The paper presents the results of investigation the novel and simple method of synthesis of silicon carbide. As raw material for synthesis was used shungite, natural mineral rich in carbon and silica. The synthesis of SiC is possible in relatively low temperature in range 1500–1600°C. It is worth emphasising that compared to the most popular method of SiC synthesis (Acheson method where the temperature of synthesis is about 2500°C) the proposed method is much more effective. The basic properties of products obtained from different form of shungite and in wide range of synthesis temperature were investigated. The process of silicon carbide formation was proposed and discussed. In the case of synthesis SiC from powder of raw materials the product is also in powder form and not requires any additional process (crushing, milling, etc.). Obtained products are pure and after grain classification may be used as abrasive and polishing powders.
Este trabajo presenta un método novedoso de obtención de carburo de silicio a partir de shunguita, mineral natural rico en carbono, y sílice. La síntesis se realiza a temperatura relativamente baja (1.500-1.600°C) respecto a las temperaturas (∼2500°C) requeridas en el método Acheson. Se describen la propiedades básicas de los productos obtenidos a partir de diferentes formas de shunguita y utilizando una amplia gama de temperaturas de síntesis. Se propone un proceso de formación del carburo de silicio. Cuando se obtiene el carburo de silicio a partir de materias primas en forma de polvo, el producto tiene también esta forma y no requiere ningún proceso adicional (trituración, molienda, etc.). Una vez clasificados los tamaños, el producto puede utilizarse directamente como abrasivo.
Shungite is a unique coal-like mineral resource discovered in 1879 in the Onega region, which is located near the village of Shunga in Karelia, Russia. According to one of the definitions, shungite is a type of amorphous coal ranked between anthracite and graphite [1]. Therefore, carbon is the one of the main chemical component of shungite; the amount of carbon may range from 10% to 100%. Depending on carbon content in shungite, it is possible to distinguish shungite-1 with a carbon content of 98–100%; shungite-2 with a carbon content of 35–80%; shungite-3 with a carbon content of 20–35%; shungite-4 with a carbon content of 10–20% and shungite-5, in which carbon content is less than 10%. Chemical composition of shungite is determined by the location of deposit. In addition, shungite composition may include SiO2, Al2O3, TiO2, Fe2O3, MgO, K2O and S. Shungite has been known in Karelia since the middle of the 18th century. It was used for the production of paints and as a construction and decorative material in the Cathedral of the Icon of the Mother of God, the Winter Palace and the State Russian Museum in Saint Petersburg. In the 19th and 20th centuries, several attempts were made to use shungite as a substitute for hard coal. In the 1970s, an extensive use of shungite as an insulating material had its beginning. Shungite aggregates sourced from the Nigozero deposit, once subject to thermal processing at temperatures ranging from 1090 to 1130°C, become ball-shaped particles of a low-density of 0.25–0.50g/cm3; the particles are successfully used as light filler materials in concrete structures. Other applications of shungite include electrochemical production of P, Cu, Ni and Co ferroalloys, as a substitute for coke and fusing agents for steel melting, an acid-resistant filler and fire-resistant material, a substitute for graphite in fire-resistant paints and plaster as well as a conductive filler material [1].
There are few papers [2,3] about possibility (in favourable conditions) of carbothermic reduction of silica resulting in the formation of silicon carbide (reaction 1) or metallic silicon (reaction 2).
The natural SiC – Moissanite – has no technological importance. Because of many useful properties of SiC their synthesis is very important and various methods for obtaining silicon carbide in used [4]. The Acheson method is the most popular and well developed SiC synthesis. Also in this method, carbothermic reduction of silica is the basic reaction for SiC formation. At the initial stage, primary SiC (cubic polymorphic 3C form) is obtained. At high temperature above 2500°C, cubic SiC undergoes decomposition and then the secondary SiC in hexagonal polymorphic 6H and 4H forms is crystallised from gaseous phase. Disadvantage of this method is necessary of crushing and milling of coarse-grained product [4].
In this paper the results of investigation the silicon carbide synthesis by carbothermic reduction of silica from shungite are presented. The syntheses were performed with various types of reaction beds, i.e. powder, aggregates and pellets. The different temperature of reaction and temperature rate were applied. Full characteristics of synthesis products were obtained, mainly in terms of phase composition.
Materials and methodsThe chemical composition of used shungite is presented in Table 1. The composition of shungite was determined using the X-ray fluorescence method (XRF) and in case of carbon the thermogravimetric method was used.
