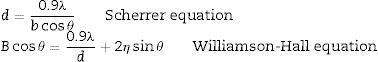In this paper, Al2O3–SiC composites were produced by SPS at temperatures of 1600°C for 10min under vacuum atmosphere. For preparing samples, Al2O3 with the second phase including of micro and nano-sized SiC powder were milled for 5h. The milled powders were sintered in a SPS machine. After sintering process, phase studies, densification and mechanical properties of Al2O3–SiC composites were examined. Results showed that the specimens containing micro-sized SiC have an important effect on bulk density, hardness and strength. The highest relative density, hardness and strength were 99.7%, 324.6 HV and 2329MPa, respectively, in Al2O3–20wt% SiCmicro composite. Due to short time sintering, the growth was limited and grains still remained in nano-meter scale.
En este trabajo se muestran compuestos de Al2O3-SiC producidos por SPS, en vacío, a 1.600°C durante 10min. Para la preparación de muestras, se molieron polvos de Al2O3 durante 5h con la segunda fase de micro-y-nano polvo de SiC. Posteriormente, estos polvos molidos se sinterizaron mediante SPS. Después del proceso de sinterización, se realizaron estudios de fase, densificación y propiedades mecánicas de los compuestos de Al2O3-SiC obtenidos. Los resultados mostraron que micro-SiC en las muestras tiene un efecto importante en su densidad aparente, dureza y resistencia. La mayor densidad relativa, dureza y resistencia fueron respectivamente del 99,7%, 324,6 HV y 2.329MPa para Al2O3 con un 20% en peso micro-SiC. Debido al corto tiempo de sinterización, el crecimiento los granos fue limitado y se mantuvieron en escala nanométrica.
Thermal and chemical stability, relatively high strength, thermal and electrical insulator alongside the availability and abundance of aluminum oxide, lead to use of this material in engineering applications [1–5]. Despite the mentioned advantages, low fracture toughness of this material lead to limitation of its application. Composites are one of the methods which over come to this limitation. In this technique alumina matrix is reinforced by particles or fibers as secondary phase, which can be metal, polymer or ceramic. Silicon carbide (SiC) as a ceramic material can be one of the option which leads to improvement of alumina matrix [6–10]. Nihara et al. reported that sintering of Al2O3–SiC composite was done successfully. They found out that adding a little amount of SiC to alumina matrix can improve mechanical properties of composite significantly in comparison with non-composite materials. They increased strength and fracture toughness from 350 to 1520MPa and 3.5 to 4.8MPam1/2, respectively by adding 5vol.% SiC [11].
There are different methods of sinter this composite. Non-pressure and hot press sintering are the most common method of sintering for this composite, but new technique which is considerable today is spark plasma sintering (SPS) [6,12–15]. On the base of spark plasma, which is created by a pulsed direct current, SPS leads to quick increasing of mold's and the powder's temperature. High heating rate, using pressure and electrical current is the specifications of this technique which distinguish this technique in comparison with other method. In addition to reduction of particle's coarsening, high heating rate increased condensation through the elimination of surface diffusion mechanism and creating of extra driving force by high temperature gradient. Pressure applying during the heating can increase the driving force of process and facilitate the sintering process. Electrical current can condense the powder in mold by creating of many sparks between particles and creating of plasma environment. Effect of plasma on surface's cleanness of particles and improvement of sintering process has been reported by researchers [16–21].
Synthesis of Al2O3–SiC composite by SPS has been investigated by a few researchers [16,17], but the effect of particle size on the densification, mechanical and wear properties has not been reported until now. So in this paper sintering process and properties of Al2O3 matrix reinforced by micro and nano-sized SiC will be examined.
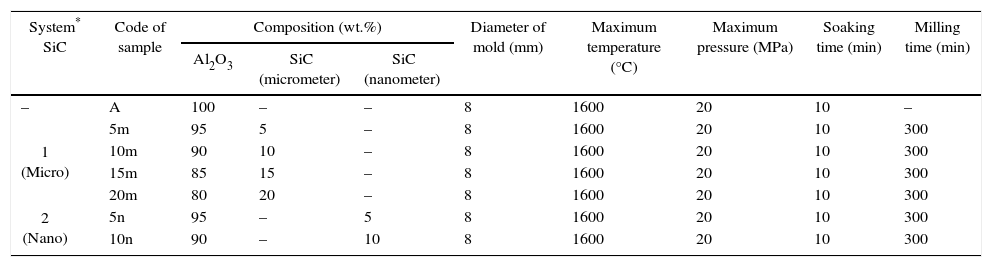
ExperimentalAl2O3 and SiC powders in micro and nano-meter scale with purity 99.8%, 99.5% and 99.9% and mean particle size of 1.5μm, 10μm and 50nm, respectively were used as raw materials. Al2O3 powder with two sources of SiC (micro and nano as systems 1 and 2, respectively) powder were milled in a planetary ball mill (as a high energy ball mill) for 5h in distilled water. Ball to powder ratio was 10 to 1 in all tests. In the following, prepared powders were sintered in a mold with 8cm in diameter under specific conditions according to Table 1 by SPS machine. The sintering process was done under high pulsed direct current (1000–3500A) in vacuum. A uniaxial pressure of 10MPa was used during the reaction and increased to 20MPa after reaching to the expected temperature and maintained during holding time. After holding time the uniaxial pressure decreased to 10MPa and maintained during cooling.
Coding and sintering conditions of samples.
| System* SiC | Code of sample | Composition (wt.%) | Diameter of mold (mm) | Maximum temperature (°C) | Maximum pressure (MPa) | Soaking time (min) | Milling time (min) | ||
|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | SiC (micrometer) | SiC (nanometer) | |||||||
| – | A | 100 | – | – | 8 | 1600 | 20 | 10 | – |
| 1 (Micro) | 5m | 95 | 5 | – | 8 | 1600 | 20 | 10 | 300 |
| 10m | 90 | 10 | – | 8 | 1600 | 20 | 10 | 300 | |
| 15m | 85 | 15 | – | 8 | 1600 | 20 | 10 | 300 | |
| 20m | 80 | 20 | – | 8 | 1600 | 20 | 10 | 300 | |
| 2 (Nano) | 5n | 95 | – | 5 | 8 | 1600 | 20 | 10 | 300 |
| 10n | 90 | – | 10 | 8 | 1600 | 20 | 10 | 300 | |
After sintering process, the samples were polished and cut. In order to detect of phases in sample's structure and evaluate of properties XRD (Siemens, 30kV, 25mA, Cu Kα) was used. The crystallite size and strain were evaluated through Scherrer and Williamson–Hall methods applying the following equations [18]:
where B, θ, λ, d and η are the full width of the peak at half intensity (rad.), position of peak in the pattern (rad.), the wavelength of X-ray (nm), crystalline size (nm), and mean internal strain, respectively.Samples density, open and closed porosity and water absorption were estimated by Archimedes method [19]. Strength of the samples was measured through three points test. Five samples with dimension of 3×4×45mm were prepared and average of strength reported [20]. Hardness of the sample was determined through Vickers method. 5 tests were done for each sample and average of results were reported [21]. Microstructures of milled and sintered samples were studied by scanning electron microscope (Cambridge model). Finally, wear resistance of samples was done in order to determine the wear properties. In this test composite samples were used as a pin and alumina was used as the disc. The force on the pin tip was 15.3N. The machine was stopped in the distances of 1000m and the weight losses of the samples were with the accuracy of 0.0001g [22].
