Mullite is one of the preferred aluminosilicates for both traditional and advanced ceramics. This paper reports the use of simplex-centroid mixture design to prepare mullite–glass ceramics by reactive sintering. The phase composition and technological properties of formulations containing kaolinitic clay, kaolin waste and alumina were investigated after sintering at 1400°C. The sintering behavior was assessed by dilatometry. Microstructural analysis indicated the crystalline phases mullite, residual quartz, cristobalite, and α-alumina. A glassy phase was also identified as a matrix embedding mullite grains (as seen by X-ray diffractometry and scanning electron microscopy). In kaolin waste-rich formulations, a liquid phase sintering mechanism favored the densification process and the mechanical resistance. Highly significant statistical models allow correlating the concentrations of raw materials with linear firing shrinkage, water absorption and apparent porosity. Overall results stand out for the potential of using kaolin waste in the preparation of mullite–glass ceramics.
La mullita es uno de los aluminosilicatos preferidos para la producción de cerámicas tradicionales como avanzadas. Este artículo informa sobre el uso del diseño de mezclas simplex con centroide para preparar materiales cerámicos de mullita-vidrio a través de la sinterización reactiva. Las fases presentes en el material y las propiedades de las formulaciones que contienen arcilla caolinítica, residuos de caolín y alúmina se investigaron después de la sinterización a 1.400°C. El comportamiento del material cerámico sinterizado se evaluó por dilatometría. Por otra parte, el análisis microestructural mostró la presencia de fases cristalinas de mullita, cuarzo residual, cristobalita y α-alúmina. También se identificó una fase vítrea como una matriz que incorpora granos de mullita (se demostró por difractometría de rayos X y microscopía electrónica de barrido). En las formulaciones ricas en residuo de caolín, la sinterización en fase líquida favoreció el proceso de densificación y la resistencia mecánica. Los modelos estadísticos altamente significativos permiten relacionar las concentraciones de materias primas con la contracción de cocción lineal, la absorción de agua y la porosidad aparente. Los resultados generales destacan el uso de residuo de caolín en la preparación de materiales cerámicos tipo mullita-vidrio.
Mullite is one of the most essential aluminosilicates, being the only stable intermediate compound in the SiO2–Al2O3 system, with the composition 3Al2O3·2SiO2 corresponding to 71.8wt.% Al2O3[1]. The technological importance, combined with the rare occurrence in nature, highlights the necessity of research on mullite synthesis [2]. Several routes, such as sol–gel process [3], hydrothermal processes [4], chemical vapor deposition [5], and reactive sintering [6] have been used for the synthesis of this mineral. On the other hand, expensive chemical precursors are commonly used. Therefore, it is necessary to use economically viable precursors as a way of reducing production costs, highlighting the potential of kaolin waste for this application [7,8].
The kaolin waste comes from the kaolin mining and processing industry, in which it produces thousands of tons of kaolin per year, being an important economical segment [9]. The recycling and reuse of waste must be seen not only as economic feasibility but also from an environmental point of view. With this in mind, the need to provide a higher added value to the obtained products gains evidence and importance, encouraging the absorption of waste in the productive environment and favoring the culture of reuse [10,11]. As discussed above, the stoichiometric mullite has a molar ratio of 3:2 for alumina:silica; however, kaolin waste and kaolinitic clay have been reported with higher SiO2 content than Al2O3[12]. In this regard, highly reactive alumina has been used with these raw materials to achieve 3Al2O3·2SiO2[13]. The mixture of raw materials is of fundamental importance for several technological sectors, and the properties of the final product can be optimized through the appropriate formulation of raw materials [14]. In this sense, the use of designed experiments has found broad application, both in laboratory research and in industrial developments [15,16]. In a previous work [13], 22 factorial designs were used to study the factors applied pressing pressure and firing temperature for the optimization of solid-state synthesis and characterization of alumina-based composites from kaolinitic clay+aluminum hydroxide and kaolin waste+aluminum hydroxide; nonetheless, the levels of one factor are independent of the levels of another factor. In mixture experiments, the factors are components of a mixture and, as a consequence, their levels are not independent [17]. With this in mind, the simplex-centroid mixture design was used in this work to prepare ceramic formulations containing kaolinitic clay, kaolin waste and alumina. In this regard, a triaxial diagram was adopted using such raw materials and the analysis of variance (ANOVA) and response surface methodology were applied to evaluate the physical properties. A comprehensive compositional, microstructural, and technological analysis was carried out. Our results stand out for the potential of using kaolin waste in the development of high value-added products, such as mullite–glass ceramics.