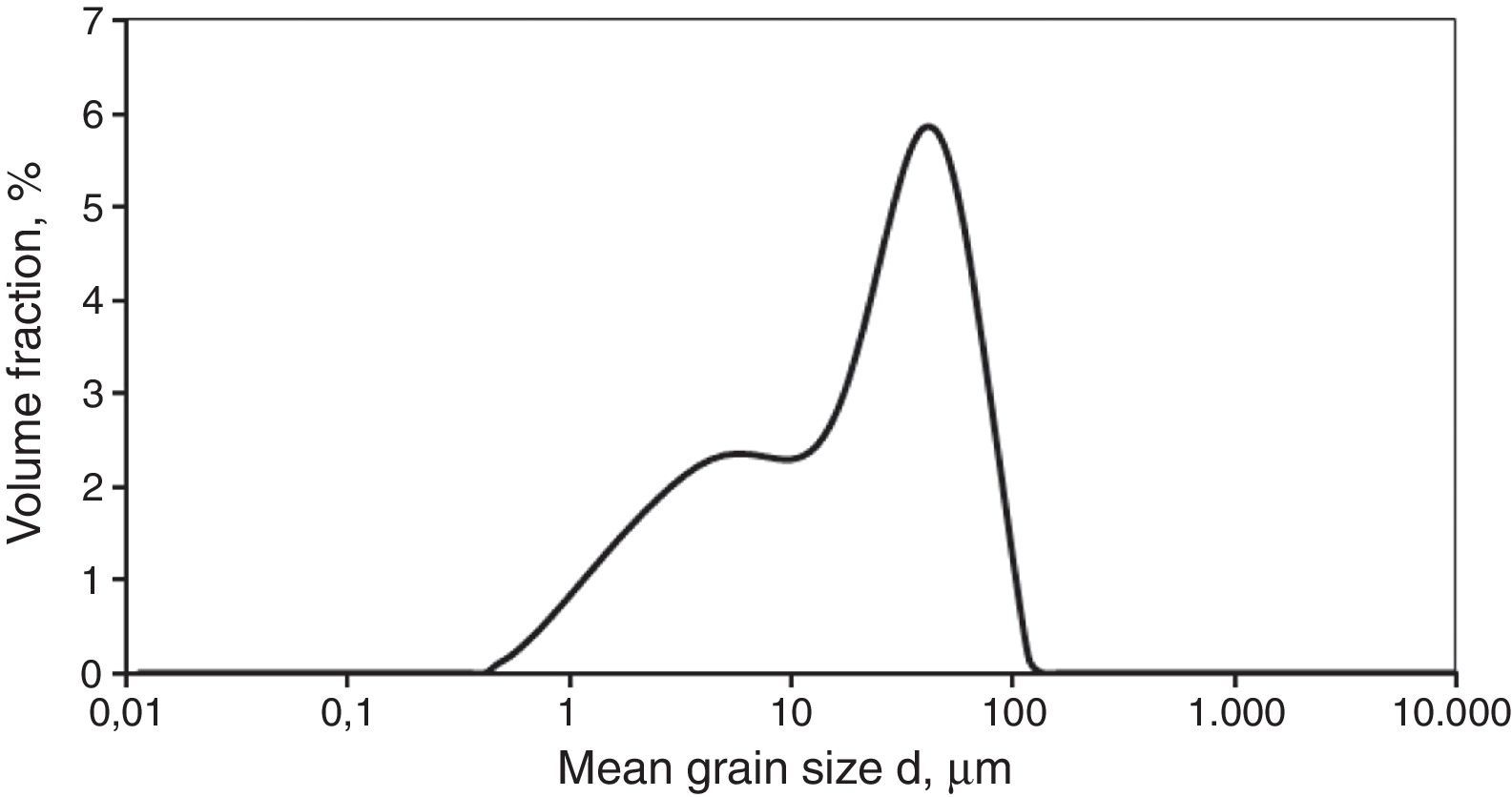
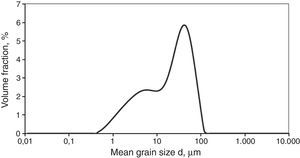
The shungite used for the tests had the form of aggregates, the dimensions of which were from 5 to 30mm. First, the aggregates were crushed to obtain approx. 1–2mm, and then were ground in a rotating-vibration mill in ethyl alcohol for 20h with ZrO2 as the grinding media. The particle size distribution of the obtained shungite powder is presented in Fig. 1.
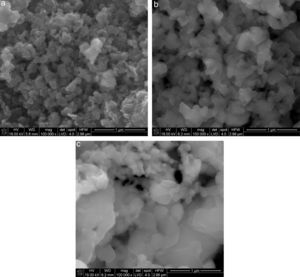
Calculated – from reaction (1) – content of carbon which is required for complete silica reduction in shungite, is 33.8% by mass and is closed to the amount of carbon determined by a thermogravimetric analysis (34.3%), thus any additives were not used. Also the amount of available silicon to reaction with carbon at high temperature was lower due to high partial pressure of silicon (II) oxide and silicon. The syntheses were initially performed in a high-temperature reactor at temperatures 1600, 1800 and 2000°C in the flow of argon. The temperature rate was 15°C per minute. Samples were held in final temperature for one hour. The syntheses were performed on various reaction beds, i.e. shungite aggregates (approx. 10mm in diameter), pellets about 3–4mm in height and 13mm in diameter, formed by uniaxial pressing under pressure of approx. 80MPa and also powder in a SiC crucible. In second case the temperature 1500, 1550 and 1600°C with temperature rate 2°C per minute were applied. The products were characterised in terms of phase composition using the X-ray diffraction (XRD) method. Quantitative phase composition of the synthesis products was determined by the Rietveld method. The crystallites were observed using a SEM microscope (Nova Nano SEM 200; FEI Company). SEM microphotographs are collected in Fig. 3. In order to obtain full characteristics of the SiC syntheses using shungite, the synthesis products and the initial powder were tested using the spectroscopic methods, i.e. infrared absorption spectroscopy (Brucker 70V) and Raman spectroscopy (Horriba Yvon Jobin LabRAM HR). With reference to the aggregates and pellets, measurements of porosity were taken by mercury intrusion porosimetry (MIP) method (Poremaster 60; Quantachrome).