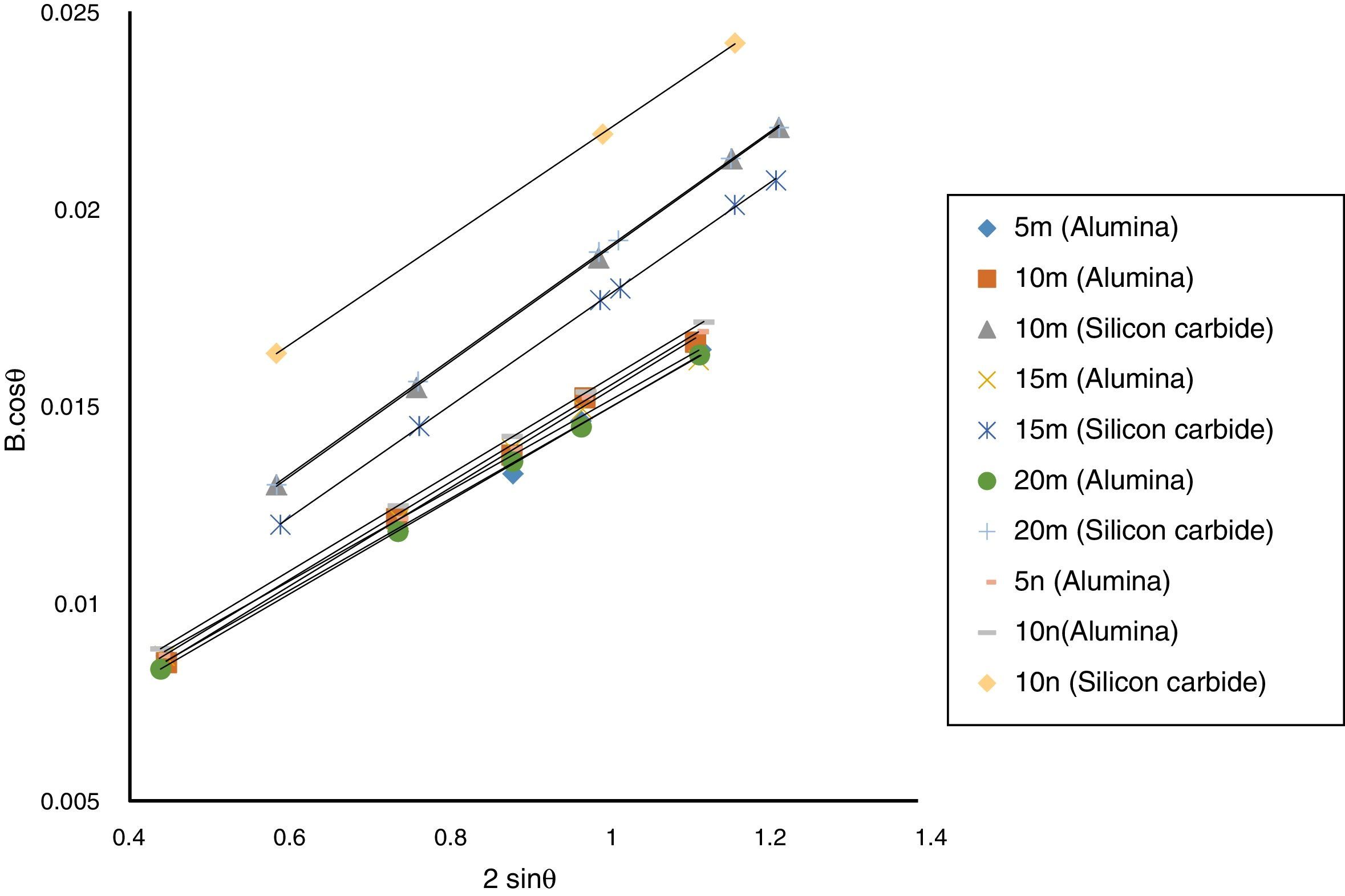
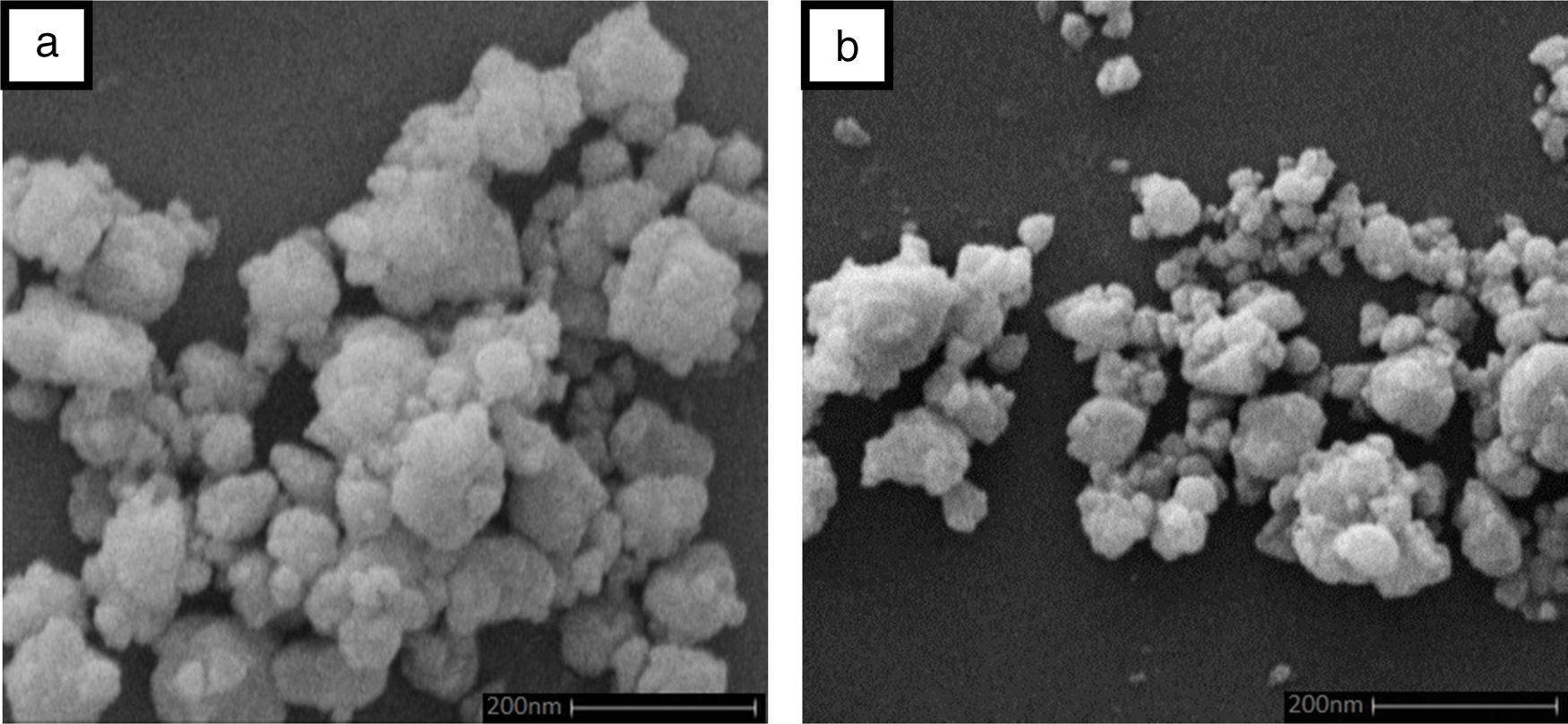
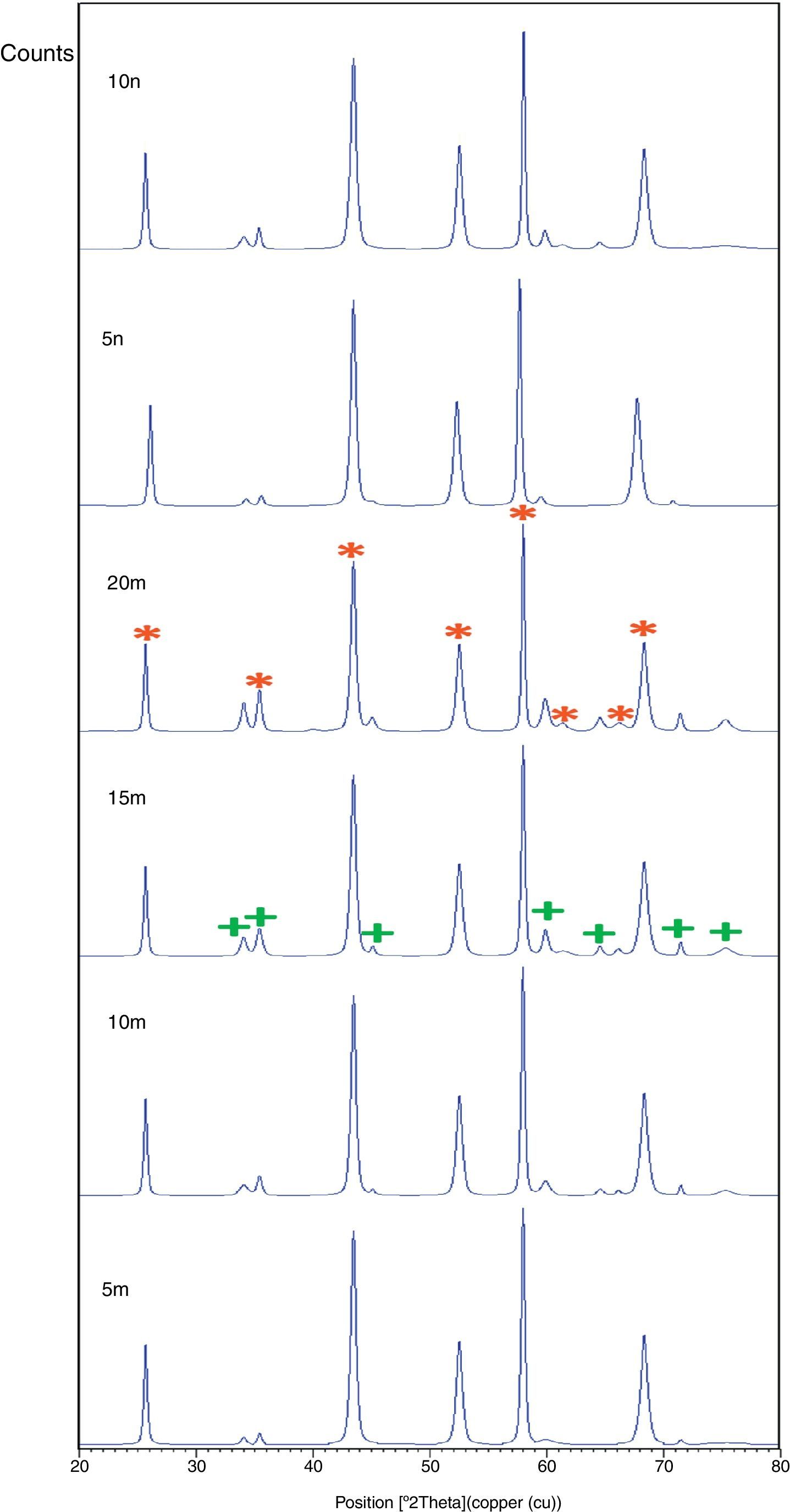
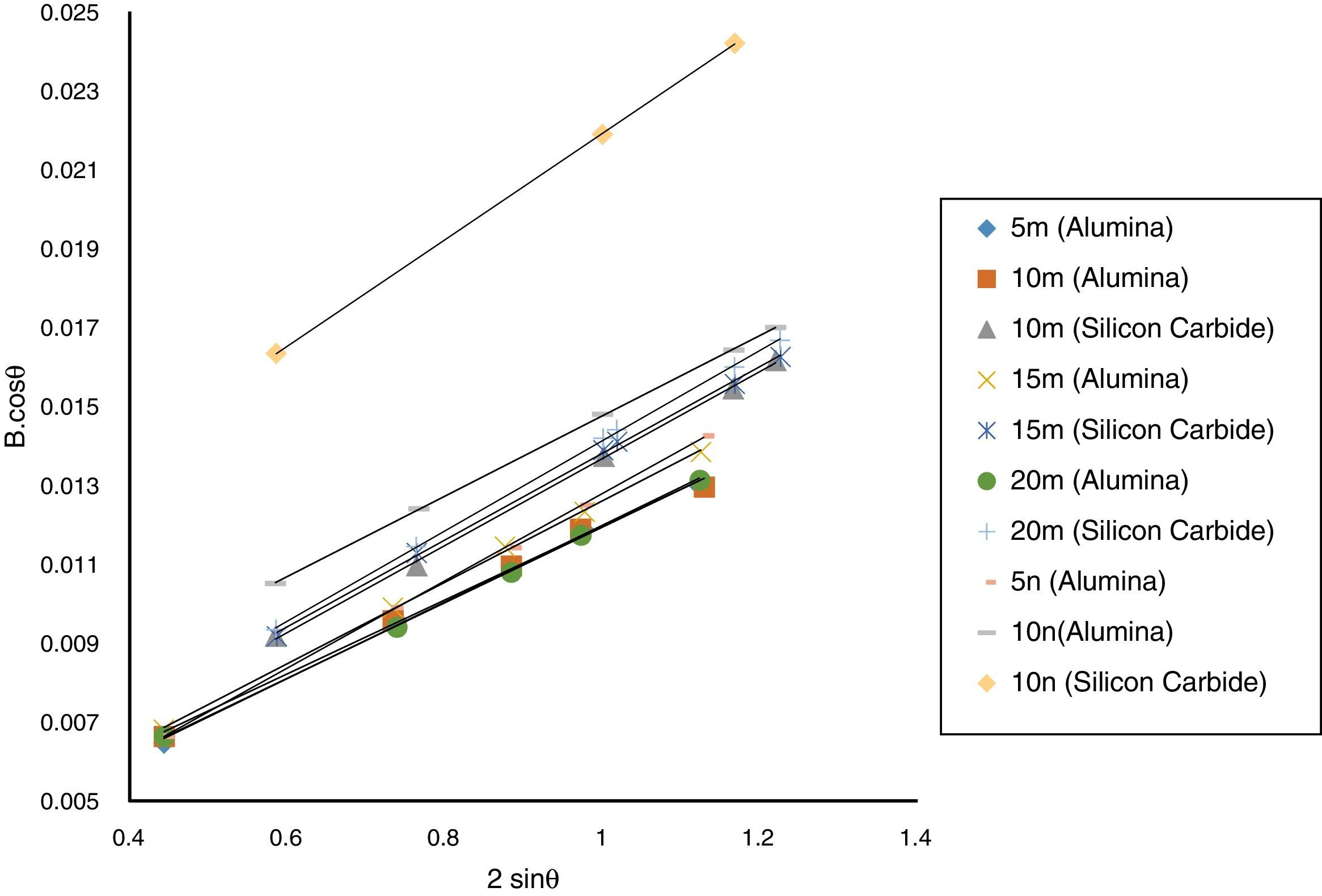
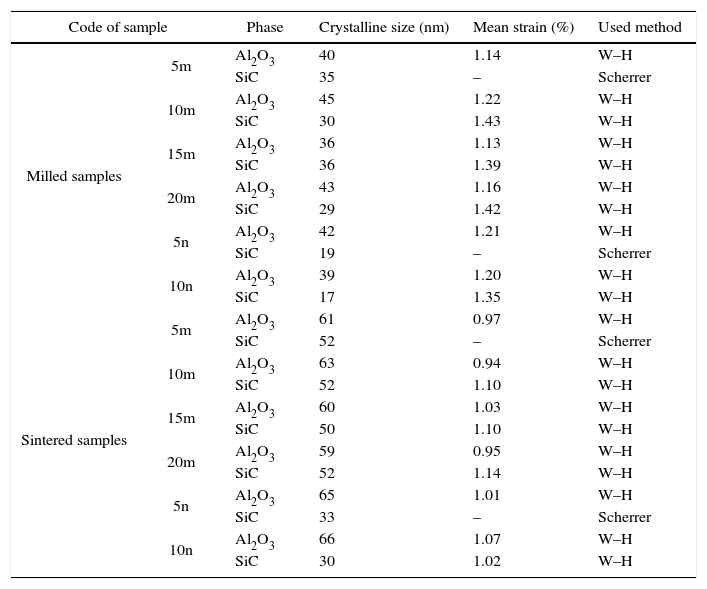
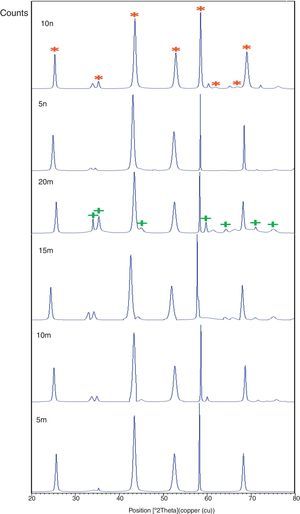
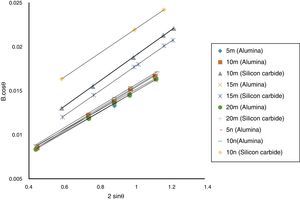
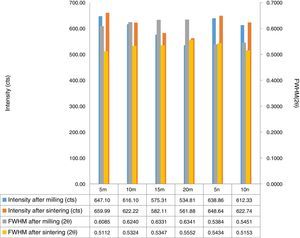
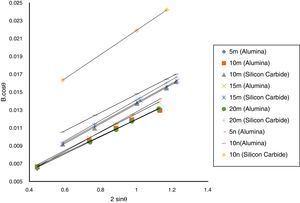
Result and discussionPatterns of X-ray diffraction of milled powders are illustrated in Fig. 1. As it is seen in these patterns, milling has not led to phase transfer in raw materials, and identified phases are Al2O3 and SiC with 1125-071-01 and 00-002-1048 reference code, respectively. As it stands, by increasing of SiC phase, intensity of their peaks has been increased. Crystalline size (d) and mean strain (η) of milled powder were measured. The changes of B·cosθ to 2sinθ are seen in Fig. 2. Calculation results are brought in Table 2, as it is seen in this table crystalline size of milled powder in all compounds for both phases are in nano-meter scale. The crystalline sizes of milled samples (which all of them have been treated in a similar way) have a range between 36 and 40nm and 17 and 36nm for Al2O3 and SiC phases, respectively and no significant difference between sizes. As it stands, size of phases in the second system is finer than first system, that can be attributed to two reasons: the first one is using of SiC with nano scale in the second system and the next one is the presence of more finer SiC particles in the second system at equal weight fraction which can operate as fine balls and facilitate of milling process. These particles increases milling energy and lead to crush of particles [23]. SEM image form milled samples are presented in Fig. 3. As it is obvious in this figure, milling lead to decreasing the size of particles in both systems and the mean particle sizes are in nanometer scale. The size of particles in the system containing nano SiC are lower than the system without that.
