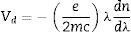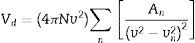High heavy metal oxides (60–100mol.%) ternary PbO–Bi2O3–B2O3 (PBB) glasses were fabricated and characterized. Using a homemade single lightway DC magnetic setup, Verdet constants of PBB glasses were measured to be 0.0923–0.1664min/Gcm at 633nm wavelengths. Glasses with substitution of PbO by Bi2O3 were studied in terms of their Faraday effects. PbO–Bi2O3–B2O3=50–40–10mol.% exhibited good thermal stability, high Verdet constant (0.1503min/Gcm) and good figure of merit (0.071). Based on this glass, a magneto optical current sensor prototype was constructed and its sensitivity at different currents was evaluated to be 8.31nW/A.
Se fabricaron y se calificaron vidrios ternarios PbO-Bi2O3-B2O3 (PBB) de óxidos metálicos muy pesados (60-100mol%). Con una sencilla y simple instalación magnética de corriente continua se midieron las constantes de Verdet de los vidrios de PBB de 0,0923-0,1664min/Gcm, a longitudes de onda de 633nm. Se estudiaron los efectos Faraday en los vidrios con una sustitución de PbO por Bi2O3. PbO-Bi2O3-B2O3=50-40-10mol% mostró una buena estabilidad térmica, una constante de Verdet elevada (0,1503min/Gcm) y un buen factor de mérito (0,071). Sobre la base de este vidrio se construyó un prototipo de sensor de corriente magneto-óptico y se evaluó su sensibilidad a diferentes corrientes para llegar a 8,31nW/A.
Magneto-optical current transducers (MOCT) based on Faraday effect have been developed worldwide as alternative to conventional optical ones [1] because MOCTs are compact and lightweight, immune to electromagnetic noise, and they offer a wide measurement range and long distance signal transmission [2]. Based on principle of Faraday effect, high rotation material is fundamental for getting a high sensitivity. Currently used high rotation materials are crystals (Bi:YIG etc.), or garnets and rare earth doped paramagnetic glasses, However, these expensive materials have limited magneto optical response to currents due to their temperature-dependent property [3–5].
Unlike its paramagnetic counterpart, diamagnetic heavy metal oxide (HMO) glasses, such as Bi2O3 and PbO, due to their mass and high polarizability of ions Pb2+ and Bi3+, are appealing for magneto optical sensors because of their temperature-independent Faraday effect, small phonon energy, large refractive index and low-melting properties [6].
Bi2O3-based glasses have attracted a great deal of research interest because of their high optical transmission into the far-infrared region (in the range 0.5–8.7mm), non-linear optical behavior and efficient luminescent applications in lasers. The modifier oxide, PbO, when added to bismuth borate glasses, the glasses are expected to become highly stable against devitrification and chemically inert [7] since PbO, in contrast with the conventional alkali/alkaline earth oxide/halide modifiers, form the stable glasses due to its dual role—modifier (with PbO6 structural units) and glass network former in both covalent and ionic bonding with PbO4/2 pyramidal units connected in puckered layers or frame structure [7,8]. Although Bi2O3 and PbO do not form glass by their own, they modify a vitreous network to form glass when they were combined with B2O3[9,10] which is a good glass forming oxide for technological applications.
Most studies on PbO, Bi2O3 based glasses are multicomponents. Reports on PBB glass doped with other elements, such as SiO2, Er/Yb [11–13], Tm, Tb/Ce [14], V2O5 Tb/Dy [15] and TiO2[16] etc. can be found for laser, luminescence [17], photosensitivity, non-linearity and dielectric dispersion applications [18]. Magneto-optical properties of multicomponents PbO–Bi2O3 doped with GeO2[6,19], ferrimagnetic FeO [20]/Fe3O4[21], TeO2[22], CdO/MnO and Ga2O3[23] etc. were reported. Spectral study of binary glass system PbO–B2O3, Bi2O3–B2O3 and PbO–Bi2O3[24] can be found as gamma-radiation shielding materials [25] and ion conductor.