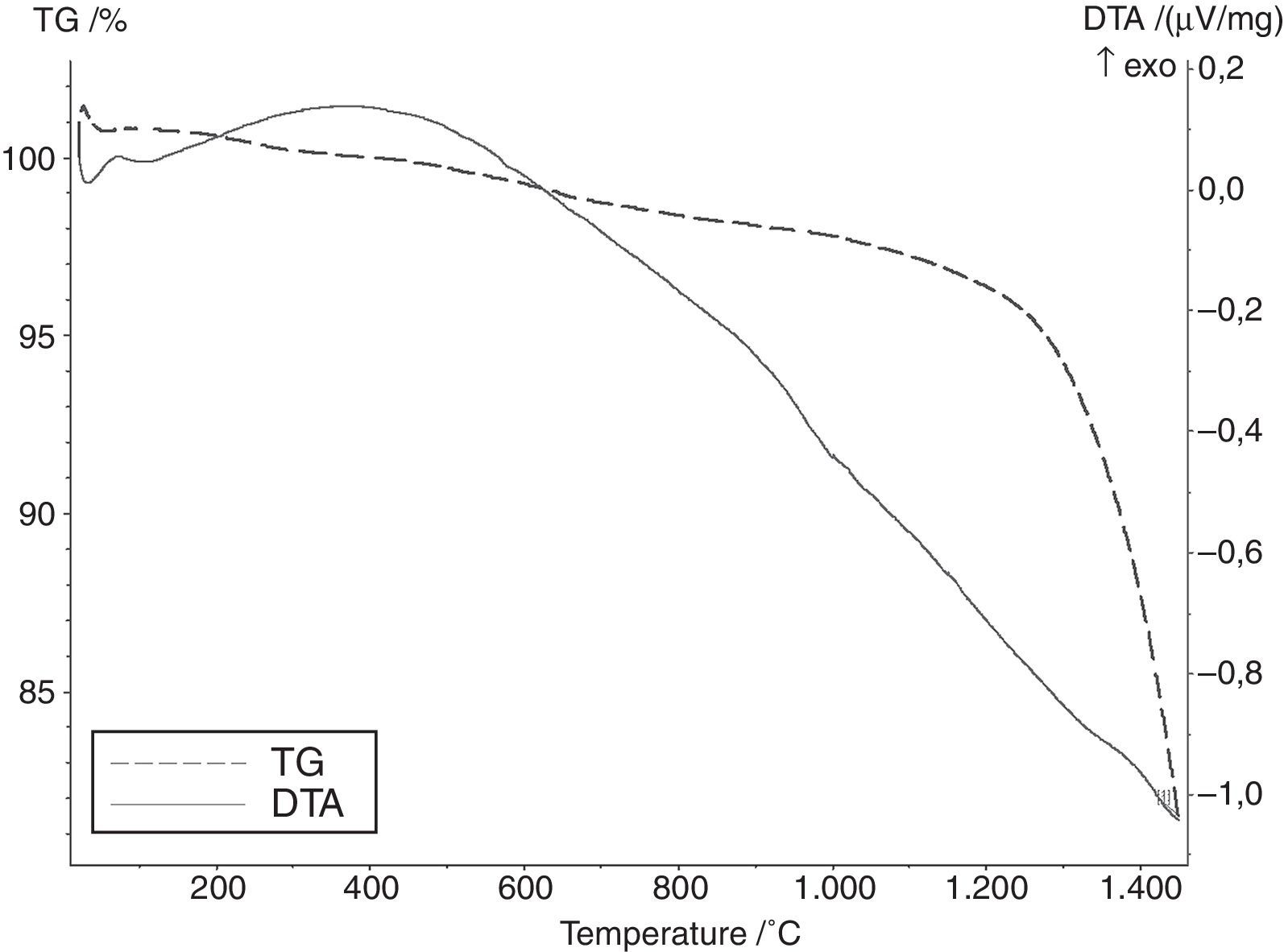
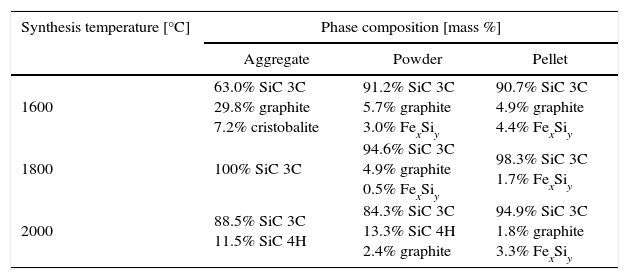
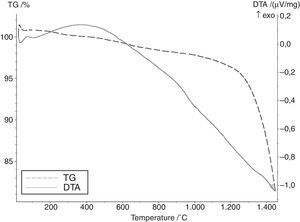
Results and discussionAccording to the literature and calculated data relating to free enthalpy of silica carbothermic reduction (1), the initial temperature of the reaction ranges from 1200 to 1350°C [3]. According to DTA curve presented in Fig. 2 any effects indicating SiC formation were observed up to 1450°C. Results of the phase analyses syntheses carried out at 1600, 1800 and 2000°C are summarised in Table 2.