Results of calculations of crystalline sizes and mean strain of milled and sintered samples.
| Code of sample | Phase | Crystalline size (nm) | Mean strain (%) | Used method | |
|---|---|---|---|---|---|
| Milled samples | 5m | Al2O3 | 40 | 1.14 | W–H |
| SiC | 35 | – | Scherrer | ||
| 10m | Al2O3 | 45 | 1.22 | W–H | |
| SiC | 30 | 1.43 | W–H | ||
| 15m | Al2O3 | 36 | 1.13 | W–H | |
| SiC | 36 | 1.39 | W–H | ||
| 20m | Al2O3 | 43 | 1.16 | W–H | |
| SiC | 29 | 1.42 | W–H | ||
| 5n | Al2O3 | 42 | 1.21 | W–H | |
| SiC | 19 | – | Scherrer | ||
| 10n | Al2O3 | 39 | 1.20 | W–H | |
| SiC | 17 | 1.35 | W–H | ||
| Sintered samples | 5m | Al2O3 | 61 | 0.97 | W–H |
| SiC | 52 | – | Scherrer | ||
| 10m | Al2O3 | 63 | 0.94 | W–H | |
| SiC | 52 | 1.10 | W–H | ||
| 15m | Al2O3 | 60 | 1.03 | W–H | |
| SiC | 50 | 1.10 | W–H | ||
| 20m | Al2O3 | 59 | 0.95 | W–H | |
| SiC | 52 | 1.14 | W–H | ||
| 5n | Al2O3 | 65 | 1.01 | W–H | |
| SiC | 33 | – | Scherrer | ||
| 10n | Al2O3 | 66 | 1.07 | W–H | |
| SiC | 30 | 1.02 | W–H | ||
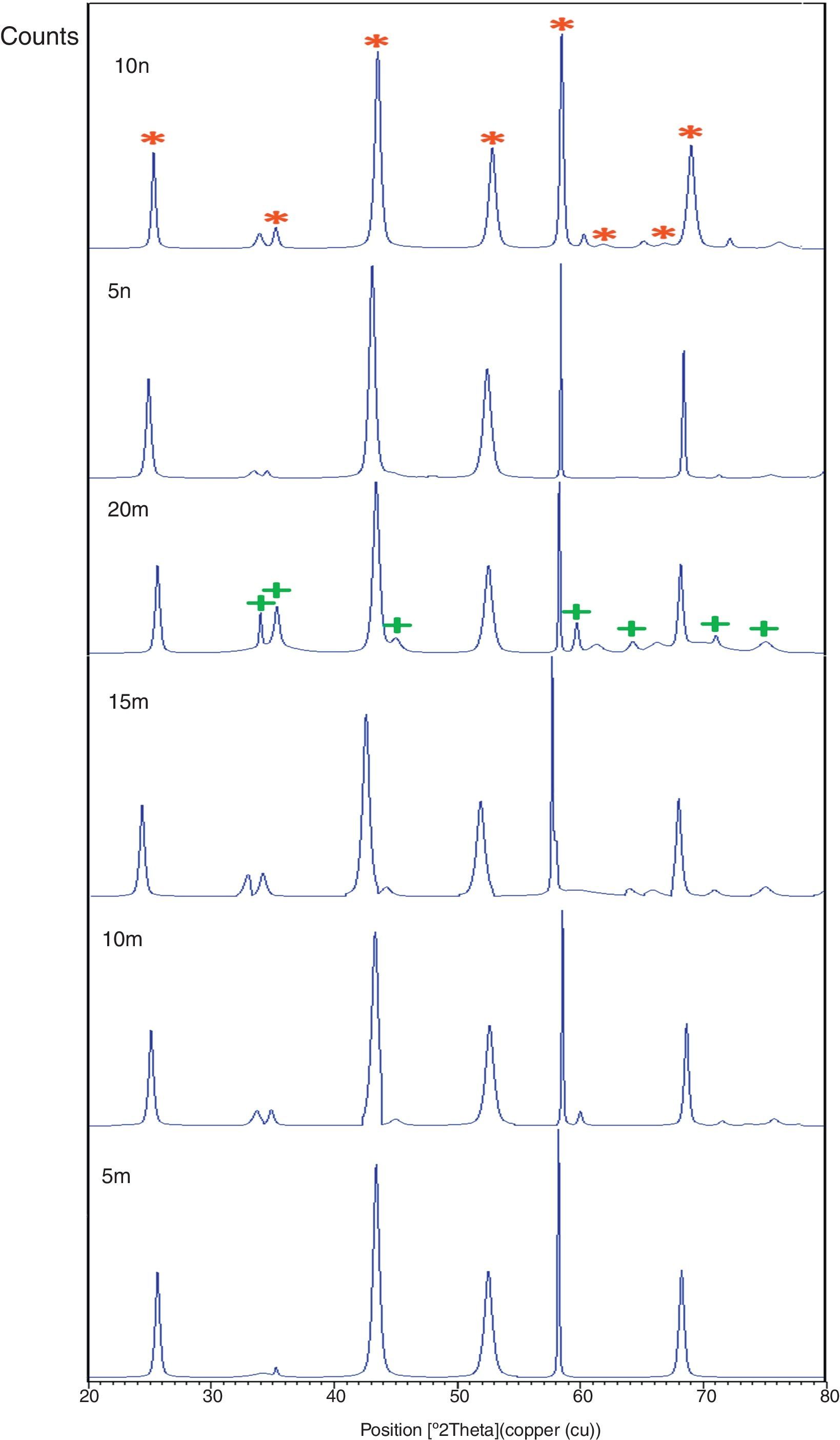
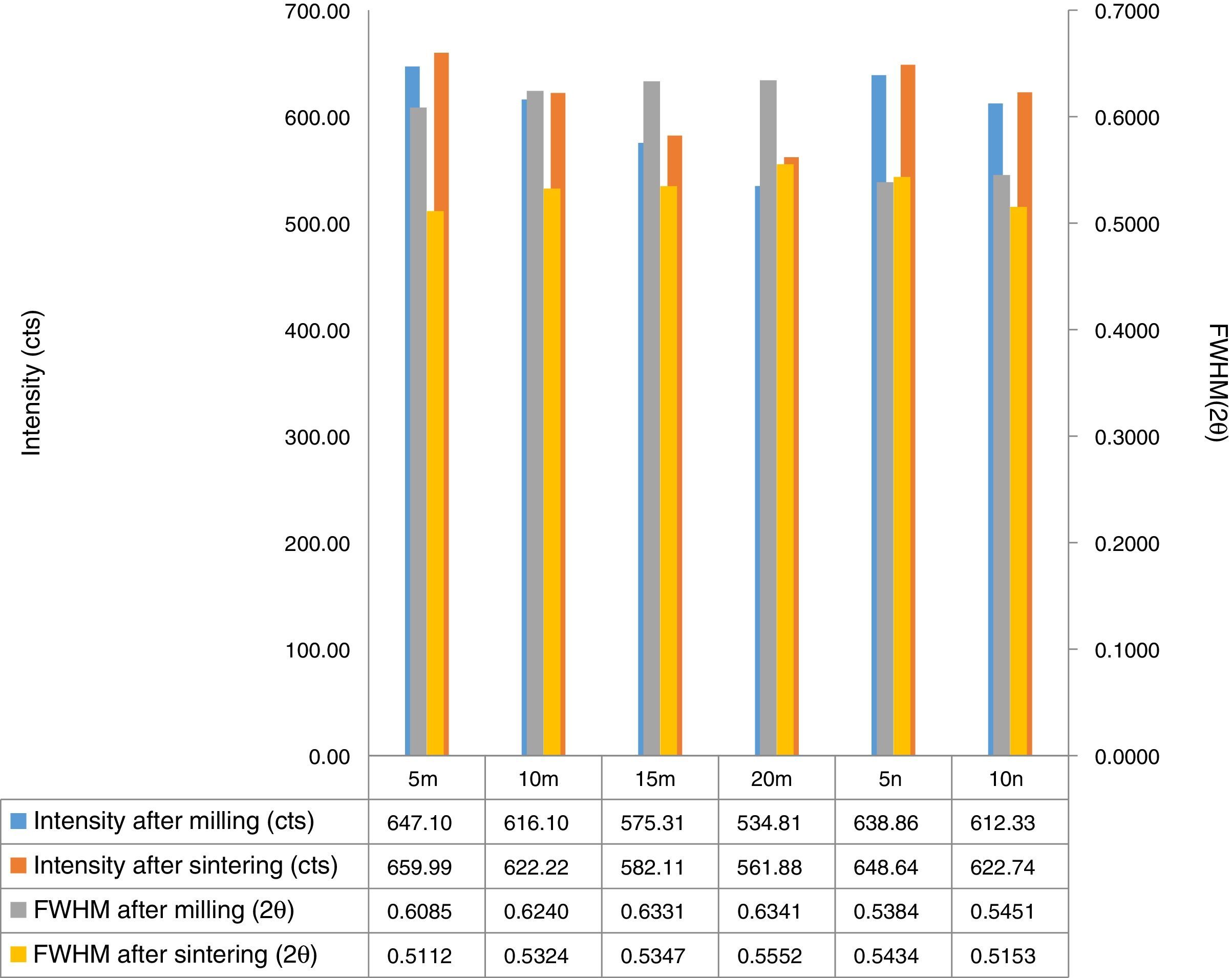
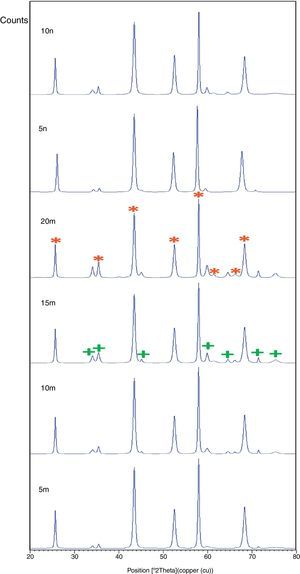
X-ray pattern of sintered sample is brought in Fig. 4, as it is seen, there is no change in phases of sintered samples and in milled sample (Fig. 1), Al2O3 and SiC are identified phases. As it is obvious in Fig. 5, the only considerable point in comparison of this pattern with milled sample pattern is increasing of peak's intensity and decreasing of peak's width in sintered sample. Increasing of temperature during the sintering can lead to growth of crystals and reducing of mean lattice strain [24,25].
The changes of B·cosθ to 2sinθ in sintered samples are shown in Fig. 6. The results of these calculations are brought in Table 2. As these calculations show, although the crystalline sizes are in nanometer scale with the range of 59–66nm and 30–52nm for Al2O3 and SiC phases, respectively but sintering leads to growth of crystals and decreasing of mean lattice strain slightly. Unlike common methods of sintering, which follow extreme growth, sintering though SPS has a little growth, so that the crystals are in nano scale yet. When the sintering (including heating and keeping processes) is completed in a few minutes, the crystals could be small [26].