Till now, two literature reported on Raman spectra/optical [26] and dispersion [27] property of ternary PbO–Bi2O3–B2O3 for non-linear and optical coating application, respectively. The magneto-optical properties of ternary PBB glass were investigated by a new Faraday measurement method by this group [28]. Further study such as doping Fe3O4 nanoparticles [21] and GeO2[29] into ternary PBB glass were continued. Detailed study on this system is of big interests because this system has many potential applications in photonics and magneto-optical fields. The main challenge is the synthesis of PBB glass, because it is known that Bi2O3 and PbO are not traditional glass former, but Bi3+ and Pb2+ are highly polarizable ions and the asymmetry of their polyhedral inhibits the crystallization processes in the melts in PBB system [14]. Also the processing conditions of the HMO glasses influence the optical quality and color of samples [30]. In addition, the steep viscosity–temperature relationship and high thermal expansion in high lead/bismuth glasses [31] also hinder the study on ternary PBB glass.
Based on our previous study [21,22,28–30] on the processing condition and Faraday rotation measurement method, in this article, we directed a more systematically study on PBB glasses with a high HMO concentrations ranging from 60% to 90% in mol., and focus on their Faraday rotation in magneto optical sensors application. The aim of this study is to find a PBB with the best thermal and magneto optical performance for MOCT prototype construction. The sensitivity of MOCT was evaluated under different currents to verify the Faraday performance of selected glass.
ExperimentGlasses of nominal compositions were fabricated by melt-quenching method. Optical grade reagents (Aldrich, purity 99.9%) PbO, B2O3 and Bi2O3 were weighted and mixed in Al2O3 crucibles at melting temperatures ranging from 900°C to 1100°C for 1h and were cast on a 200°C preheated brass plate. The cast bulk glasses were annealed for 2h at temperature ranging from 300°C to 350°C at 1°C/min heating/cooling rate. The fabricated glasses were bubble-free, highly homogeneous and transparent with a yellow color. The sample with the optimum composition, assuring the best glass forming and physical properties, was chosen as sensing element for MOCT. The annealed glasses were cut into parallel slabs with a thickness of 2.5mm and optically polished using a polishing instrument (λ – Logitech PM5).