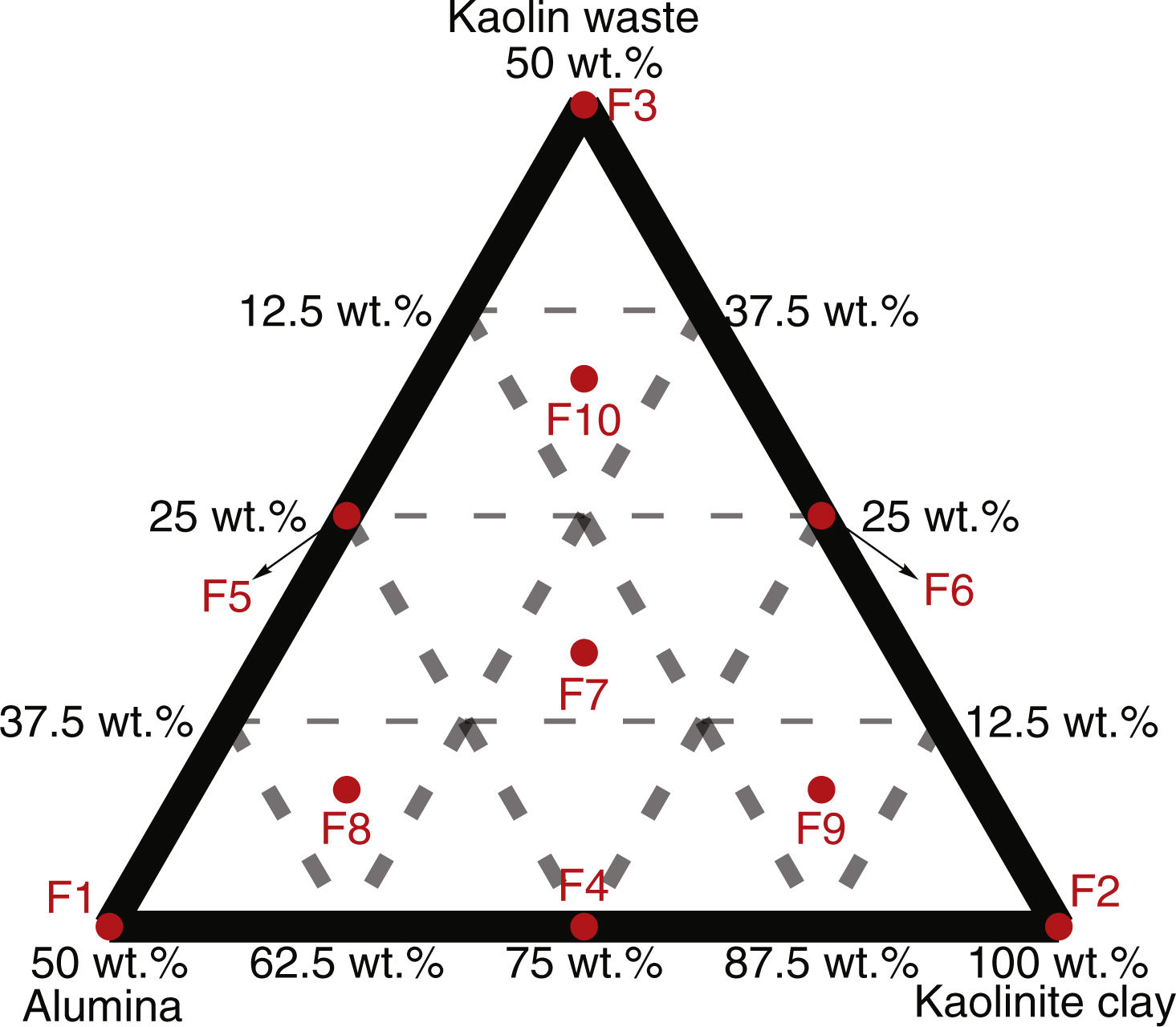
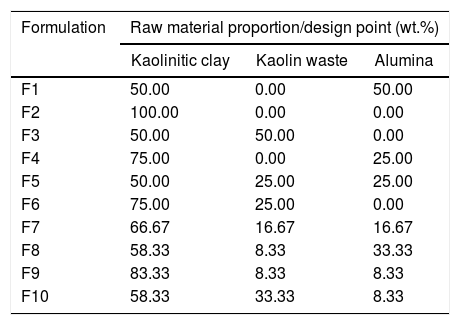
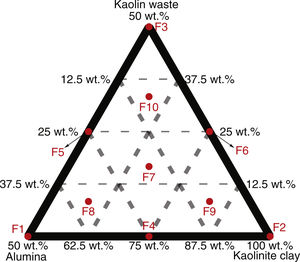
Materials and methodsCeramic formulations were prepared based on the simplex-centroid mixture design using kaolinitic clay, kaolin waste (both from the state of Paraíba – Brazil) and alumina as starting materials. The alumina powder was obtained from the calcination of aluminum hydroxide at 1000°C, as reported in previous works [18,19]. Restrictions related to the amount of each raw material were used as provided in Table S1. Kaolin is a good candidate to prepare mullite-based ceramics by conventional sintering [20,21]. Our group has reported the increase in the amount of glassy phase with increasing the kaolin waste content in mixtures with clay [8,22]. In order to obtain mullite–glass composites, kaolinitic clay content ranged from 50 to 100wt.% in formulations with kaolin waste and extra alumina to react with SiO2. The used formulations are shown in Table 1. Four replicates were run for each formulation. The design points obtained according to a {3,2} simplex-centroid design augmented with interior points are schematized in Fig. 1.
Ceramic formulations using an augmented simplex-centroid design.
| Formulation | Raw material proportion/design point (wt.%) | ||
|---|---|---|---|
| Kaolinitic clay | Kaolin waste | Alumina | |
| F1 | 50.00 | 0.00 | 50.00 |
| F2 | 100.00 | 0.00 | 0.00 |
| F3 | 50.00 | 50.00 | 0.00 |
| F4 | 75.00 | 0.00 | 25.00 |
| F5 | 50.00 | 25.00 | 25.00 |
| F6 | 75.00 | 25.00 | 0.00 |
| F7 | 66.67 | 16.67 | 16.67 |
| F8 | 58.33 | 8.33 | 33.33 |
| F9 | 83.33 | 8.33 | 8.33 |
| F10 | 58.33 | 33.33 | 8.33 |
Starting materials comprising a kaolinitic clay, kaolin waste and alumina were ball milled in aqueous medium for 5h using a weight ratio of powder to alumina balls of 1:3. Suspensions were dried at 110°C for 24h and the resulting powders were deagglomerated and sieved. Rectangular ceramic bodies (61×21×7.6mm, using 6.5wt.% of water as a binding agent) were shaped by uniaxial pressing at 40MPa and subsequently fired at 1400°C for 3h in air using a heating rate of 3°C/min.
The chemical composition of starting powders was obtained by X-ray fluorescence spectroscopy (EDX – Shimadzu, EDX-700). Mineralogical characterization of the raw materials (reported in a previous work [12]) and sintered samples was performed by powder X-ray diffractometry. (XRD – Shimadzu, XRD 7000, using Cu-Kα radiation, 30mA and 40kV). The crystalline phases were identified by comparing the experimental data with patterns registered in the ICDD (International Center for Diffraction Data). Microstructural analysis of fracture samples (without any surface treatment) was carried out using scanning electron microscopy (Supra 35-VP Model, Carl Zeiss and Quanta 450, FEI). Sintering shrinkage behaviors of green rectangular samples were measured by dilatometry using a horizontal pushrod Netzsch DIL 402 PC dilatometer in air from 30 to 1500°C with an Al2O3 Netzsch standard as reference.
The apparent porosity (AP), density (AD) and water absorption (WA) of sintered samples (rectangular bodies) were determined following the Archimedes’ principle in distilled water. Linear firing shrinkage (ΔL) was determined by geometric measurements before and after the sintering process. The mechanical properties of sintered samples were investigated by using the three-point bending test following the ASTM C674 [23]. The physical-mechanical properties were acquired using the relations described Eqs. (1)–(5)[24].