The changes of sample's thickness to time for sample which contain different amount of SiC include three zones. At first, the time of less than 35min, which sample has been heated and has a little expansion. In the following by increasing the temperature, sintering process occurred and samples were contracted quickly during about 5min and then change of displacement will be constant. As it stands, the sintering process was completed at the end of second zone and samples were dense. According to the changes of sample thickness and temperature versus sintering time plots, beginning temperature of sintering (beginning of second zone) is determined. This information is brought in Table 3. Decrease in thickness of the samples and the starting of second zone is due to the overcoming of contraction of sintering on thermal expansion of the samples.
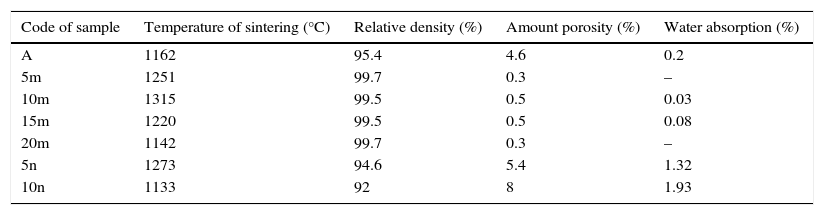
Temperature of sintering with physical properties of sintered samples.
| Code of sample | Temperature of sintering (°C) | Relative density (%) | Amount porosity (%) | Water absorption (%) |
|---|---|---|---|---|
| A | 1162 | 95.4 | 4.6 | 0.2 |
| 5m | 1251 | 99.7 | 0.3 | – |
| 10m | 1315 | 99.5 | 0.5 | 0.03 |
| 15m | 1220 | 99.5 | 0.5 | 0.08 |
| 20m | 1142 | 99.7 | 0.3 | – |
| 5n | 1273 | 94.6 | 5.4 | 1.32 |
| 10n | 1133 | 92 | 8 | 1.93 |
As it is seen in Table 3, in the first system by increasing of SiC to 10wt.%, beginning temperature of sintering has been increased and then decreased. SiC with higher melting point than Al2O3, increase the sintering temperature of Al2O3. But increasing of SiC which has lower thermal expansion (TEC) coefficient in comparison with Al2O3 leads to decreasing of thermal expansion coefficient of composite (melting point and thermal expansion of Al2O3 and SiC are 2072°C and 8.1×10−6/°C and 2730°C and 4.0×10−6/°C, respectively) [27]. Hence the early expansion has been decreased during the heating and as a result beginning temperature of contraction is decreased too. So, after 10m sample, decrease of sintering temperature is seen. This treatment is similarly seen in second system too. Only in this system, overcoming of the second phenomenon to first one is quicker than and is happened in less amount of SiC.
The changes of relative density of sintered samples for the two systems are presented in Table 3. As it is obvious, in system 1, addition of different amount of SiC to Al2O3 matrix leads to completing of sintering and achievement to samples with nearly full density. Lower sintering temperature, short temperature and holding times have prepare it possible to produce nano-composite of SiC–Al2O3 to near theoretical density with little crystal growth [28]. To attainment full density by common sintering technique, higher temperature and soaking time is needed. Shi et al. succeeded to sinter Al2O3–SiC composite with relative density of 100% via hot press technique at temperature over 1700°C. They reported sintering by hot press leaded to abnormal growth in some samples [29].