Glass transition temperature (Tg) and crystallization temperature (Tx) were determined by differential scanning calorimetry (Perkin-Elmer DSC7), under N2 atmosphere at a heating rate of 10°C/min. The density was calculated at room temperature following the Archimedes’ principle using water as immersion liquid. The refractive index (n) was measured under different wavelengths by the prism coupling method using Metricon 2010. The UV absorption spectra were recorded in the wavelength range of 200–800nm by means of a UV-VIS spectrophotometer (Varian Cary 500) using optically polished samples with a thickness of 2.5mm. Using samples thickness, the absorption coefficient can be calculated by equation: α=log(I0/I)/z=A/z, where α is the absorption coefficient, A is the absorbance obtained from UV spectra, z is the sample thickness (z=2.5mm). The cut off is defined as the wavelength at which light ceases to propagate in the medium, it is normally calculated as the one at which the transmission decreases to 50% of its maximum. Fourier transforms infrared spectra (FT-IR) measurements from 1500 to 4000cm−1 wave number were carried out using a Varian Cary 500 spectrophotometer.
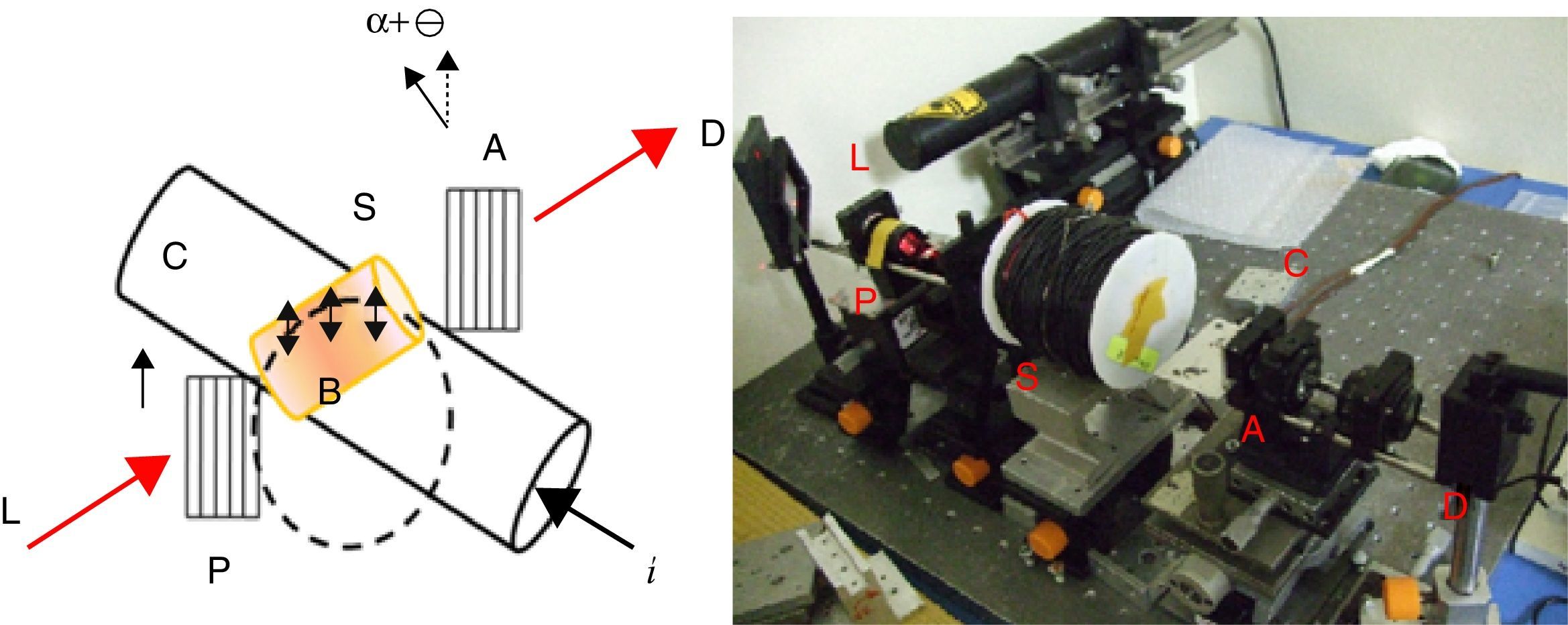
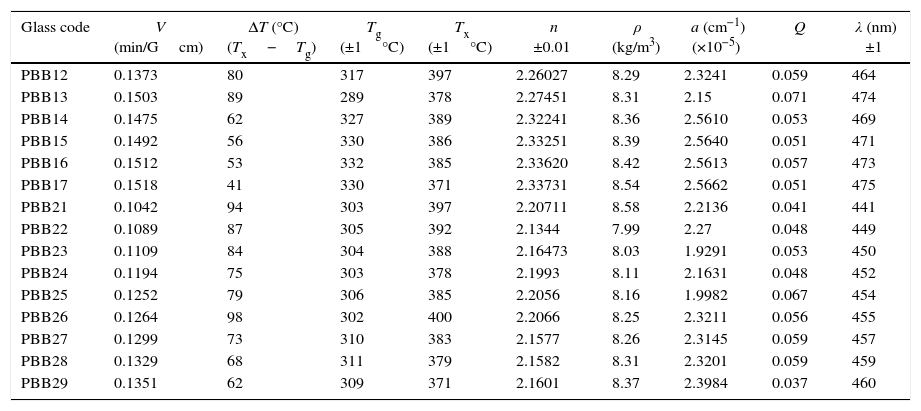
The Verdet constants of glasses were measured using a home-made optical bench as shown in Fig. 1. A He–Ne laser, emitting 1.8mW in a linearly polarized laser beam about 1mm in diameter, was focused on the glass using a 10× microscope objective with NA=0.28, resulting in a launching efficiency of 29%. The polarization extinction ratio of the laser beam was measured to be better than 1:5000. Glass samples were mounted in a solenoid which wrapped with a copper electrical wire, 2mm in diameter, coiled into 220 turns around a 19cm long a polymeric tube with a radius of 11.5mm. The overall outer radius of the electrical coil was 17mm.
As demonstrated in Fig. 1, the polarized beam propagates through the glass and then passes through an analyzer, which was mounted on a rotational stage graduated with a precision of 3×10−4rad. The power of the output beam was then measured using a photo detector (Ophir PD300) having a dynamic range of 30dB down to a power level of 0.02nW. A pure silica (with a known Verdet constant in literature [32,33]) is used as the reference for the sake of precise.
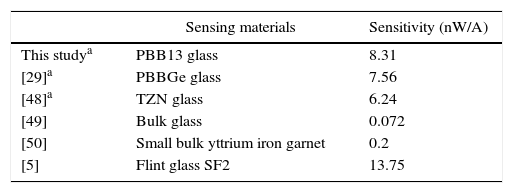
A MOCT prototype was constructed using PBB glass as sensing element as shown in Fig. 2. The setup consists of two linear polarizers, PBB glass, the conductor (Ø=34mm), laser and photodiode detector. The sensing head was fixed within the conductor. A DC current supplier was connected in series to the conductor for current supplying. The response signal was collected by photo-detector through which the signal can be converted to electric intensity. The sensitivity to current can be calculated and expressed by output intensity response to applied current [29].
