition behavior and lack long range structural periodicity [1]. However, the most generally know glass materials are those with SiO2 matrix. Generally, glass is a very strong material than most metal and alloys with estimated theoretical strength of 7000MPa but due to inherent defects and micro-cracks fails in service applications below their theoretical strength value [2]. Owing to this, strengthening of glass has been the usual norm in recent times in other to improve the structure and strength property so as to find suitable applications in buildings, automobiles, aircraft and chemical plants. Glass strengthening by toughening either chemically (through ion exchange) or thermally has been employed to improve the strength property of glasses over time to initiate surface compression stresses which impede growth of flaws [3,4]. In chemical toughening, glasses are immersed inside potassium nitrate (KNO3) salt in a salt bath and heat at a temperature higher than 380°C for several hours during which an ionic exchange occurred between the potassium ions (K+) in the salt and the sodium ions (Na+) on the glass surface resulting into surface compression stresses that inhibits growth of flaw and thus enhancing the strength of the glass [5]. In thermal tempering, glasses are subjected to heating inside a furnace at a elevated temperature superior to 600°C and suddenly cooled either by a blast of air or dropping inside a viscous liquid during which the glass surface is under compressive stresses with balancing inner tensile stresses. However, it has been discovered over the years that chemically toughened glasses (CTGs) possessed high strength but have poor fragmentation while fully toughened glasses (FTGs) by heat possessed low strength but break safely. As a result, researchers have found that there is possibility for an ideal glass to be developed which combines the strength characteristics of a CTGs with safe fragmentation of a FTGs processed through combination of chemical and thermal tempering referred to as bi-treated glass (BTG) [4]. The first developed bi-treated glass (BTG) process was investigated and patented by Hess et al. [6] but it was not clearly stated if and how it actually performed well than either CTGs or FTGs. A more recent work was however carried out by Zaccaria and Overend [4] and reported that BTG can offer a higher strength than FTG or a safe failure, but it was not possible to achieve both characteristics at the same time.
In this view, this paper attempts to investigate experimentally the microstructure and mechanical behavior of a developed bi-treated soda-lime glass (BTG) and also making comparison with fully toughened glass (FTG) and chemically toughened glass (CTG) at different temperatures and immersion times.
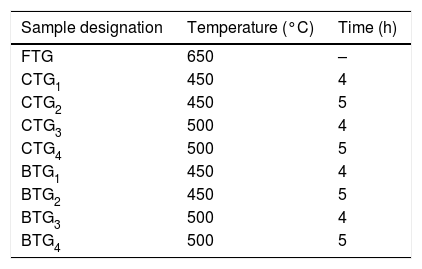
Materials and methodGlass characteristics and sample preparationIn this work, soda-lime silica flat glass having a nominal thickness of 4mm which was obtained commercially in its as-received condition. The composition of the glass taken into consideration is based on the typical composition of the soda-lime flat glass: SiO2 – 72.6%, Na2O – 13.6%, Al2O3− – 0.7%, CaO – 8.6%, MgO – 4.1%, K2O – 0.3%. The as-received glass was first cleansed from surface dirt by rinsing with distilled water and afterward dried. A total number of 26 glass samples measuring 120×50×4mm were cut from the flat glass and prepared for tempering procedures outline in Table 1.