Phase compositions (XRD) of synthesis products at 1600–2000°C.
| Synthesis temperature [°C] | Phase composition [mass %] | ||
|---|---|---|---|
| Aggregate | Powder | Pellet | |
| 1600 | 63.0% SiC 3C 29.8% graphite 7.2% cristobalite | 91.2% SiC 3C 5.7% graphite 3.0% FexSiy | 90.7% SiC 3C 4.9% graphite 4.4% FexSiy |
| 1800 | 100% SiC 3C | 94.6% SiC 3C 4.9% graphite 0.5% FexSiy | 98.3% SiC 3C 1.7% FexSiy |
| 2000 | 88.5% SiC 3C 11.5% SiC 4H | 84.3% SiC 3C 13.3% SiC 4H 2.4% graphite | 94.9% SiC 3C 1.8% graphite 3.3% FexSiy |
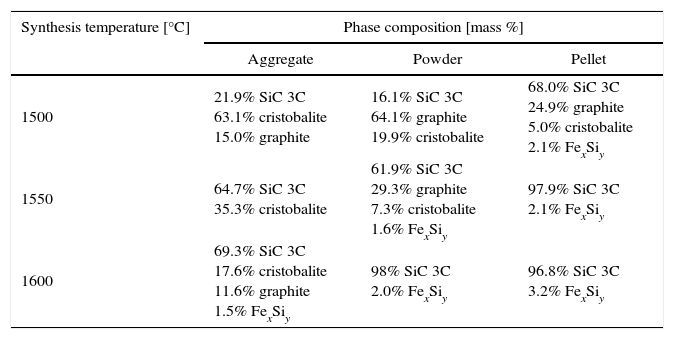
In products obtained at the synthesis temperature of 1600°C silicon carbide is the major phase, for pellets and aggregates and reaction was efficient. The amount of SiC is about 90%. It was the reason for performed the syntheses at lower temperature, even 1500°C but with temperature rate 2°C per minute. In case of aggregates and pellets the products are weak and able to disintegrate under small pressure. Phase composition of samples obtained at lower temperature (1500–1600°C) is collected in Table 3.
Phase compositions (XRD) of synthesis products at 1500–1600°C.
| Synthesis temperature [°C] | Phase composition [mass %] | ||
|---|---|---|---|
| Aggregate | Powder | Pellet | |
| 1500 | 21.9% SiC 3C 63.1% cristobalite 15.0% graphite | 16.1% SiC 3C 64.1% graphite 19.9% cristobalite | 68.0% SiC 3C 24.9% graphite 5.0% cristobalite 2.1% FexSiy |
| 1550 | 64.7% SiC 3C 35.3% cristobalite | 61.9% SiC 3C 29.3% graphite 7.3% cristobalite 1.6% FexSiy | 97.9% SiC 3C 2.1% FexSiy |