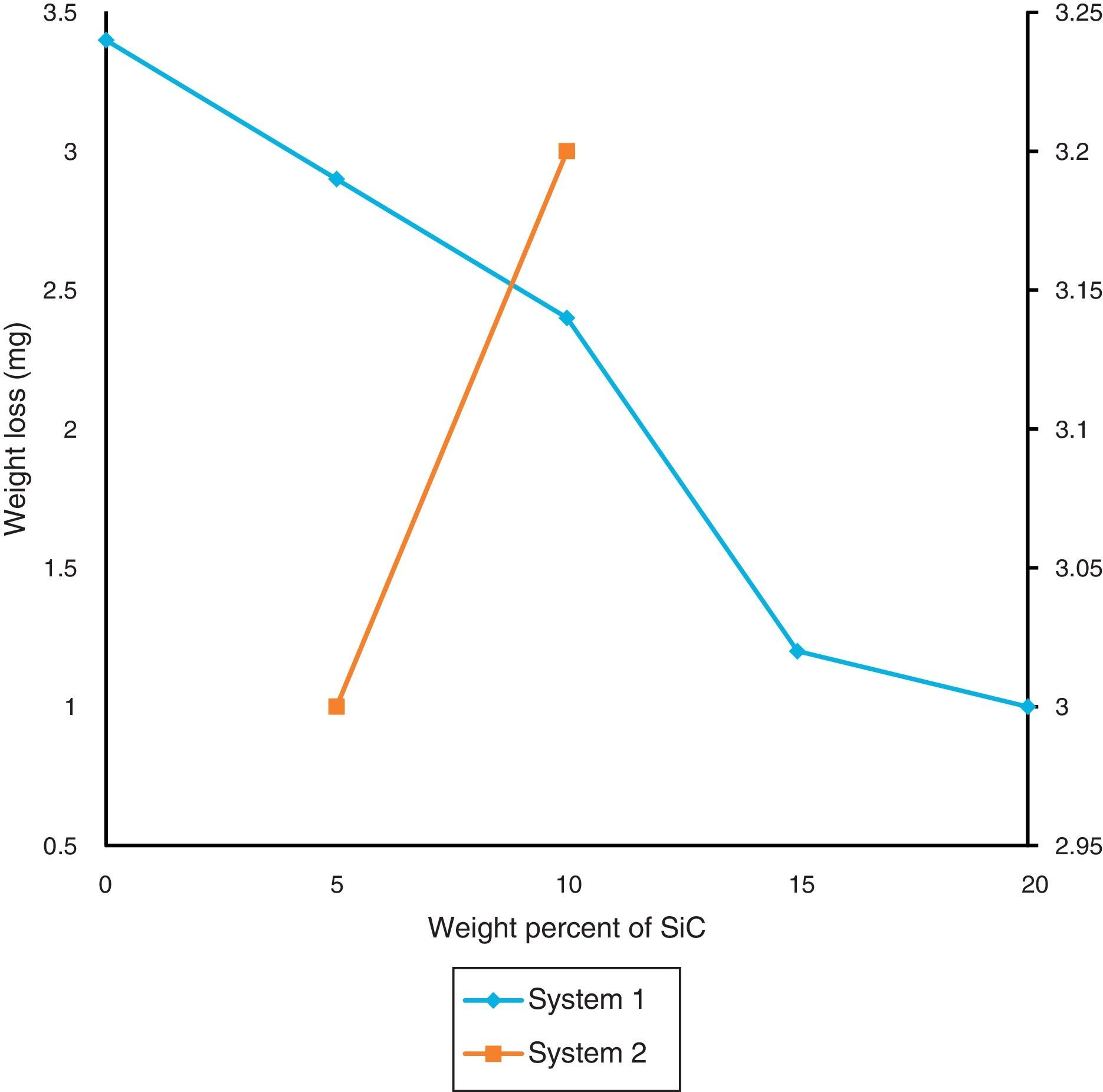
Against, presence of nano-sized SiC in system 2 could not obtain a sample with high density. A great difference between particle size of matrix and the reinforcement phase in the second system decreased packing and leads to decrease of final density. There is evidence when a SiC as fine component are added to the Al2O3 particles, it adhere to the large particles strongly and delay the penetration of fine components to the mixtures [30]. Wide distribution of particles in system 2 confirms this point (Fig. 3b). Furthermore, as it is obvious in Table 3, by increasing the weight percent of SiC, density is decreased. This could be due to the poor sintering property of SiC at examined temperatures in this paper. Similar result was reported by other researchers [29,31,32].
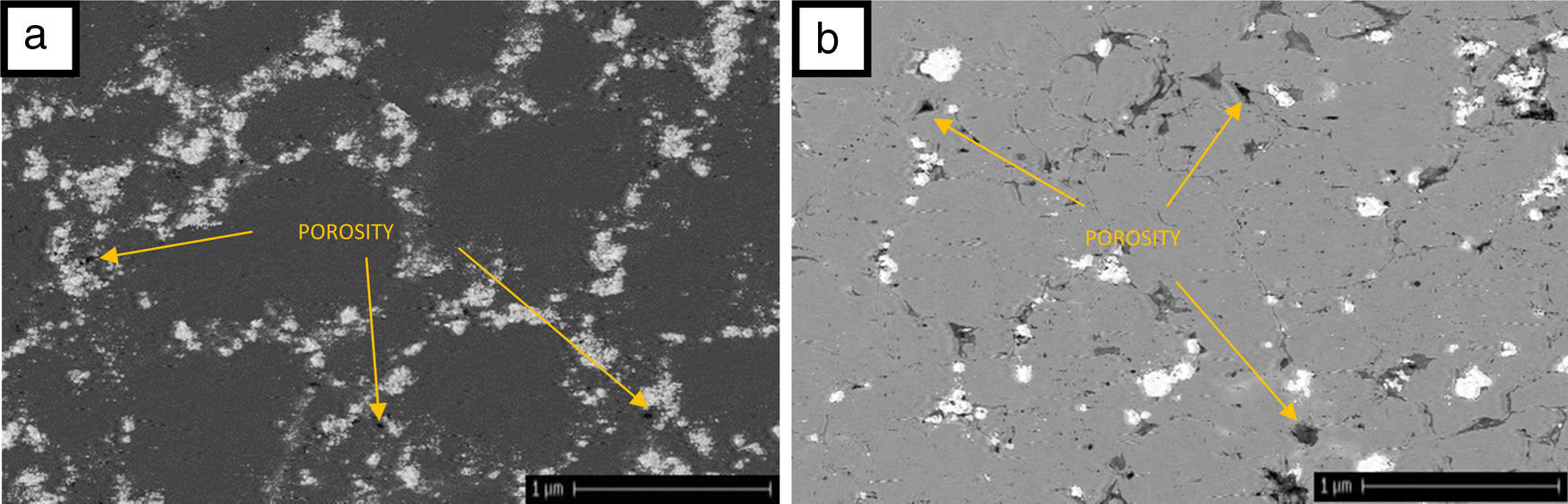
Fig. 7 shows the SEM images for the microstructure of the sintered compacts. There are two different phases in both systems, i.e., dark and light phase. As it is seen from Fig. 4 and as it was discussed earlier, XRD pattern implied that there was no reaction between the raw materials. So Al2O3 and SiC are only phases in sintered samples. According to these images, SiC with lower mass absorption coefficient than Al2O3 as light and dark phases, respectively were dispersed in the matrix [33,34]. As it was calculated porosity by Archimedes method, porosity can be seen in microstructural images of samples. The amount and sizes of porosity in system 2 is higher than system 1.