Tempering processThree different types of tempering processes were utilized in the course of this research, which are thermal tempering (FTG), chemical tempering (CTG) and bi-treatment (BTG) respectively. The thermal tempering to obtain a fully toughened glass (FTG) was carried out inside the muffle furnace at a fixed temperature of 650°C used as a standard in most industries and soak for few minutes at that temperature. The thermal toughened glass was then brought out and quench rapidly in viscous SAE 40 oil bath at room temperature which resulted into surface compressive stresses with a balancing internal tensile stress and the sample labeled FTG. For the CTG, prepared glass samples were embedded in KNO3 salt inside a salt bath and heated in the muffle furnace at varying temperatures of 450°C and 500°C for immersion period of 4 and 5h at each temperature respectively. The glass samples were then brought out of the molten salt and were labeled CTG1–CTG4 as shown in Table 1. For the BTG, some of the CTG samples obtained at varying temperatures and immersion times were then selected and further subjected to thermal tempering at a fixed temperature of 450°C below glass transition temperature (530°C) so as not to nullify the strengthening from the ion exchange and then rapidly quench in viscous SAE 40 oil bath under room temperature to obtained the developed bi-treated glass samples (combining chemical and thermal tempering) and were labeled accordingly as BTG1–BTG4 as shown in Table 1.
CharacterizationThe microstructures of the glasses were examined by scanning electron microscope (SEM, ASPEX 3020) with acceleration voltage set at 16.0kV. Representative glass samples were selected and clamped respectively on the sample holder attached to the machine and the surface to be viewed was then exposed at varying display magnification.