| 1600 | 69.3% SiC 3C 17.6% cristobalite 11.6% graphite 1.5% FexSiy | 98% SiC 3C 2.0% FexSiy | 96.8% SiC 3C 3.2% FexSiy |
The calculations and measurements justify the use of low temperatures during the synthesis. As far as the results of phase composition measurements (Table 3) are concerned, they confirm that the SiC synthesis, beginning at a temperature of 1550°C, is possible and effective.
For pellets (as shown by the results of phase composition analyses), silicon carbide starts to appear of reaction bed products at a temperature as low as 1500°C, and the amount is up to 70% by mass. For higher temperature (above 1550°C) completely reaction of SiC formation occurs. At the highest synthesis temperature (1600°C), complete reaction is obtained also for powder. Only for aggregates degree of reaction is smaller and is about 70% at the highest temperature. A by-product of the SiC synthesis is obtained in the form of iron silicides produced as a result of reactions of the iron oxides in the bed with the formed SiC [3].
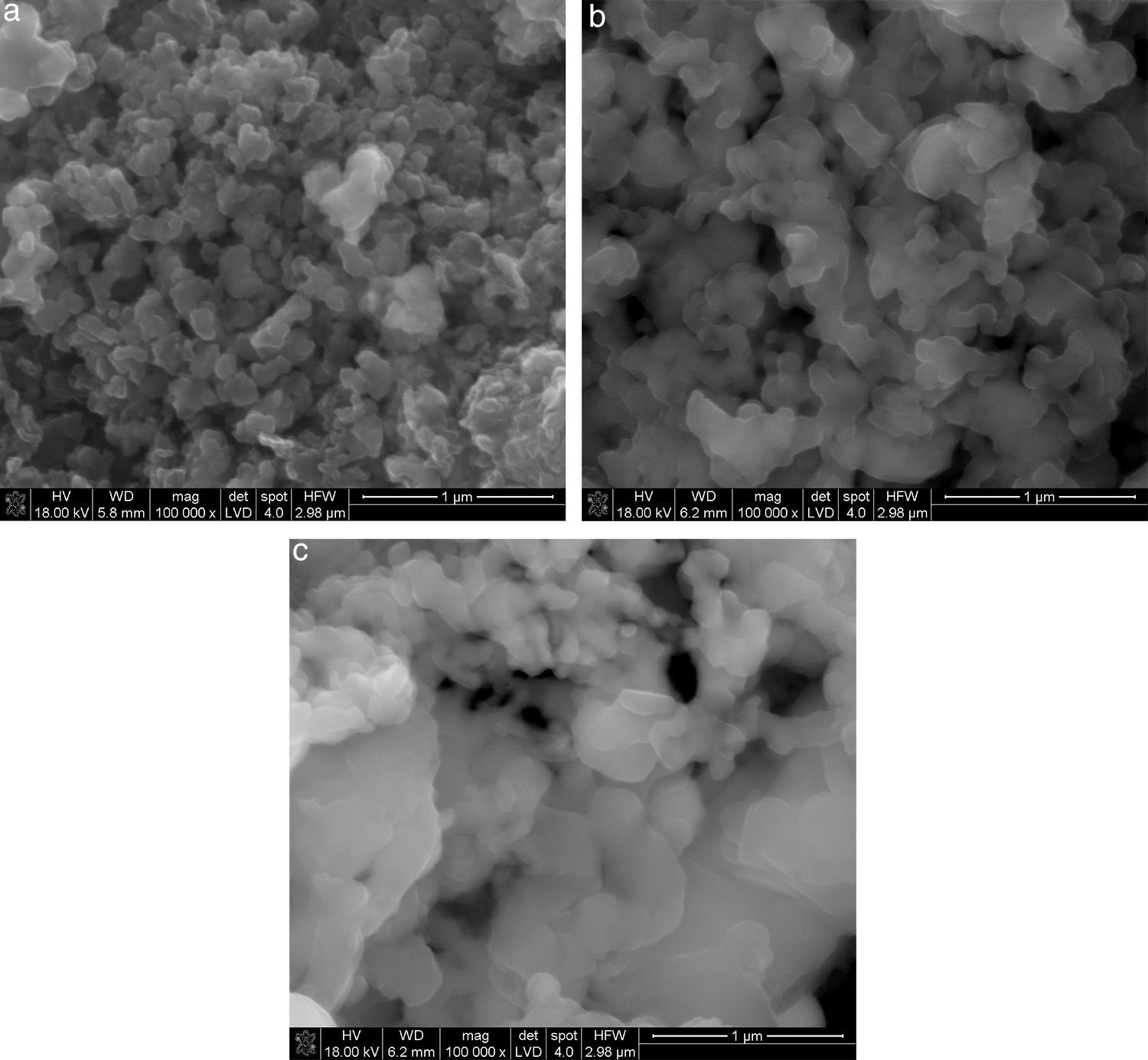
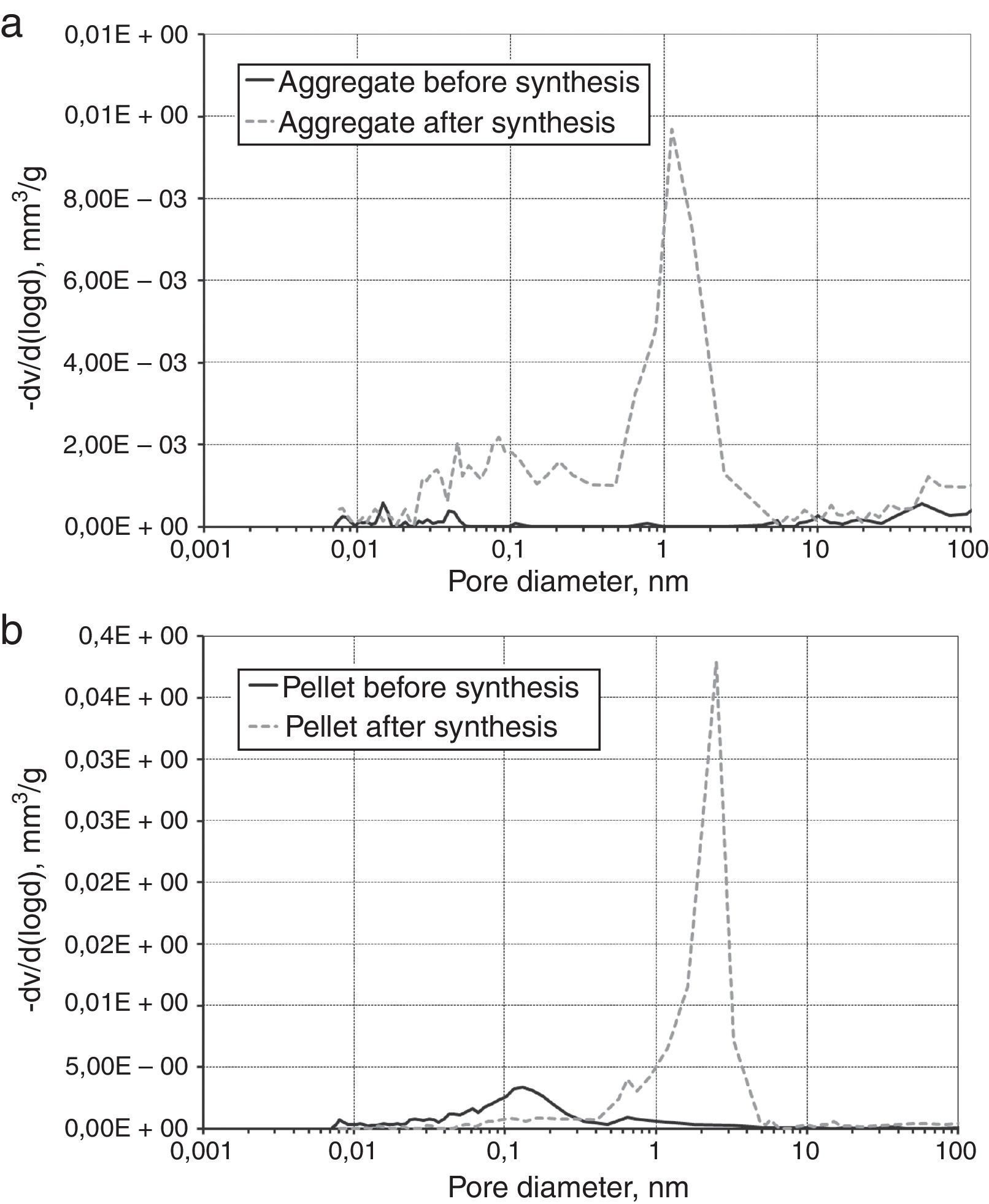
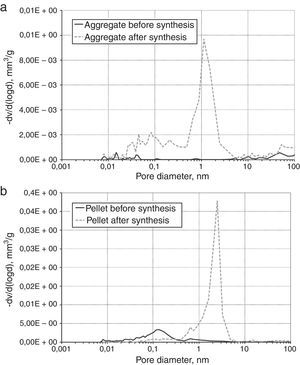
The morphologies of synthesis products in Fig. 3, with the different beds distinguished. Typical form of SiC obtained by carbothermic reduction was observed. For products obtained from solid beds (aggregates and pellets) an open porosity is much higher than before reaction and the large amount of pores about 1μm is visible (Fig. 4).