W is the sample weight (g) and ρ is the density of water at room temperature (considered as 1g/cm3 at 25°C). L is the sample length (mm). F is the applied force, Lss is the length of the support span (mm); b and d are width (mm) and thickness (mm) of the sample, respectively.
Fitted models were evaluated in terms of the relative content of each component in the mixture. Regression models and coefficients were determined at the 5% level of significance. Four simplex-centroid designs (for each physical property) were carried out to evaluate the responses measured from the Archimedes’ principle (AP, AD, WA, and ΔL) and the response surface methodology was used for the modeling and analysis of the results.
Results and discussionCompositional and microstructural analysisThe chemical compositions of the raw materials are presented in Table S2. The kaolinitic clay and kaolin waste are essentially composed of silica (SiO2), alumina (Al2O3) and potassium oxide (K2O). The high content of SiO2 and Al2O3 of the kaolinitic clay and kaolin waste highlights the starting materials as potential candidates to be used in the processing of mullite-based ceramics. The purity of the alumina powder derived from calcination of aluminum hydroxide at 1000°C is found to be around 95%. The alumina is an additional source of ions Al3+ in the sintering process of mullite ceramics, due to its insufficient amount in the kaolinitic clay and kaolin waste to form stoichiometric mullite (3Al2O3·2SiO2) [13,19,25]. The small increment of K2O in the waste in comparison to kaolinitic clay (4.82wt.% vs. 0.55wt.%) may explain the relative higher content of mica in the waste material (40.5wt.% vs. 4.3wt.%), as previously discussed by Alves et al. [12]. The loss on ignition (LoI) at 1000°C for the clay (19wt.%) is significantly higher than for the kaolin waste (5.5wt.%), which is associated with the burn-out of organic matter and dehydroxylation of kaolinite [26]. Kaolinitic clay and kaolin waste are composed of kaolinite, mica and quartz, as can be seen in detail in our previous work [12].
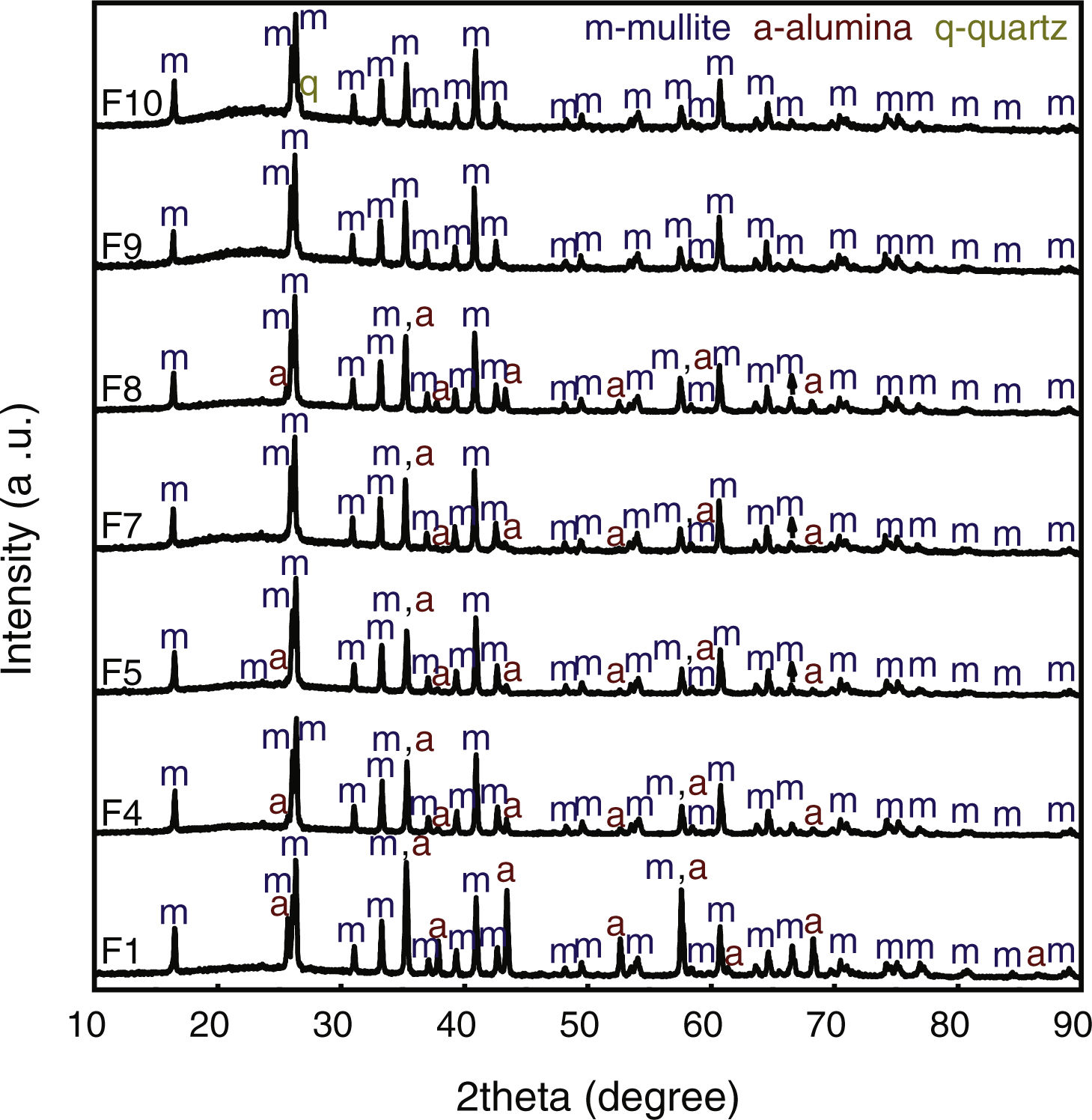
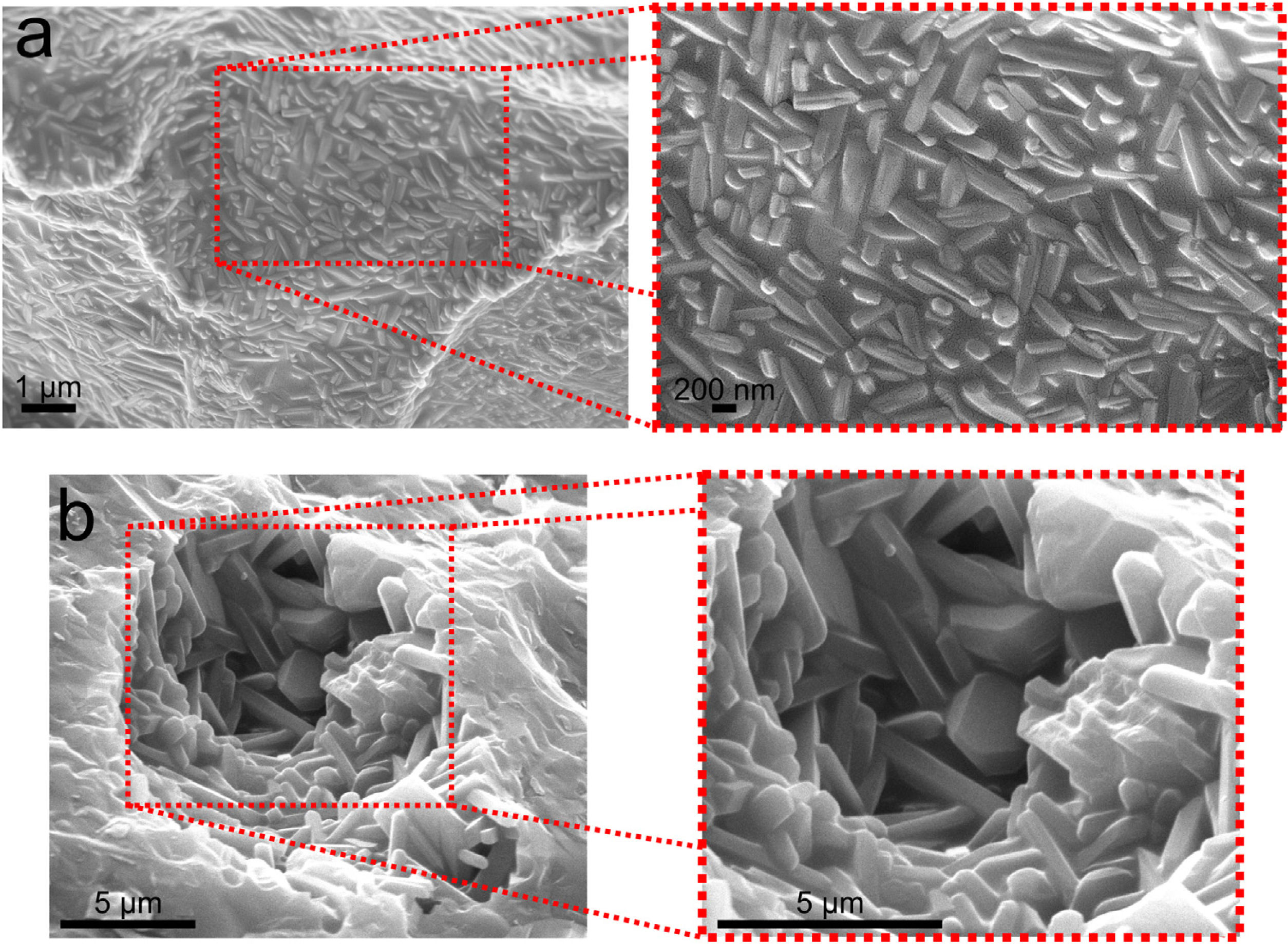
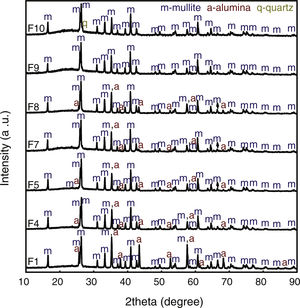
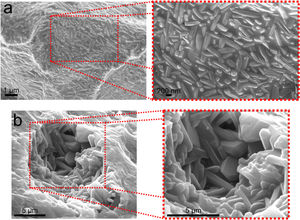