The mechanical behavior such as hardness, flexural strength and impact strength was examined by Brinell hardness indenter (BHN), Universal testing machine and Izod impact tester respectively. The sample preparation and the test procedure for the hardness evaluation was carried out in accordance with ASTM E-92 standard in which the samples were exposed to a direct load of 250kg for about 15s after which the indented diameter was measured by a special low-powered microscope utilizing a scale. The hardness values were then obtained using a conversion table according to standard. Flexural strength was performed and evaluated in accordance with ASTM D790 standard at 25°C (room temperature) by a 3-point bending strength test using Instron universal testing machine (Instron 3369, 50kN load capacity) operated at a strain/load rate of 5mm/min, and a support radius of 2mm. The sample glasses were placed respectively on the two supporting rods of the machine, in a way that the two projecting ribs of the samples had equal distance from the two supporting rods with the span between supports set at 65±0.2mm. Load was then applied on the sample to obtain a rate of stress increase of 0.2N/mm per sec. The load was applied until the sample fracture and force at the point of fracture noted and the flexural strength automatically obtained from the machine. Impact strength test was conducted and evaluated using Izod impact tester at room temperature under stipulated parameters of specimen dimension, mounting, notching and pendulum velocity at impact as stated by ASTM D256. Samples of dimension 75×6×6mm were prepared and fixed respectively and an arm held at a specified height was released, this arm hits the sample and breaks it. The impact strength was then determined from the absorbed energy by the sample. Both the flexural strength and impact strength tests were carried out in triplicate to ensure the repeatability and reliability of the results generated.
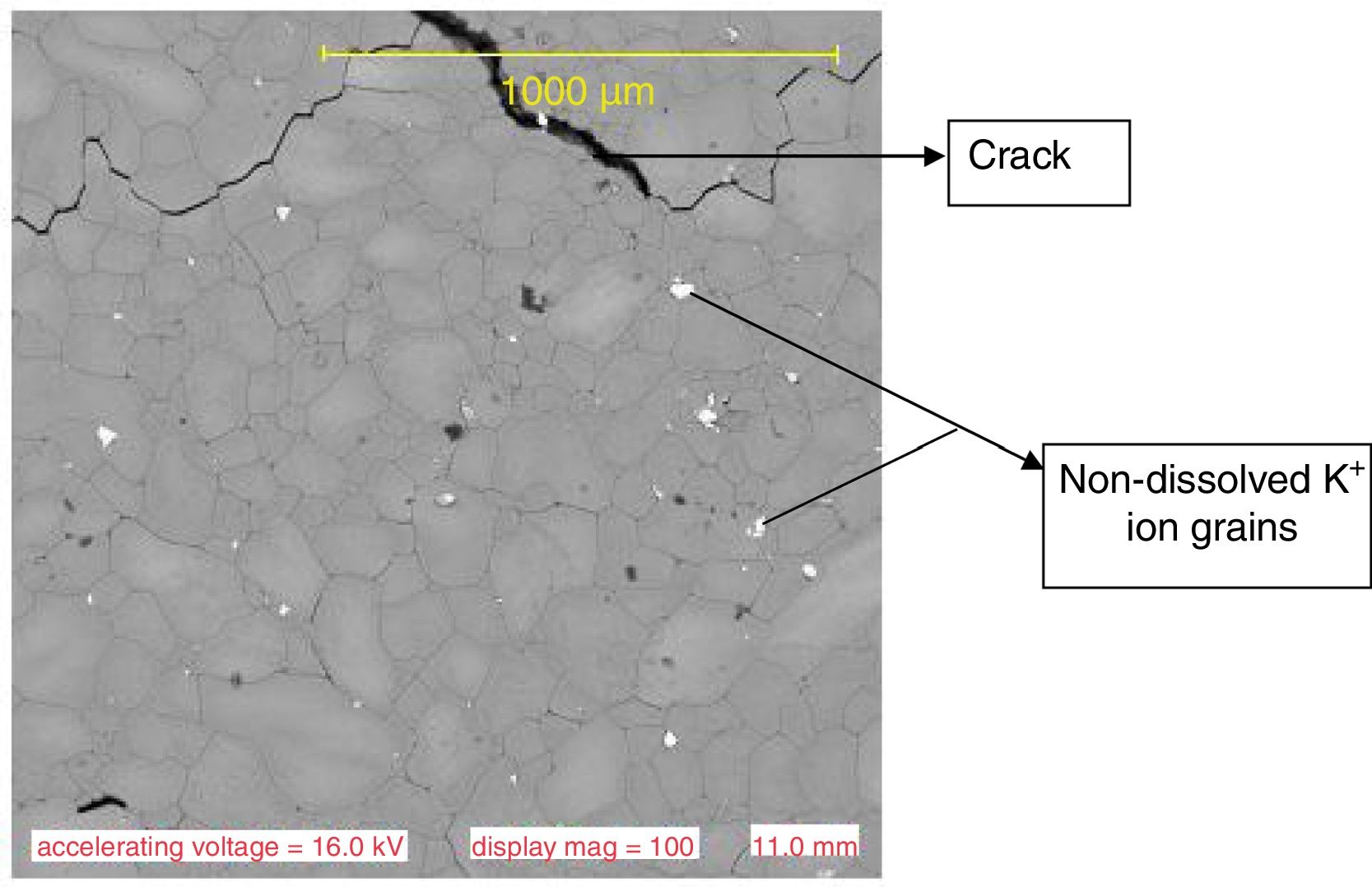
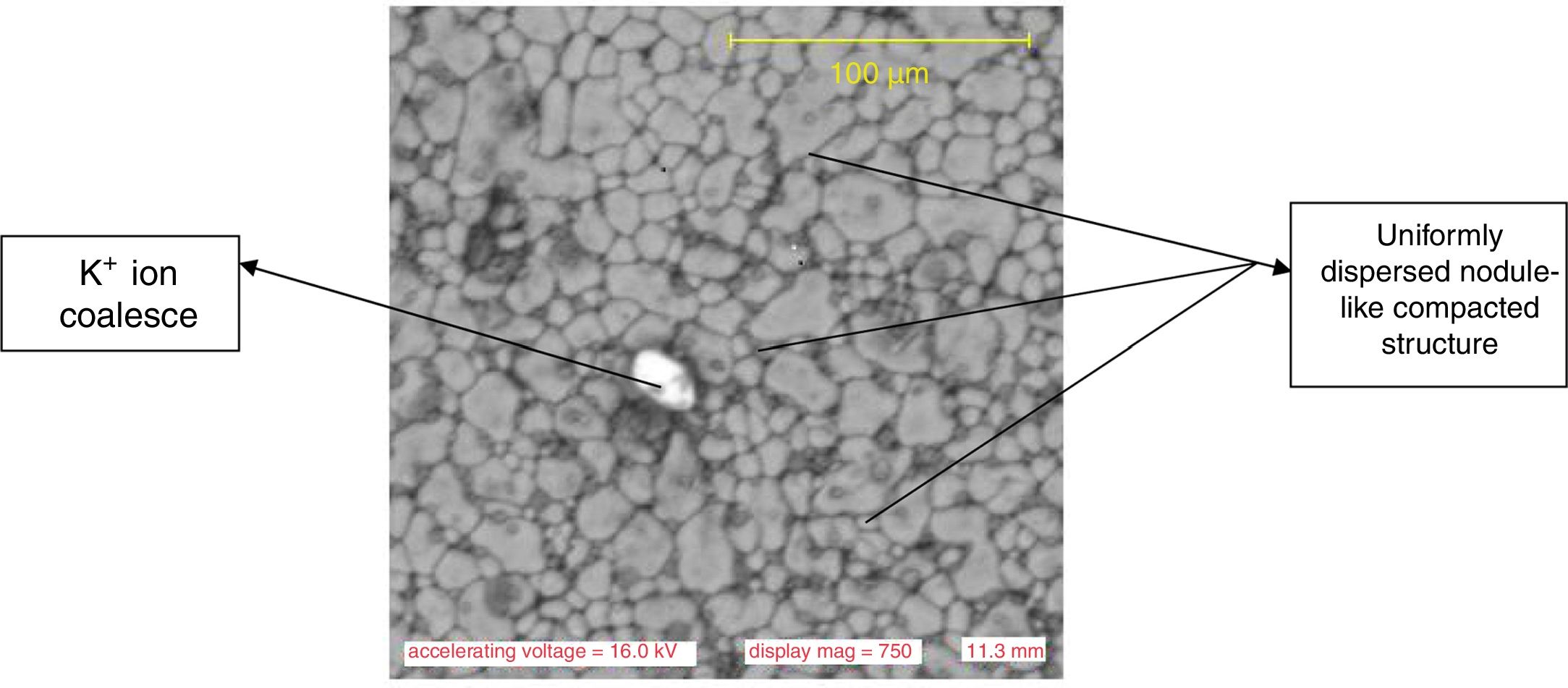
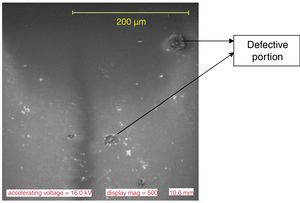