When the beds are porous (powder and pellet) the free exchange of gaseous products is possible. Consequently the synthesis of SiC is more effective. In case of more compacted aggregates the products are formed at boundaries of interphase contact which creates a diffusion barrier and hinders further reaction of the SiC formation. A consequence of this phenomenon is the presence of unreacted of SiO2 and graphite, as proven by spectroscopic and phase composition investigations.
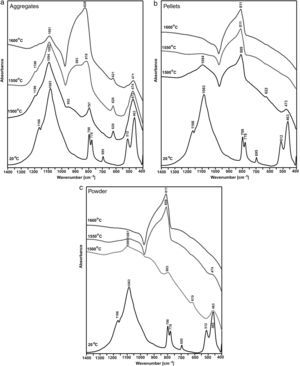
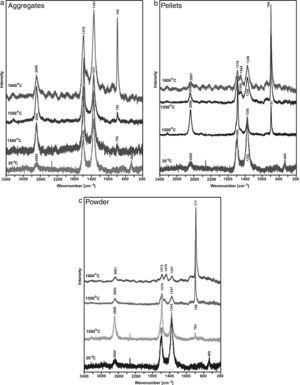
The determination is confirmed by spectroscopic tests presented in Figs. 5 and 6.
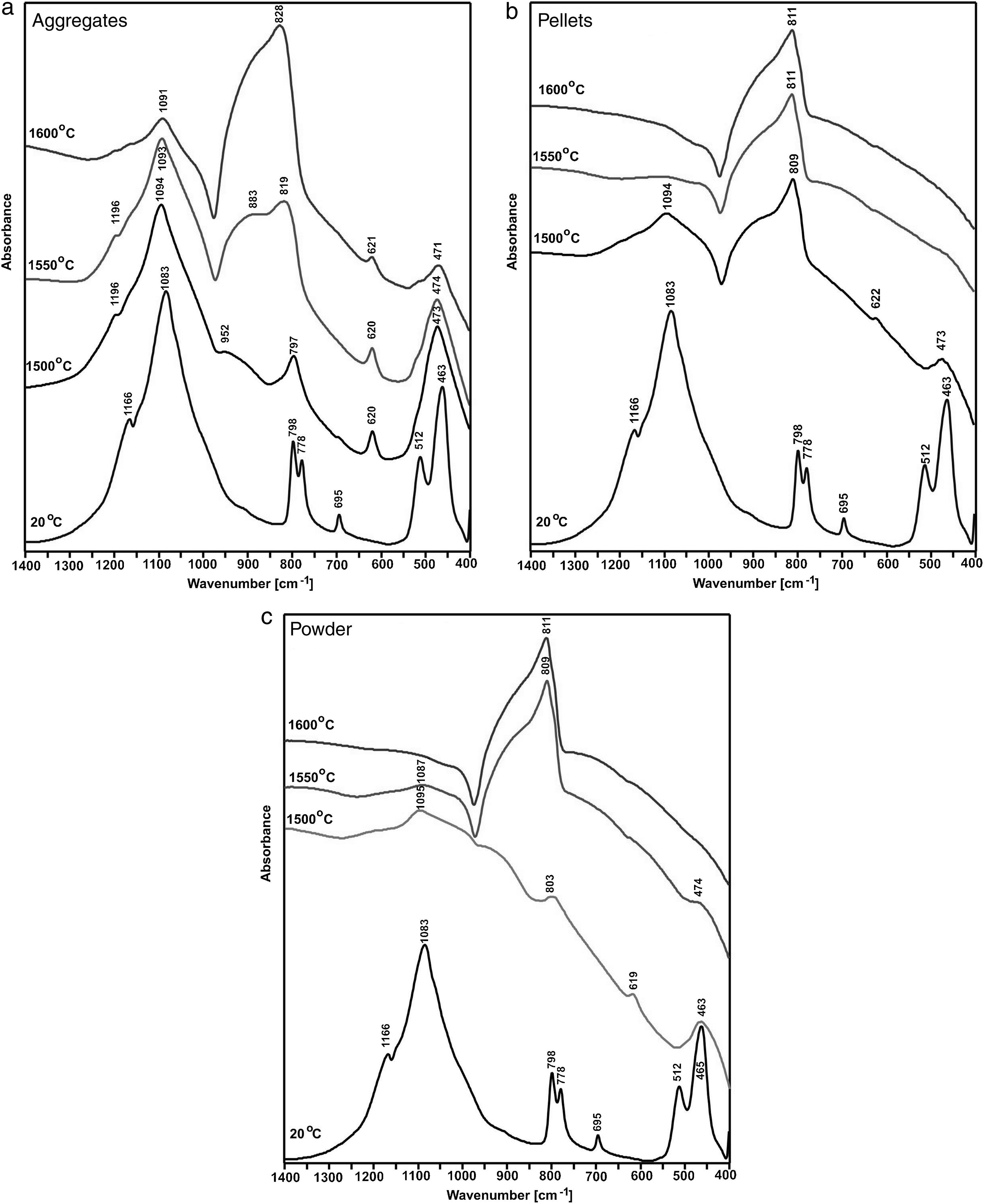
Fig. 5 presents an MIR spectrum of shungite before the reaction and MIR spectra of the reaction products at the synthesis temperatures, i.e. 1500, 1550 and 1600°C. Prior to the reaction, pure β-quartz was identified. The spectrum presents only bands characteristic for β-quartz, which are associated with OSiO bending vibration as well as stretching vibration of SiO(Si) and SiO. The synthesis conducted on aggregates at a temperature of 1500°C results only in polymorphous conversion of β-quartz into β-cristobalite, as indicated by the characteristic bands of β-cristobalite (1196, 1094, 797, 620 and 473cm−1) [5]. The formation of SiC at this temperature may be confirmed by the wide band in the range 950–850cm−1[6,7]. By increasing the synthesis temperature to 1550°C, the amount of SiC being formed is increased as is indicated by the bands in the range 950–820cm−1. The β-cristobalite remains to be the main phase. Further increase in the synthesis temperature by 50°C up to 1600°C in the case of aggregates results in a high increase of SiC being formed. Silicon carbide becomes the main phase, which is indicated by the wide band in the range 950–820cm−1, but the characteristic bands of β-cristobalite are still visible despite the temperature increase. At all MIR spectra of the aggregates after the synthesis the band at c.a. 620cm−1 is present which unequivocally indicates the presence of β-crystobalite.
The measurements for powder and pellets present a different situation, wherein a mixture of SiC and β-cristobalite is formed at the synthesis temperature as low as 1500°C. At a higher synthesis temperature (1550°C), SiC is the dominant phase as indicated by the wide band in the range 950–820cm−1. However, it is possible to identify traces of β-cristobalite as indicated by the bands at 474 and 1087cm−1. Holding at a temperature of 1600°C results in the production of pure SiC (only the wide band in the range 950–820cm−1 is visible). Infrared spectroscopy tests are recommended for compounds, in which the ion component of the bond is dominant. Therefore, the tests reflect the SiO2 reduction process well. A method which is complementary to the infrared absorption spectroscopy is the Raman spectroscopy, which provides identification of compounds, in which the covalent component of the bond is dominant.