XRD patterns of samples sintered at 1400°C are shown in Fig. 2. XRD data of F2, F3 and F6 have been published in a previous article [12] and they are as Supplementary Material (Fig. S1). The crystalline phases identified were mullite (m, reference code 15-0776), quartz (q, reference code 46-1045), cristobalite (c, reference code 03-0270), and α-alumina (a, reference code 46-1212). The peaks regarding crystalline phases in formulations 1 and 4 (containing only kaolinitic clay and alumina) were indexed as mullite and alumina. In this situation, the SiO2 from kaolinitic clay (crystalized as quartz and cristobalite) react with alumina forming mullite and the excess of Al2O3 crystallizes as α-alumina [27,28]. In F2 (kaolinitic clay), the crystalline phases are mullite, quartz and cristobalite. Quartz is transformed into amorphous silica and partially crystallized into cristobalite [29,30]. In alumina-free formulations (2, 3 and 6), the dissolution of silica during sintering saturates the liquid phase, promoting the crystallization of silica polymorphs at the quartz-liquid interface. The two mullite reflections at 2θ≈26° (planes (120) and (210)) are classically attributed to the presence of orthorhombic mullite (secondary mullite) [31]. Formulations 5, 7 and 8 have the same crystalline phases (mullite and alumina). Formulation 9 has only mullite at 1400°C due to the low amount of alumina (8.33wt.%). The same behavior was observed in formulation 10, but it also has a residual amount of quartz. The mullitization reaction occurs between the particles of Al2O3 and SiO2 by diffusing Al+3 and Si+4 ions through the crystalline lattice [32]. Formulation 10, having more kaolin waste and less alumina than formulation 8, presents the residual quartz phase (2θ≈26.7°). In the XRD patterns, the presence of an amorphous silica-rich phase is observed by the amorphous halo in the region of 2θ=15°–30°. Fig. 3 depicts needle-like mullite embedded in the amorphous silica-rich phase for sample F3 (Fig. 3a) and mullite and alumina grains for sample F1 (Fig. 3b). In all formulations with kaolin waste (the vast majority), mullite grains embedded in a glassy phase are expected, as depicts in Fig. 3a. Raw materials with mica in their compositions present an increased amount of liquid phase during the sintering process, which contributes to the dissolution of silica and a glassy phase is then formed [22]. As predicted by the SiO2-Al2O3-K2O phase diagram, K2O promotes the occurrence of a peritectic liquid at temperatures higher than 1140°C [33]. Kim et al. [34] studied the K2O–Al2O3–SiO2 system with a thermodynamic optimization of experimental phase diagrams. In their study, the liquid phase was described using the Modified Quasichemical Model with the KAlO2 associate component.