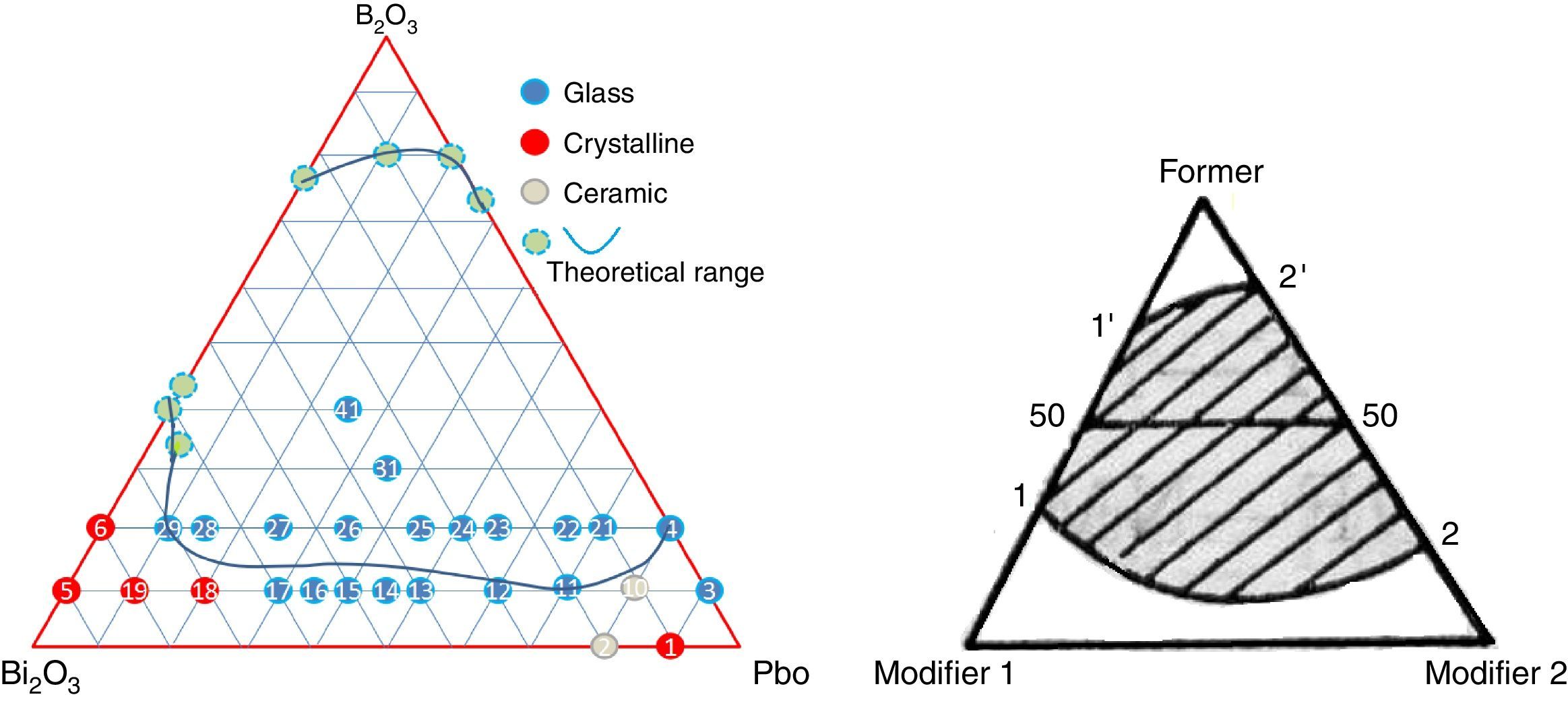
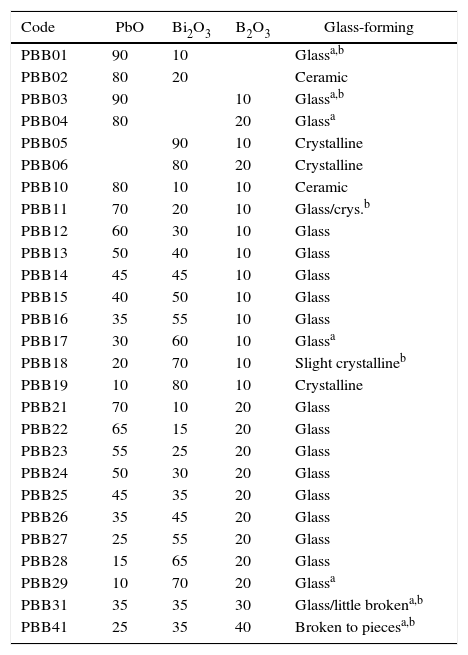
Results and discussionGlass formingAll of the obtained glasses are transparent, free of bubbles, yellow in color with good quality. For the aim of investigate the influence of PbO and Bi2O3 to the glass forming ability, 6 binary glasses PBB01–PBB06 were fabricated as comparison to ternary. From Table 1, it can be seen that binary PbO and Bi2O3 could not form glass (i.e. PBB01, PBB02) by themselves, PbO had a wider glass formation ability with B2O3 than Bi2O3 at same concentration (PBB03 and PBB04 are glasses, but PBB05 and PBB06 could not form glass). Substitution of Bi2O3 (or PbO) with PbO(or Bi2O3) in binary PbO–B2O3 and Bi2O3–B2O3 systems [11] resulted much stable ternary PBB glasses (PBB12–PBB17 and PBB21–PBB29). This good glass forming ability of ternary PBB came from contribution from B3+ and from the high polarizability of the Pb2+ and Bi3+ ions, which have outer shells of eighteen electrons.
Composition (mol.%) of the prepared PBB glasses and glass forming appearance.
| Code | PbO | Bi2O3 | B2O3 | Glass-forming |