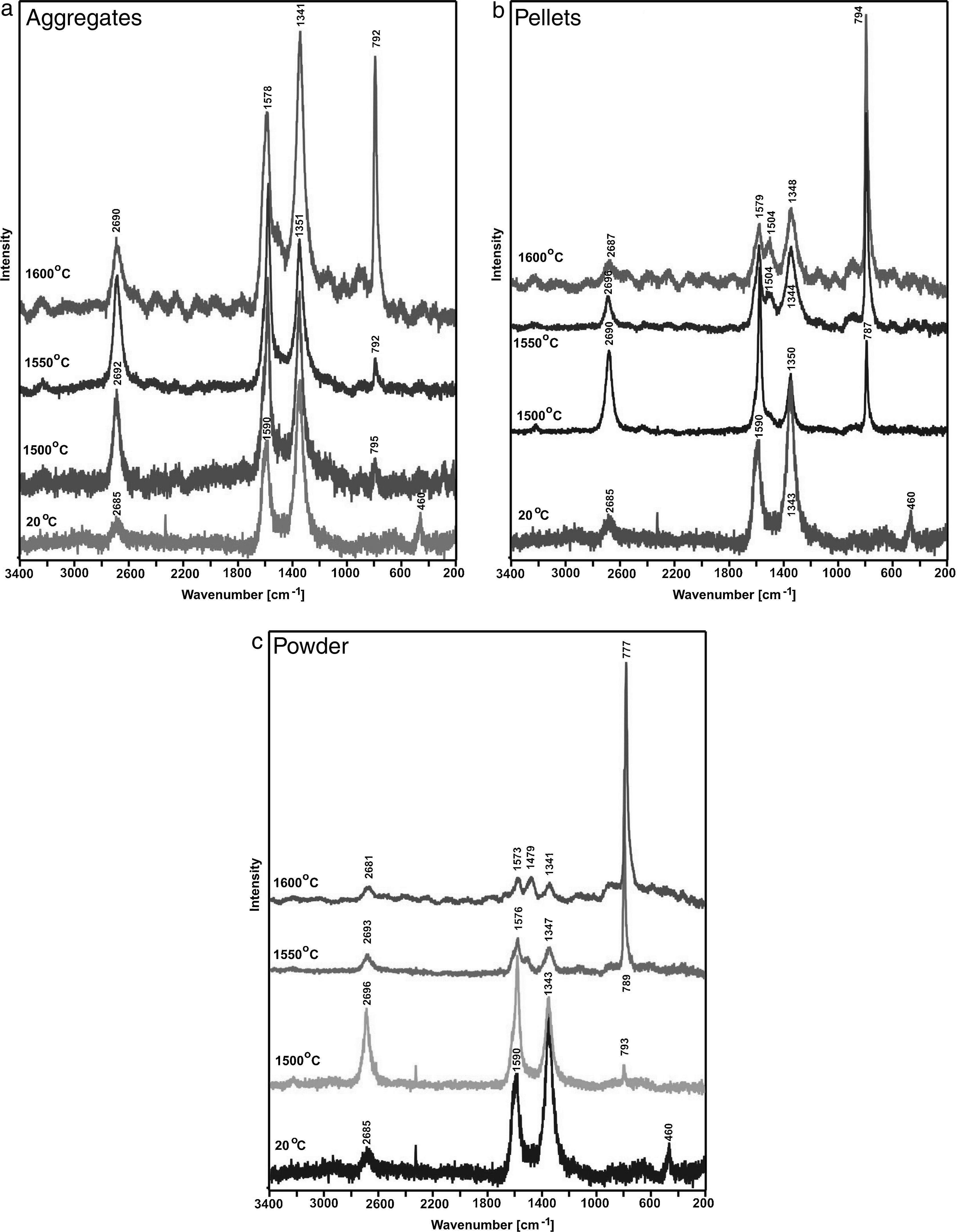
Fig. 6 shows results of the measurements taken for shungite before (20°C) and after the synthesis at the three listed temperatures 1500, 1550 and 1600°C for different reaction beds, i.e. powder, pellets and aggregates. The Raman spectra obtained for shungite before the reaction (at 20°C) show G bands (approx. 1580–1570cm−1) [8,9] and G′ bands (approx. 2690cm−1) [10], which indicates the presence of carbon with sp2 hybridisation. However, this is not crystalline, structurally ordered graphite, but defected one as a very intensive band at approx. 1340cm−1 (the so-called D band) is visible. This various degree of graphite defectiveness is indicated by various ratios of the bands: G (approx. 1580–1570cm−1) to D (approx. 1340cm−1) [11,12]. Holding the samples at a temperature of 1500°C results in the formation of SiC, and a band at approx. 790cm−1[13] appears in the spectra. The band is weak visible for aggregates but best identifiable for pellets. An increase in the synthesis temperature for powder and pellets is the reason why the bands indicating the presence of defective graphite (G and D bands) disappear and the band indicating the presence of SiC at approx. 790cm−1 becomes more intensive. The higher the synthesis temperature, the weaker bands originating from graphite, and stronger bands from the main phase, i.e. SiC. The measurements relating to aggregates present a different situation. As the synthesis temperature increases, the SiC characteristic band becomes more intensive, but the bands for graphite become weaker. Results of spectroscopic analyses correspond to with the phase composition analysis performed by the X-ray diffraction method; independently of the synthesis temperature, the SiC formed is present together with unreacted substrates, i.e. graphite and SiO2. This is the ground to suppose that the size reduction operations, secondary homogenisation and formation create favourable conditions for the increase in the number of contact spots between the substrates.