XRD patterns of ceramic formulations sintered at 1400°C. F1 – 50wt.% clay and 50wt.% alumina; F4 – 75wt.% clay and 25wt.% alumina; F5 – 50wt.% clay, 25wt.% waste and 25wt.% alumina; F7 – 66.67wt.% clay, 16.67wt.% waste and 16.67wt.% alumina; F8 – 58.33wt.% clay, 8.33wt.% waste and 33.33wt.% alumina; F9 – 83.33wt.% clay, 8.33wt.% waste and 8.33wt.% alumina; F10 – 58.33wt.% clay, 33.33wt.% waste and 8.33wt.% alumina.
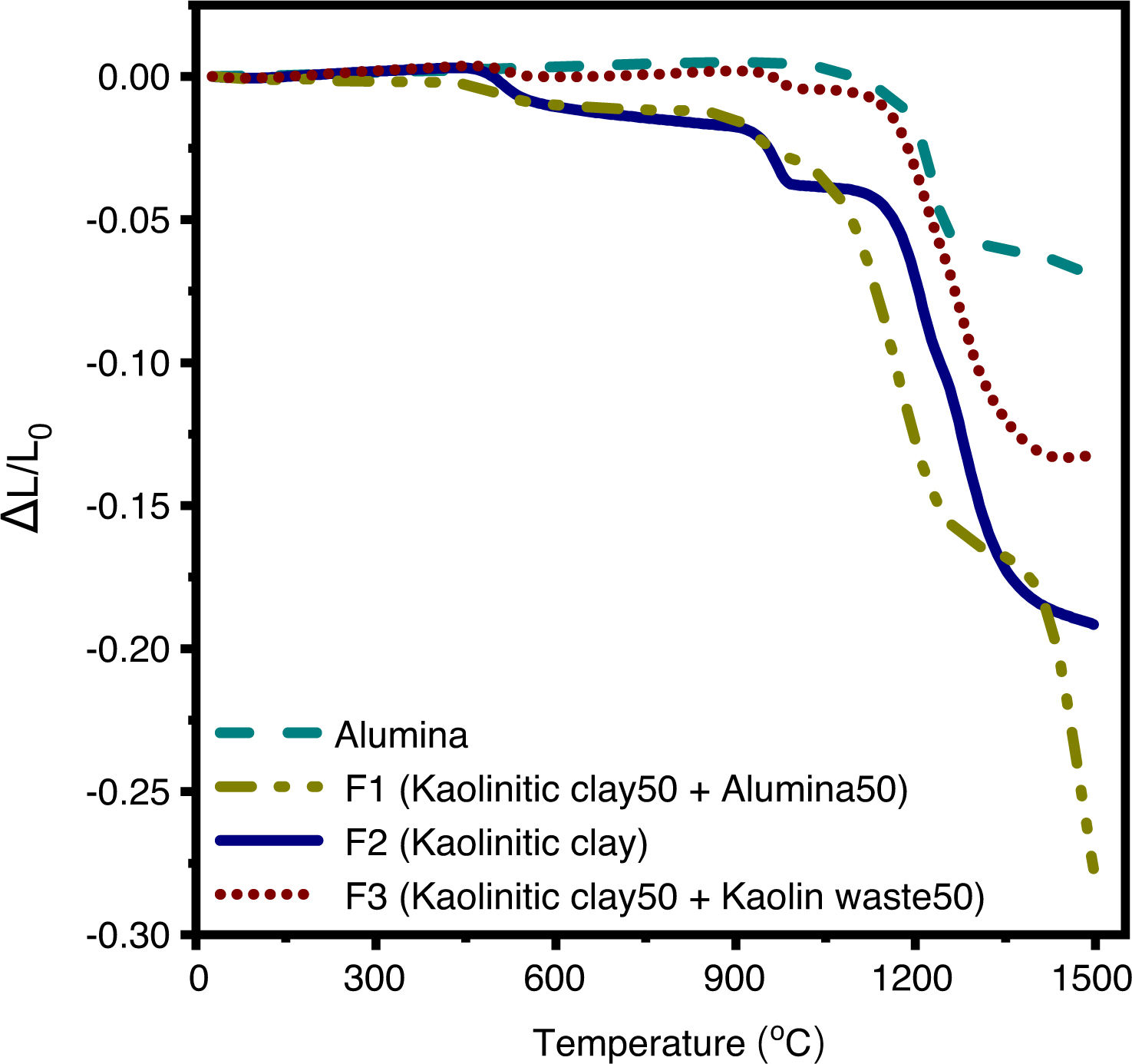
The sintering shrinkage behavior of the formulation 2 (kaolinitick clay), alumina, formulation 1 (kaolinitic clay and alumina, 1:1), and formulation 3 (kaolinitic clay and kaolin waste, 1:1) was investigated via dilatometric analysis, as seen in Fig. 4. In the formulations 1–3, linear shrinkage of about 1% was observed between 450 and 600°C. This fact is related to the transformation of kaolinite into metakaolinite [35]. F1 and F2 samples are less sensitive to this structural transformation (dehydroxylation of kaolinite) due to the lower kaolinite content when compared to the pure kaolinitic clay [12]. Between 900 and 1000°C, kaolinitic clay shows a shrinkage of around 2%, which corresponds to the nucleation of Al-Si spinel and mullite [36]. In formulations with partial substitution of kaolinitic clay by kaolin waste, this shrinkage is less pronounced. This fact can be explained by an increase in volume during the dehydroxylation of the mica [37]. The dilatometric curve of alumina shows the θ-Al2O3→α-Al2O3 phase transformation at 1100–1260°C, in good agreement with the work of Lamouri et al. [38]. As previously reported, 1500°C is not enough to promote the complete densification of alumina [38]. The sintering process starts at approximately 1100°C for the clay-based formulations (F1, F2 and F3). The lower shrinkage observed for the F3 compared to the F2 is due to the higher liquid phase content formed in F3 (formulation with kaolin waste) that accelerates the densification process through a viscous flux mechanism. As previously discussed, K2O contributes to the formation of a peritectic liquid at temperatures higher than 1140°C. As is well known, green density of ceramics is about 50–60% [39]. After the firing process, mullite–glass ceramics densify around 90% at 1400°C [8]. F1 shows events related to the phase transformation of alumina (θ to α) and the densification process; however, as well as for pure alumina, complete densification was not achieved at 1500°C due to the amount of alumina in the formulation (50wt.%).