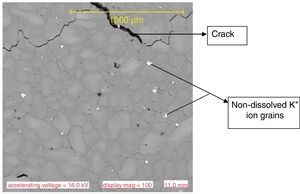
Results and discussionMicrostructure characteristicsThe results of the representative microstructure obtained showing the microstructure characteristics are shown in Figs. 1–3 respectively. Fig. 1 shows the microstructure of the non-toughened glass sample (control) while Fig. 2 represents the microstructure of CTG sample and Fig. 3 indicates the microstructure of BTG sample. From Fig. 1, it can be observed that the non-toughened glass displayed a good uniform morphology with spatial distribution of somewhat white pattern which might be due to contamination and few defects portion indicated by a dark-like bubble; which might however affect its mechanical behavior. From Fig. 2 which represents the chemically toughened glass sample (CTG) having features generally observed on the remaining chemically toughened samples, it can be observed that the stuffing of potassium ions on the glass resulted into surface compressive stresses which are uniformly distributed throughout the glass matrix indicated by several crystalline grains formation. However, a lateral crack was observed at the upper portion of the matrix which might be attributed to the contact between the tong (used to remove the glass samples) and the glass while picking from the molten salt. The lateral crack observed might in turn affect the mechanical behavior of the sample. From Fig. 3 which indicates the structure of the BTG sample with the same features generally observed on the remaining BTGs, it will be observed that the bi-treatment (combining chemical and thermal tempering) resulted into a more compacted surface compressive stresses indicated by a nodule-like compacted structure uniformly distributed throughout the entire matrix with several crystalline grains not easily vulnerable to crack propagation and which eventually contributed to the improvement of the mechanical behavior displayed by the BTGs over the FTG and the CTGs.