According to the phase composition analysis, the resulting SiC has a cubic polymorphic form. As stated in the paper by Knippenberg [14], the cubic form of SiC (3C) is unstable over the entire temperature range. Therefore, it may be taken as a surprising fact that only this polymorphic form is present in products obtained from the syntheses. In other work describing SiC crystallisation, an important role in stabilisation of the cubic polymorphic form of SiC is attributed to silicon [15]. The simplest reaction of SiC formation is the reaction of elements i.e. silicon and carbon. When the synthesis between solid compounds takes place the silicon carbide is formed due to diffusion of carbon into the silicon structure or, in the second case, the carbon is dissolve in liquid silicon [16,17]. In both cases, crystallisation is initiated by the formation of SiC nuclei in silicon and continues through the stage of formation of solid solutions or alloys [18]. Cubic silicon structure is isostructural with SiC. This is the reason why the cubic form is preferable.
As regards the SiC synthesis using shungite, it must be taken into account that the silica in shungite may be reduced to silicon and the formation of the unstable cubic SiC form is preferred. If this suggestion is accepted, the following stages of the SiC synthesis may be proposed. At the initial stage, silicon (II) oxide and carbon (II) oxide are formed in accordance with the reactions (3–5):
orNext, in accordance with assumptions made by Knippenberg [14], the reduction of silicon (II) oxide is continued, resulting in the formation of free silicon. As a consequence, the reaction of fixed or liquid silicon with carbon and the formation of the cubic polymorphic SiC form occur. The situation is represented by the following reactions (6 and 7):
As the SiC synthesis takes place at temperatures higher than the melting point of silicon – 1414°C – it may be assumed that carbon can be dissolved in liquid silicon and the alloy can be supersaturated, leading to silicon carbide crystallisation.
ConclusionsIn this paper, advisability of the SiC synthesis using shungite has been proven. Advantages of the synthesis must mainly refer to the low temperature – 1600°C – as the Acheson method used on the industrial scale requires temperatures exceeding 2500°C. It is worth noticing that in case of powder and pellets the products are also in powder form or need small forces to disintegrate samples. Obtained products can be simple applied as abrasive or polishing powders. For basic disadvantages of the synthesis, there is variability of the chemical composition in the shungite. Therefore, it is necessary to provide uniformity of the bed batch before synthesis and – if required – the correction of composition is easy to perform by using additives e.g. carbon.
The work was performed within statutory research realised on Faculty of Material Sciences and Ceramics AGH University of Materials Science, No. 11.11.160.617.