Dilatometric analysis of the samples F1 (50wt.% clay and 50wt.% alumina), F2 (100wt.% clay), F3 (50wt.% clay and 50wt.% waste), and alumina. Note: F2 and F3 data were obtained from our previous article [12] for comparison.
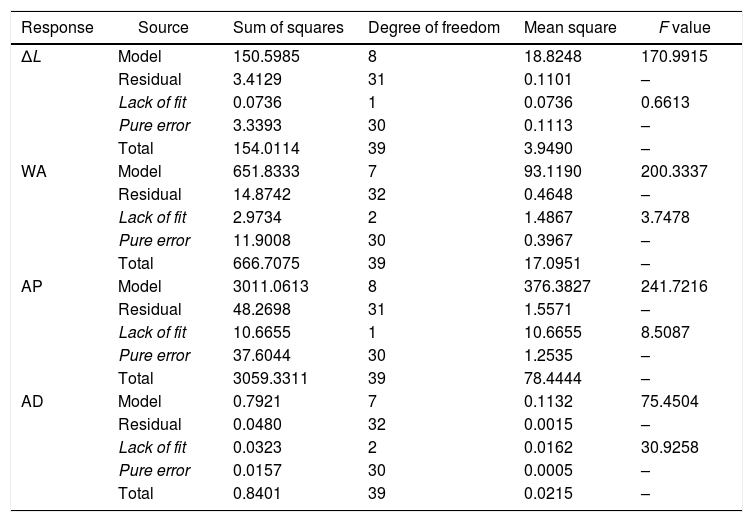
The physical properties are shown in Table S3. From the analysis of variance (ANOVA, Table 2), the experimental data were well fitted to the full cubic model.
ANOVA for the fit of the data in Table S3.
| Response | Source | Sum of squares | Degree of freedom | Mean square | F value |
|---|---|---|---|---|---|
| ΔL | Model | 150.5985 | 8 | 18.8248 | 170.9915 |
| Residual | 3.4129 | 31 | 0.1101 | – | |
| Lack of fit | 0.0736 | 1 | 0.0736 | 0.6613 | |
| Pure error | 3.3393 | 30 | 0.1113 | – | |
| Total | 154.0114 | 39 | 3.9490 | – | |
| WA | Model | 651.8333 | 7 | 93.1190 | 200.3337 |
| Residual | 14.8742 | 32 | 0.4648 | – | |
| Lack of fit | 2.9734 | 2 | 1.4867 | 3.7478 | |
| Pure error | 11.9008 | 30 | 0.3967 | – | |
| Total | 666.7075 | 39 | 17.0951 | – | |
| AP | Model | 3011.0613 | 8 | 376.3827 | 241.7216 |
| Residual | 48.2698 | 31 | 1.5571 | – | |
| Lack of fit | 10.6655 | 1 | 10.6655 | 8.5087 | |
| Pure error | 37.6044 | 30 | 1.2535 | – | |
| Total | 3059.3311 | 39 | 78.4444 | – | |
| AD | Model | 0.7921 | 7 | 0.1132 | 75.4504 |
| Residual | 0.0480 | 32 | 0.0015 | – | |
| Lack of fit | 0.0323 | 2 | 0.0162 | 30.9258 | |
| Pure error | 0.0157 | 30 | 0.0005 | – | |
| Total | 0.8401 | 39 | 0.0215 | – |
Statistical significance can be investigated through the analysis of the relevant statistical parameters (R2, P-value, and F-test). The ratio of the regression sum of squares and the total sum of squares, R2, is attributed to the correlation between the observed response and the value predicted by the adjusted model. For the fitted models, the R2 values of linear firing shrinkage, water absorption, apparent porosity and apparent density were 97.78%, 97.77%, 98.42%, and 94.29%, respectively; however, R2 must not be compared with 100% and pure error must be discounted [40]. Therefore, those R2 values must be compared with 97.83%, 98.21%, 98.77%, and 98.13%, respectively (these values were obtained as follows: (Sum of squarestotal−Sum of squarespureerror)/Sum of squarestotal). These percentages are close to 100% because the contribution of pure error is relatively small. The ratios between the model mean square and the residual mean square for the linear firing shrinkage, water absorption, apparent porosity and apparent density were 170.99, 200.33, 241.72, and 75.45, respectively. Therefore, for linear firing shrinkage (F8,31=2.26), water absorption (F7,32=2.31), apparent porosity (F8,31=2.26), and apparent density (F7,32=2.31), the regressions are highly significant, at the 5% level of significance [40]. However, for the apparent density, the ratio between the lack of fit mean square and the pure error mean square (30.93) compared to the F distribution (F2,30=3.32) showed that the model exhibits a considerable lack of fit and, in this case, the model is not effective in making reliable predictions.
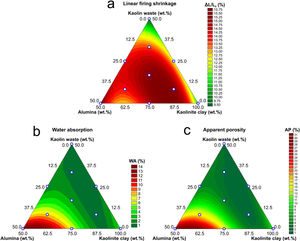
Through the results, regression equations (in terms of pseudo components) for the physical properties were obtained. These Eqs. (6)–(8) are valid for the ranges of each raw material studied here (Table S1). In these equations, A, C, and W correspond to the pseudo component values (0–1): alumina, kaolinitic clay and kaolin waste, respectively.