|---|---|---|---|---|
| PBB01 | 90 | 10 | Glassa,b | |
| PBB02 | 80 | 20 | Ceramic | |
| PBB03 | 90 | 10 | Glassa,b | |
| PBB04 | 80 | 20 | Glassa | |
| PBB05 | 90 | 10 | Crystalline | |
| PBB06 | 80 | 20 | Crystalline | |
| PBB10 | 80 | 10 | 10 | Ceramic |
| PBB11 | 70 | 20 | 10 | Glass/crys.b |
| PBB12 | 60 | 30 | 10 | Glass |
| PBB13 | 50 | 40 | 10 | Glass |
| PBB14 | 45 | 45 | 10 | Glass |
| PBB15 | 40 | 50 | 10 | Glass |
| PBB16 | 35 | 55 | 10 | Glass |
| PBB17 | 30 | 60 | 10 | Glassa |
| PBB18 | 20 | 70 | 10 | Slight crystallineb |
| PBB19 | 10 | 80 | 10 | Crystalline |
| PBB21 | 70 | 10 | 20 | Glass |
| PBB22 | 65 | 15 | 20 | Glass |
| PBB23 | 55 | 25 | 20 | Glass |
| PBB24 | 50 | 30 | 20 | Glass |
| PBB25 | 45 | 35 | 20 | Glass |
| PBB26 | 35 | 45 | 20 | Glass |
| PBB27 | 25 | 55 | 20 | Glass |
| PBB28 | 15 | 65 | 20 | Glass |
| PBB29 | 10 | 70 | 20 | Glassa |
| PBB31 | 35 | 35 | 30 | Glass/little brokena,b |
| PBB41 | 25 | 35 | 40 | Broken to piecesa,b |
Probable reasons for glass forming and crystalline of binary and ternary PBB systems are addressed from different aspects.
- 1.