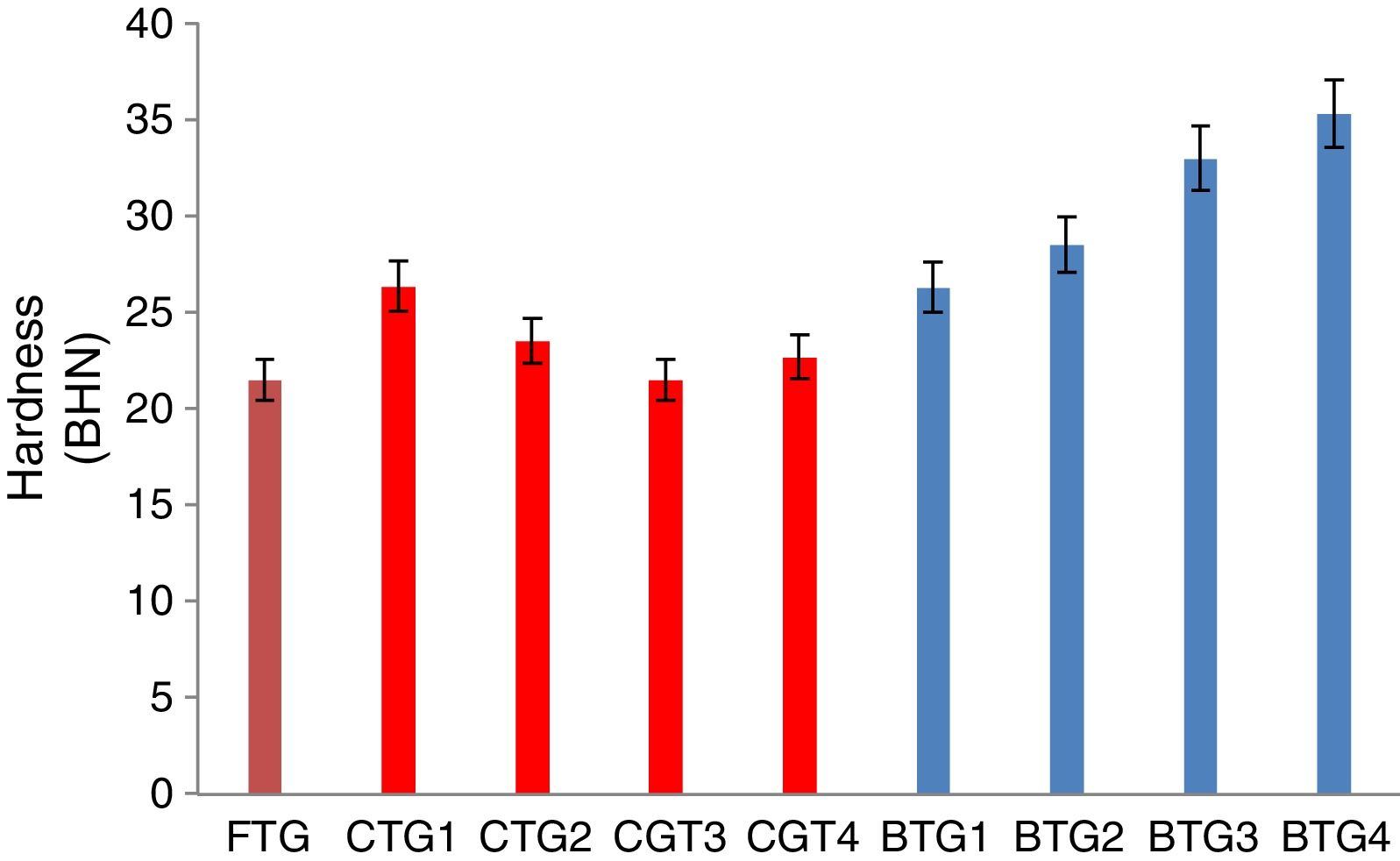
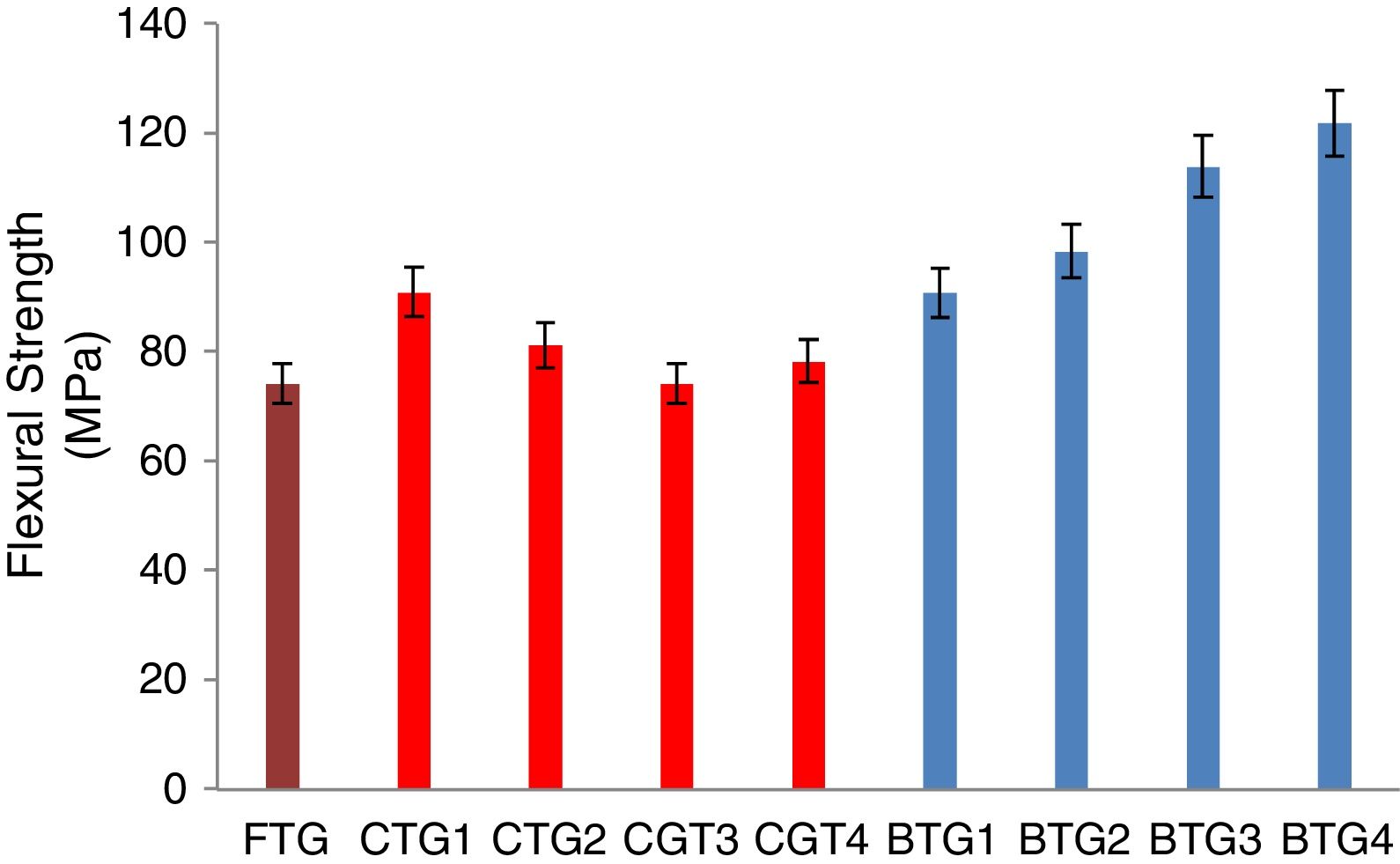
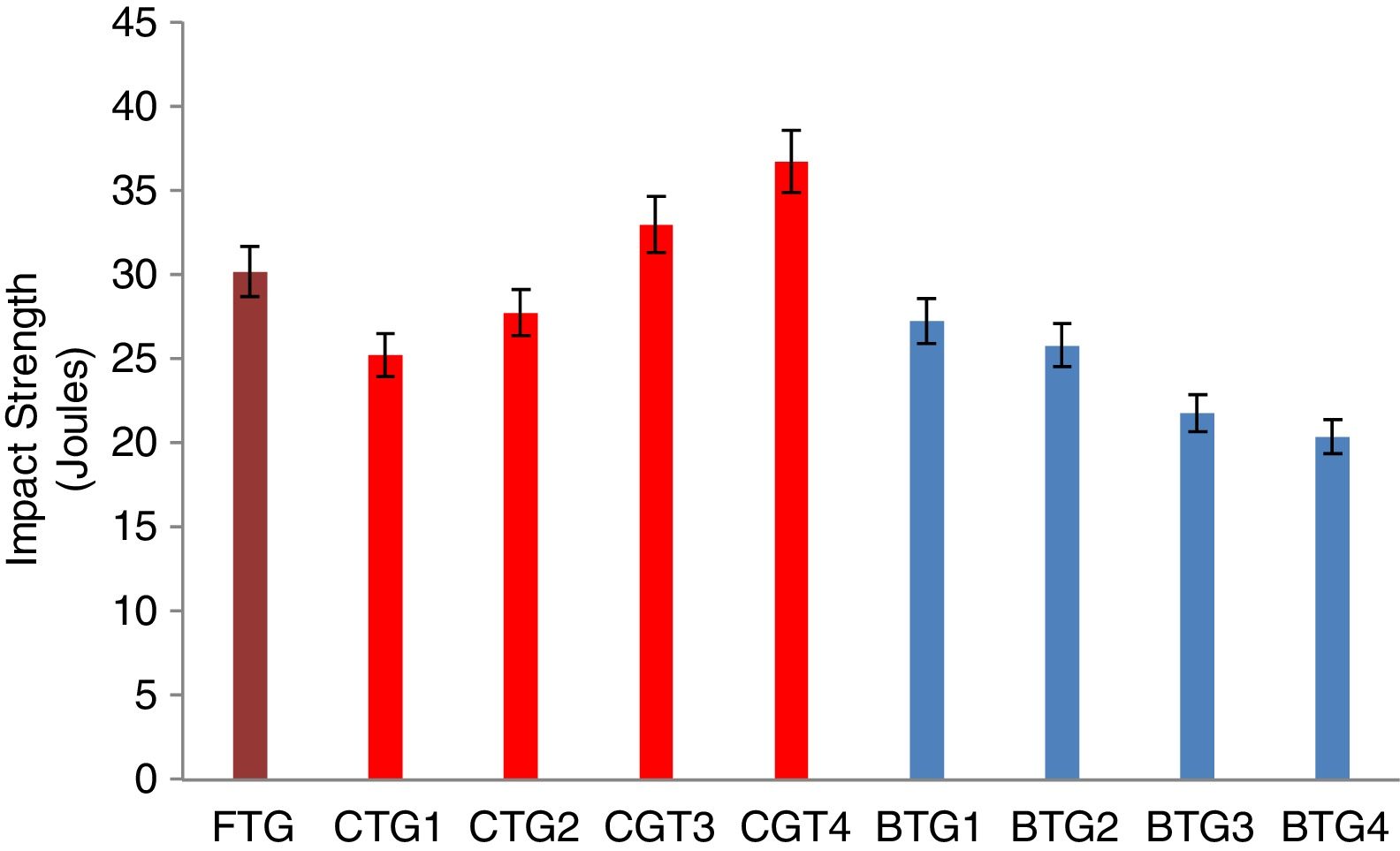
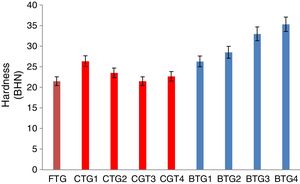
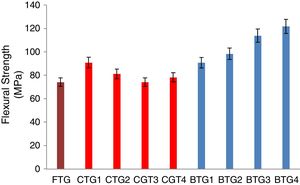
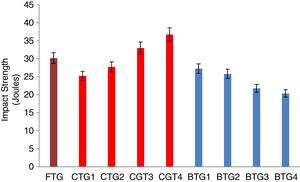
Mechanical behaviorThe results of the evaluated mechanical behaviors are presented in Figs. 4–6. Fig. 4 shows the result of the hardness values of the FTG, CTGs and the developed BTGs, Fig. 5 indicates the flexural strength of the FTG, CTGs and BTGs while Fig. 6 represents the impact strength values of the FTG, CTGs and the developed BTGs respectively.
From Fig. 4, it will observed that the all the chemically toughened glass samples (CTGs) showed superior hardness value over the FTG (fully toughened glass) with maximum hardness of 26.35 BHN observed in CTG1 which justifies reports from literatures that CTG are harder compare with