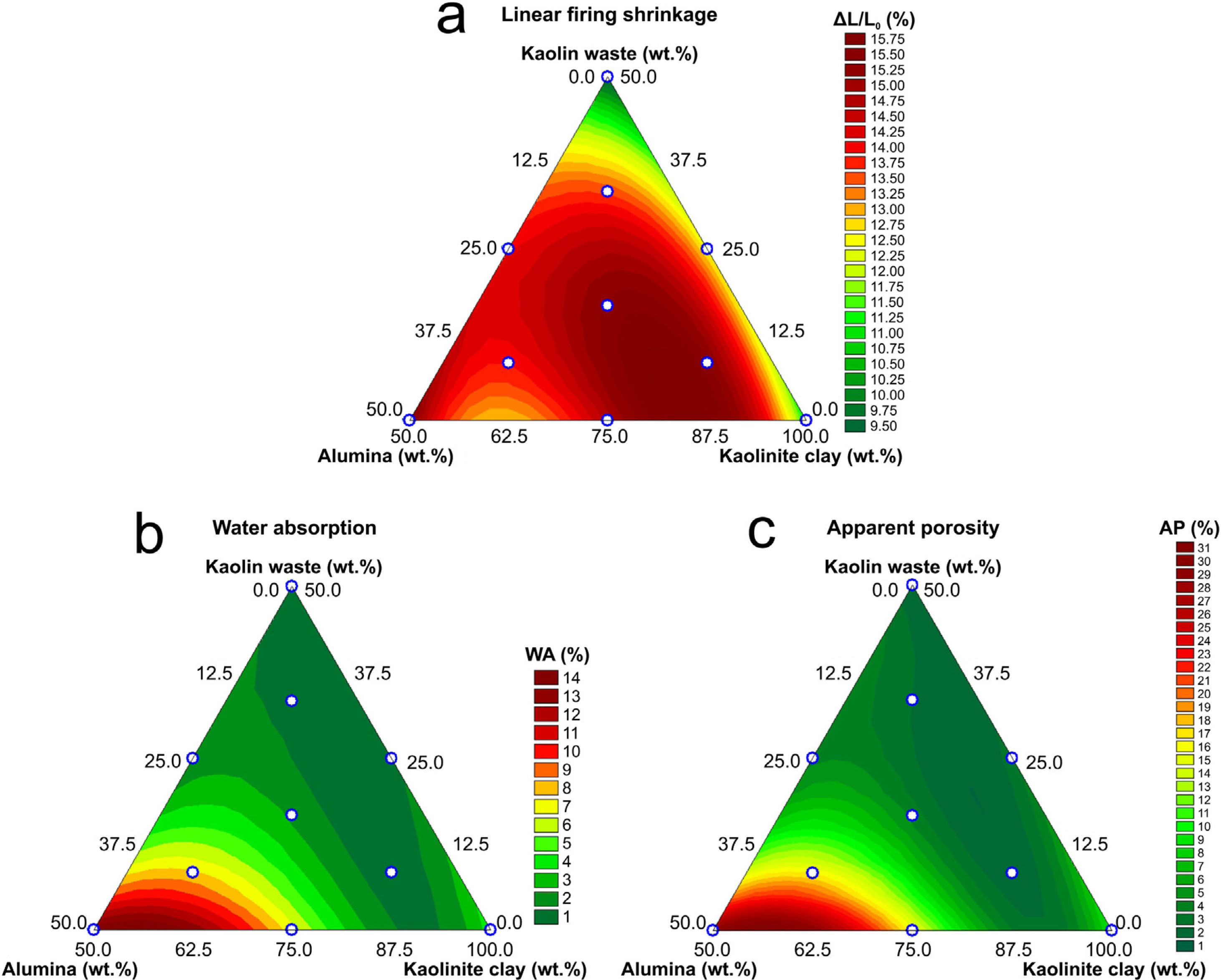
Fig. 5 shows the response surfaces described by Eqs. (6)–(8). In Fig. 5(a), it was possible to observe that formulations with higher kaolin waste content have lower firing shrinkage. This fact can be explained by the liquid phase-assisted sintering mechanism activated by the increased amount of mica from the kaolin waste and therefore filling the open pores [8,13]. From Fig. 5(b, c), a lower open porosity (thereby, water absorption) is observed in formulations with higher kaolin waste content due to the liquid phase-assisted sintering mechanism. On the other hand, solid phase-assisted sintering mechanism is observed for formulations with high alumina content (mullite–alumina composites, Fig. 3b), as reported in a previous work [13]. Formulations with a higher waste content show lower shrinkage and lower porosity due to the liquid that fills the pores, reaching complete densification faster than formulations with a higher alumina content (lower waste content).
Our group has recently studied the electrical–dielectric properties of mullite–glass composites from mixtures of a kaolinitic clay and kaolin waste [8,41]. Ribeiro et al. [42] also studied the electrical properties of mullite–alumina ceramics with the presence of a glassy phase. In all these studies, interesting microstructure-electrical relationships as a function of glass content have been highlighted in mullite-based ceramics. Therefore, from the statistical analysis, it is possible to determine the appropriate formulations according to the target application, e.g., electronics-related applications.
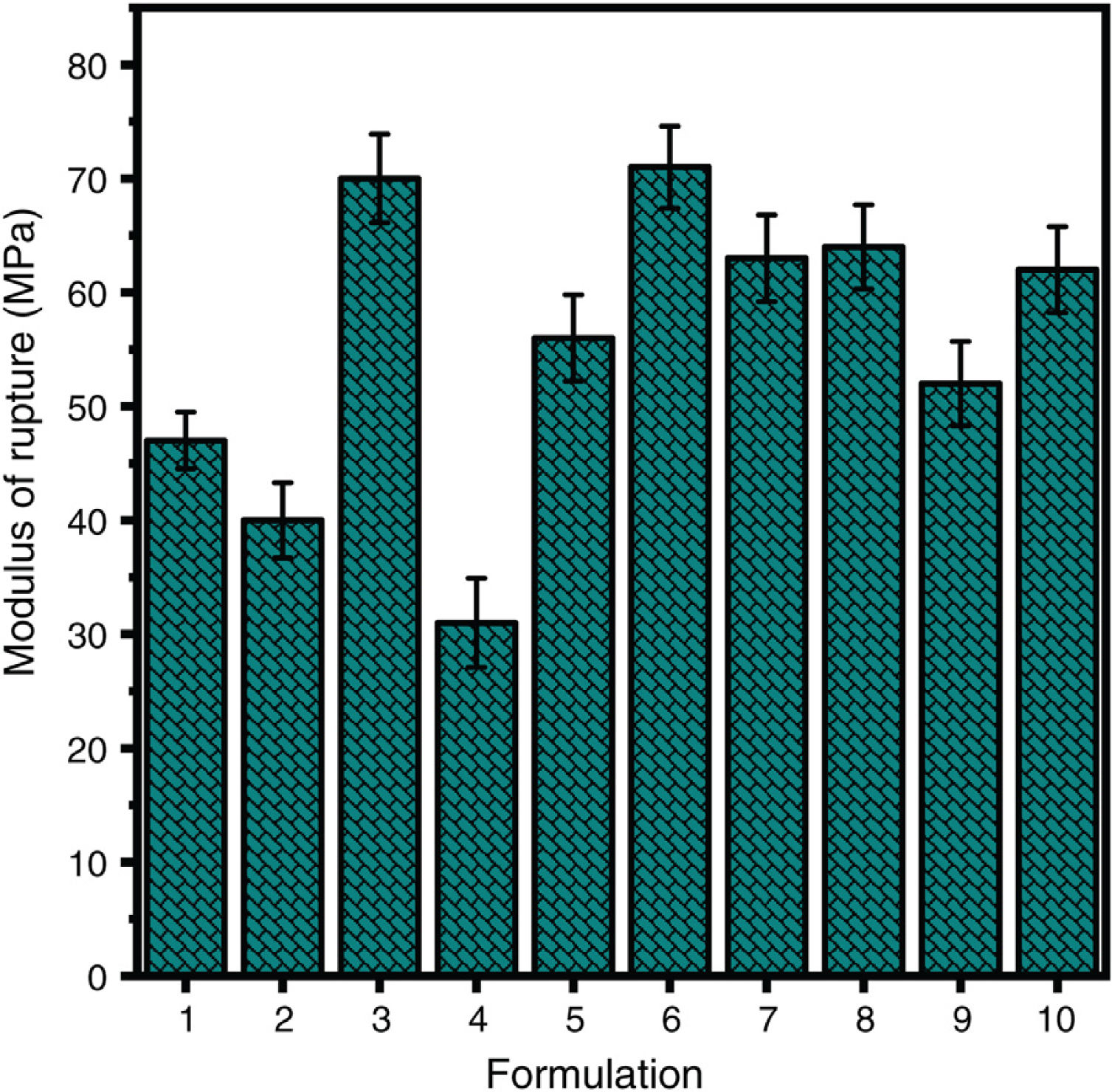
The modulus of rupture of the ceramic formulations is shown in Fig. 6. The lowest values are obtained in kaolin waste-free formulations (F1, F2 and F4). In formulations with kaolin waste, liquid phase sintering is activated and the liquid fills the open pores (as shown in Fig. 5(c)), which leads to an increase in modulus.