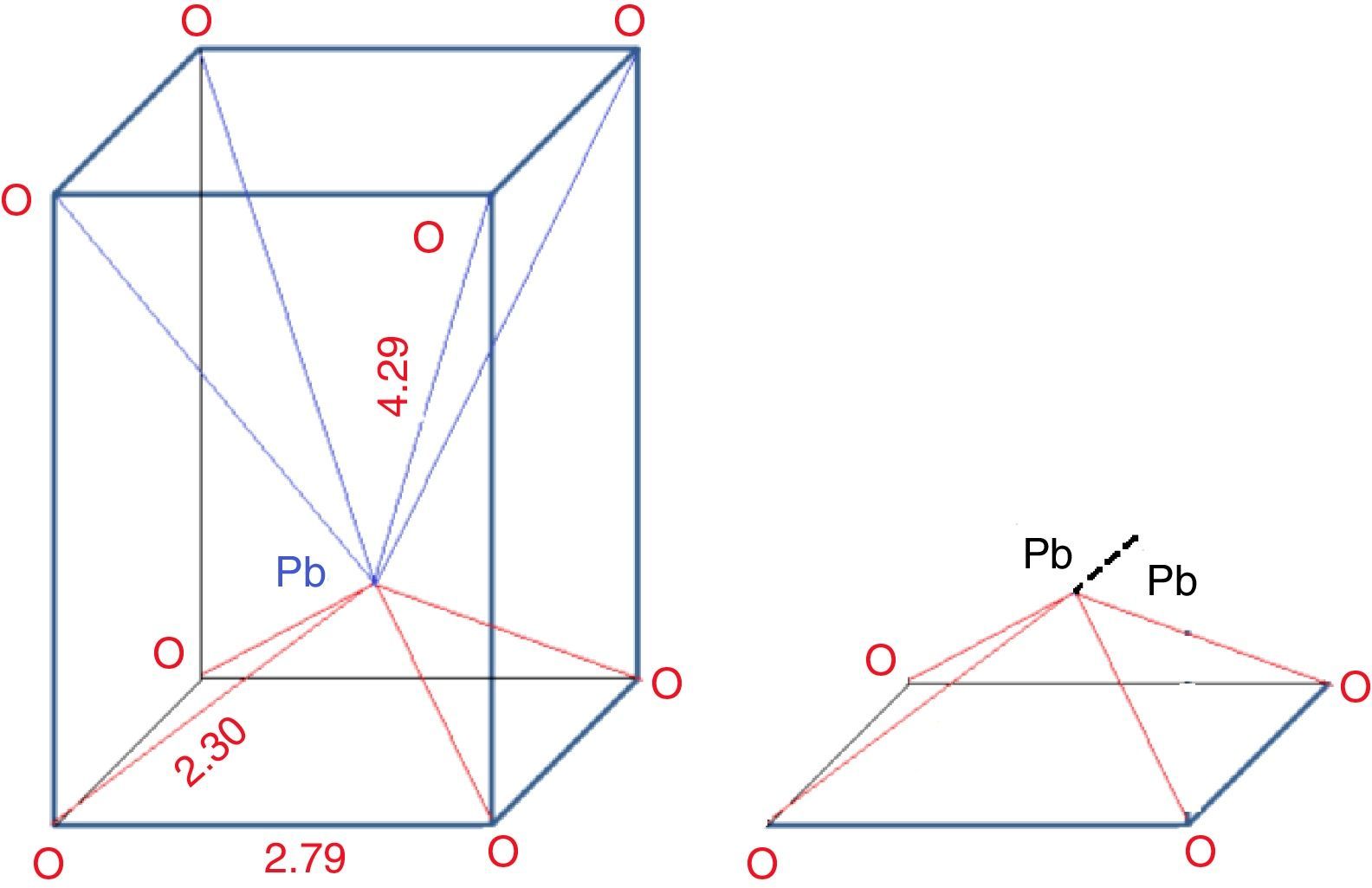
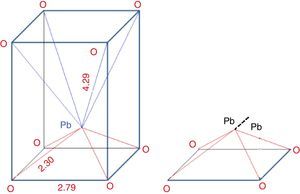
From the structure: Fig. 3 illustrates the structure of PbO, it can be seen that one Pb ion is surrounded by eight oxygen ions, in which four O2− are far from Pb (0.429nm), while the other four O2− are closer (0.23nm), thus the coordination is asymmetric. The inert electrons outside the Pb ion are rejected by the nearest oxygen which push the Pb ions to the other oxygen side, so it can be assumed losing two electrons and considered as a Pb4+ nucleus. While on the other hand, the other four O2− which are far away from the Pb, can be assumed receiving two electrons and considered as Pb0. Since the Pb locates at the top of PbO4 pyramidal unit. So the Pb2+ in the pyramidal unit can be considered as –1/2Pb4+–1/2Pb0– structure. The 1/2Pb0 termed as metal bridge decreases glass forming ability.
- 2.