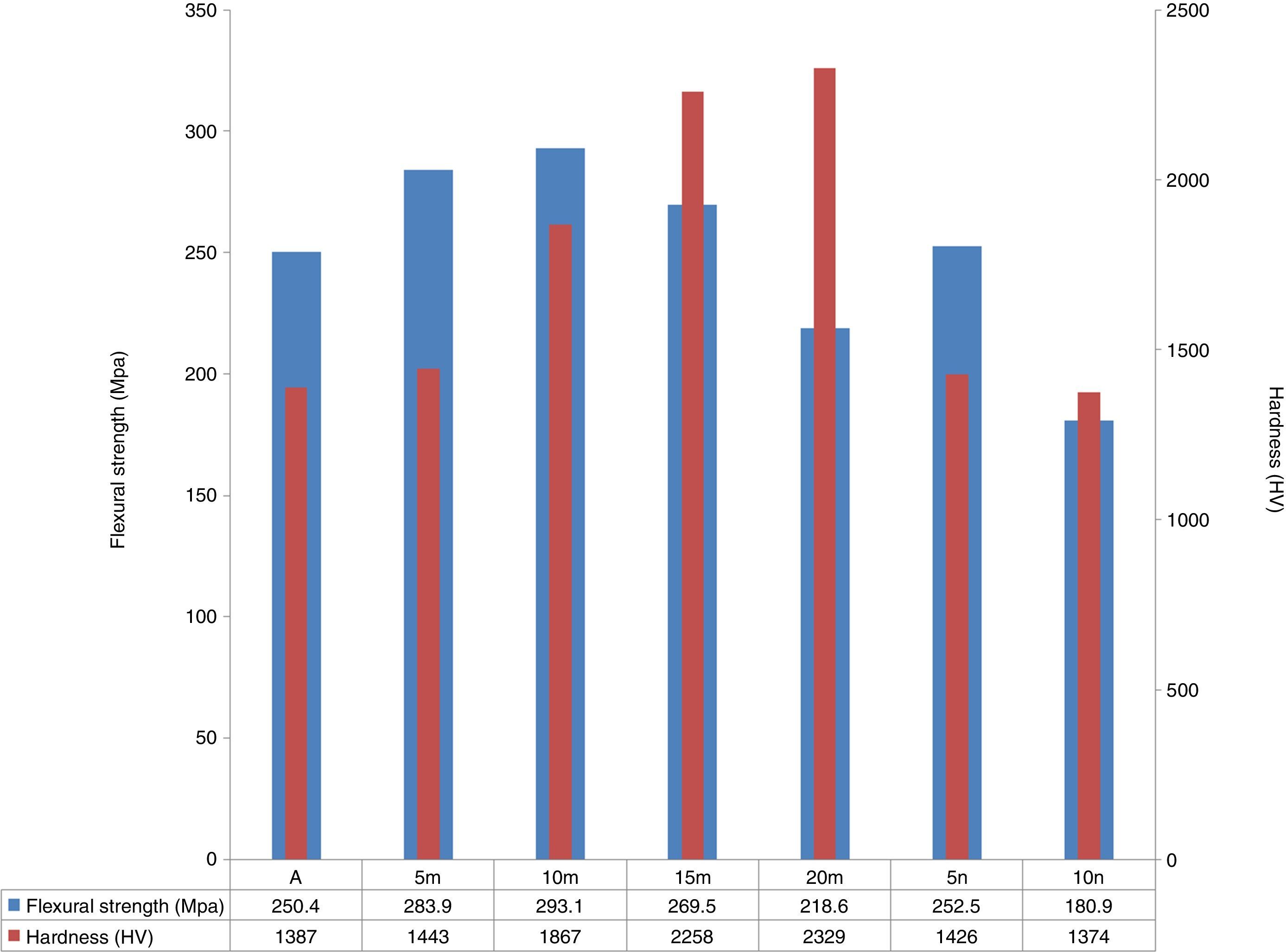
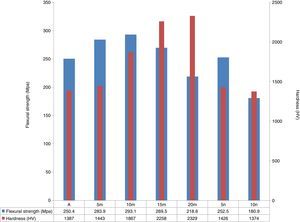
Flexural strength and hardness of sintered samples are illustrated in Fig. 8. As it stands, in system 1 with a density close to each other, the flexural strength of samples increased by increasing the amount of SiC particles up to 10% and after that adding more SiC could not increase the flexural strength. When the weight percent of SiC was more than 10, a suitable distribution of this phase cannot be seen [32,35]. Existence of hard SiC particles with a fine structure could be improve the mechanical properties [36]. Furthermore, due to residual stress from the mismatch of TEC between SiC and Al2O3, matrix is under the compressive stress during cooling. So, existence of SiC in the matrix of Al2O3 increases the strength [37]. Adding hard SiC as reinforcement phase to the Al2O3 matrix could increase the hardness numbers significantly (hardness of SiC and Al2O3 are 1175 HV and 2800 HV) [27]. Since in system 2 achievements to samples with full density were not happening, flexural strength and hardness were weaker than specimens of first system. The porosity effects on the mechanical properties of ceramic materials meaningfully [29].
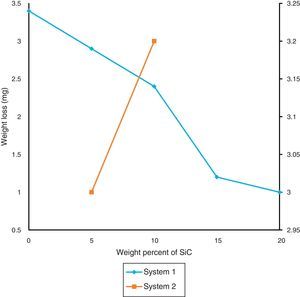
Loss of weight in the sample after wear resistance is seen in Fig. 9. Due to high hardness in sample which contain hard SiC, wear resistance of them are increased. During dry sliding, the SiC particles do not easily come out in the debris because of their reasonably good bonding with the matrix. By decreasing the particle size, bonding takes place better and the wear resistance increases. Furthermore, the formation of oxide layer on the wear surface of the composite reduced wear resistance. These oxides consist of very fine with sizes of about 10–100nm which have been compacted onto the composite's surface, preexisting surface oxide layers, or compressed coarse wear debris [38]. Because of lower density of samples in the second system, wear properties of these samples are lower than the first system.
ConclusionAl2O3–SiC composites were prepared successfully by SPS with relative density of 100%. The composites with denser structure have higher flexural strength. 293.1MPa and 2329 HV of the highest hardness and flexural strength were obtained from the samples reinforced by 10 and 20wt.% SiC, respectively. By increasing the amount of SiC, flexural strength was improved first and then decreases because of a bad distribution of seconded phase in the matrix. Due to lower density in samples containing nano-sized SiC, mechanical properties were weaker than specimens of the first system.