Modulus of rupture of the ceramic formulations sintered at 1400°C. F1 – 50wt.% clay and 50wt.% alumina; F2 – 100wt.% clay; F3 – 50wt.% clay and 50wt.% waste; F4 – 75wt.% clay and 25wt.% alumina; F5 – 50wt.% clay, 25wt.% waste and 25wt.% alumina; F6 – 75wt.% clay and 25wt.% waste; F7 – 66.67wt.% clay, 16.67wt.% waste and 16.67wt.% alumina; F8 – 58.33wt.% clay, 8.33wt.% waste and 33.33wt.% alumina; F9 – 83.33wt.% clay, 8.33wt.% waste and 8.33wt.% alumina; F10 – 58.33wt.% clay, 33.33wt.% waste and 8.33wt.% alumina. Note: Data of F2, F3 and F6 were obtained from our previous work [12] for comparison.
The simplex-centroid mixture design was successfully used to prepare mullite–glass ceramics after sintering at 1400°C. The microstructural analysis revealed mullite, residual quartz, cristobalite, and α-alumina as the main crystalline phases of the samples derived from kaolin, kaolin waste, and alumina. An amorphous silica-rich phase was observed after sintering of raw materials with the presence of mica in their compositions. Statistical analysis proved to be adequate to obtain statistical models, highly significant, which correlate the concentrations of raw materials with linear firing shrinkage, water absorption, and apparent porosity. In kaolin waste-rich formulations, a liquid phase assisted sintering mechanism favors the densification process, improving the mechanical strength of the ceramics. While high concentrations of alumina increase the water absorption and apparent porosity of the samples.
FundingThis study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.





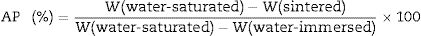
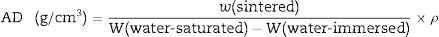
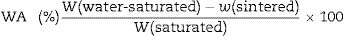
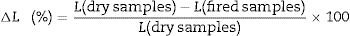
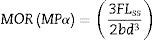
![Dilatometric analysis of the samples F1 (50wt.% clay and 50wt.% alumina), F2 (100wt.% clay), F3 (50wt.% clay and 50wt.% waste), and alumina. Note: F2 and F3 data were obtained from our previous article [12] for comparison. Dilatometric analysis of the samples F1 (50wt.% clay and 50wt.% alumina), F2 (100wt.% clay), F3 (50wt.% clay and 50wt.% waste), and alumina. Note: F2 and F3 data were obtained from our previous article [12] for comparison.](https://static.elsevier.es/multimedia/03663175/0000006100000002/v2_202206010315/S0366317520300881/v2_202206010315/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Modulus of rupture of the ceramic formulations sintered at 1400°C. F1 – 50wt.% clay and 50wt.% alumina; F2 – 100wt.% clay; F3 – 50wt.% clay and 50wt.% waste; F4 – 75wt.% clay and 25wt.% alumina; F5 – 50wt.% clay, 25wt.% waste and 25wt.% alumina; F6 – 75wt.% clay and 25wt.% waste; F7 – 66.67wt.% clay, 16.67wt.% waste and 16.67wt.% alumina; F8 – 58.33wt.% clay, 8.33wt.% waste and 33.33wt.% alumina; F9 – 83.33wt.% clay, 8.33wt.% waste and 8.33wt.% alumina; F10 – 58.33wt.% clay, 33.33wt.% waste and 8.33wt.% alumina. Note: Data of F2, F3 and F6 were obtained from our previous work [12] for comparison. Modulus of rupture of the ceramic formulations sintered at 1400°C. F1 – 50wt.% clay and 50wt.% alumina; F2 – 100wt.% clay; F3 – 50wt.% clay and 50wt.% waste; F4 – 75wt.% clay and 25wt.% alumina; F5 – 50wt.% clay, 25wt.% waste and 25wt.% alumina; F6 – 75wt.% clay and 25wt.% waste; F7 – 66.67wt.% clay, 16.67wt.% waste and 16.67wt.% alumina; F8 – 58.33wt.% clay, 8.33wt.% waste and 33.33wt.% alumina; F9 – 83.33wt.% clay, 8.33wt.% waste and 8.33wt.% alumina; F10 – 58.33wt.% clay, 33.33wt.% waste and 8.33wt.% alumina. Note: Data of F2, F3 and F6 were obtained from our previous work [12] for comparison.](https://static.elsevier.es/multimedia/03663175/0000006100000002/v2_202206010315/S0366317520300881/v2_202206010315/en/main.assets/thumbnail/gr6.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