FTG [2,4]. However, the BTGs showed better improvement in hardness values over both the CTG and FTG samples as temperature and immersion period increases with maximum hardness value of 35.30 BHN observed for BTG4. This superior hardness displayed by the BTGs might be attributed to resistance to surface plastic deformation offered by the compacted nodule-like structure uniformly distributed throughout the matrix. From Fig. 5, it can be observed that the strength values showed similar trends as observed in hardness (Fig. 4). The developed bi-treated glass samples (BTGs) displayed superior strength both over the FTG and CTGs respectively. The strength of the BTGs increases as the temperature and time increased with BTG3 and BTG4 displaying the maximum strength value of 113.85MPa and 121.79MPa respectively. The CTGs showed higher strength compare with FTG, however, the strength of the CTGs was observed to decrease with immersion time and temperature which might be due to the lateral crack observed in their structure (as shown in Fig. 2). The combining effects of chemical and thermal tempering enhance the strength behavior of the BTGs. In Fig. 6, the result of the impact strength is in contrast with the hardness and strength values as shown in Figs. 4 and 5 respectively. The BTGs suffer poor impact strength in comparison with FTG and CTGs as their impact strength decreases with temperature and immersion time, this actually justifies the concluding report from Zaccaria and Overend [4] that it might be difficult to achieve improvement in all the mechanical properties simultaneously. However, the CTGs showed increase in impact strength as temperature and time progresses over both the FTG and BTGs.
ConclusionsExperimental investigation on microstructure characteristics and mechanical behavior of a developed bi-treated soda lime glass has been investigated. The results show that:
- •
In terms of microstructure, the bi-treated soda lime glass (BTG) displayed a compacted nodule-like structure with several crystalline grains which are evenly dispersed throughout the glass matrix impeding easy crack propagation thus enhancing the mechanical behavior indicating the beneficial effect of combining chemical and thermal tempering.
- •
The bi-treated glass (BTG) displayed the highest hardness values and flexural strength which improves significantly as temperature and time increase over both FTG and CTG respectively.
- •
The BTGs suffer decrease in impact strength in comparison with FTG and CTG showing that it might be difficult to enhance all the mechanical properties simultaneously indicating future work in that aspect.