From the crystalline chemistry: The number of unpaired electrons in orbit for Pb, Bi and O elements are 2, 3 and 2, respectively, so from the point of view of the possibility of forming bond, Pb and O, Bi and O can form covalent bonds by sharing electrons. Pb, Bi and O atoms bond, in the molten state, they exist as Pb2+, Bi3+ and O2−. During cooling, due to electrostatic force these ions accumulate together, and ion crystals are formed. Therefore, it is difficult to form glass for single oxide and binary oxides of PbO and Bi2O3.
- 3.
From the point of dynamics, crystallines mainly depends on the formation of nucleus and the growth of nucleus into crystals. Because the viscosity of molten PbO and Bi2O3 is very small, so the active energy for Pb2+, Bi3+ and O2− diffusion is also small, the nucleus is easy to grow into bigger crystals.
- 4.
While for ternary PBB system, due to BO bond strength is very big (498kJ/mol), the introduced B2O3 exist in the form of isolated layered triangular [BO3] or tetrahedral [BO4] which surround the accumulated Pb2+, Bi3+ and O2− and therefore prevent their growth. On the other hand, [BO3] or tetrahedral [BO4] occupy certain space and inhibit the transfer or diffusion of Pb2+, Bi3+ and O2−, making the Pb2+, Bi3+ and O2− outside the [BO4] surrounding cannot enter and accumulate with ions inside, and finally stop the formation of crystals and easy glass-forming.
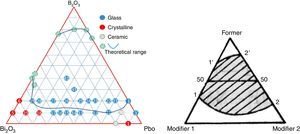
A ternary diagram was plotted according to glasses compositions in this study, also a theoretically diagram for ternary glass consisting one F (former) and two high polarized heavy metal oxides modifier (M) was presented as a reference. From Fig. 4, it can be seen that PbO and Bi2O3 cannot form binary system, while it is possible to form binary PbO–B2O3 and Bi2O3–B2O3 system. Initially, a molten glass network based on PbO and B2O3 was formed firstly, with the incorporation of Bi2O3 into the network, more free oxygen was introduced, and the melting temperature decreased. An equilibrium was reached until the lowest co-melting point appear. And a continuous ternary glass forming range (11′22′) formed. Due to the double rule of PbO, the lowest co-melting point should be close to PbO, and the binary PbO–B2O3 has a wider forming glass range (22′) than Bi2O3–B2O3 system (11′). The experimental PBB glass forming diagram agreed very well with theoretical one.
For PBB glasses with HMO of 90%, when Bi2O3 >60% or Bi2O3 <30%, the melts form ceramic or crystalline, not glass. This proved that Bi2O3 had a substantial effect on the process of glass crystallization. When Bi2O3 >70%, PBB18 began to crystalline, when Bi2O3 >80%, PBB19 became ceramic.
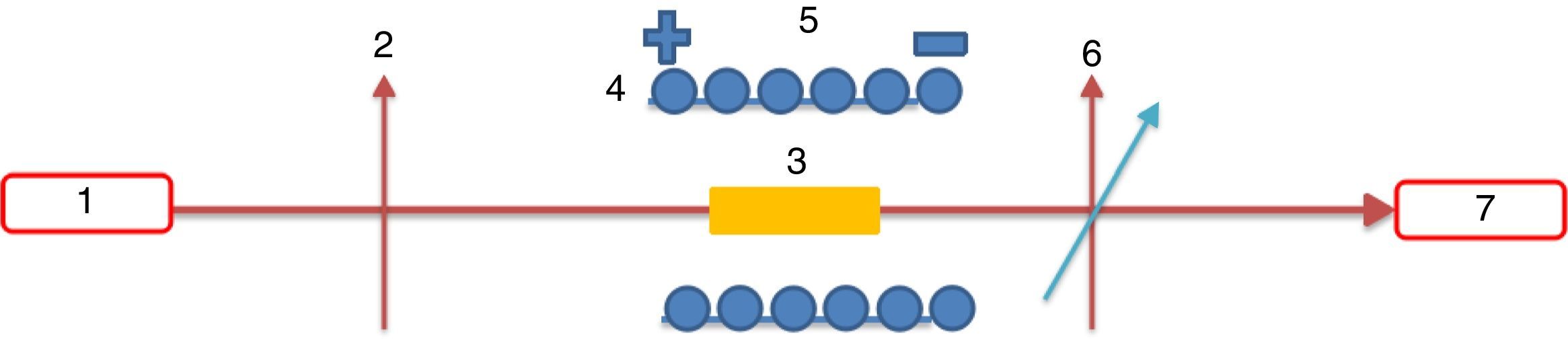
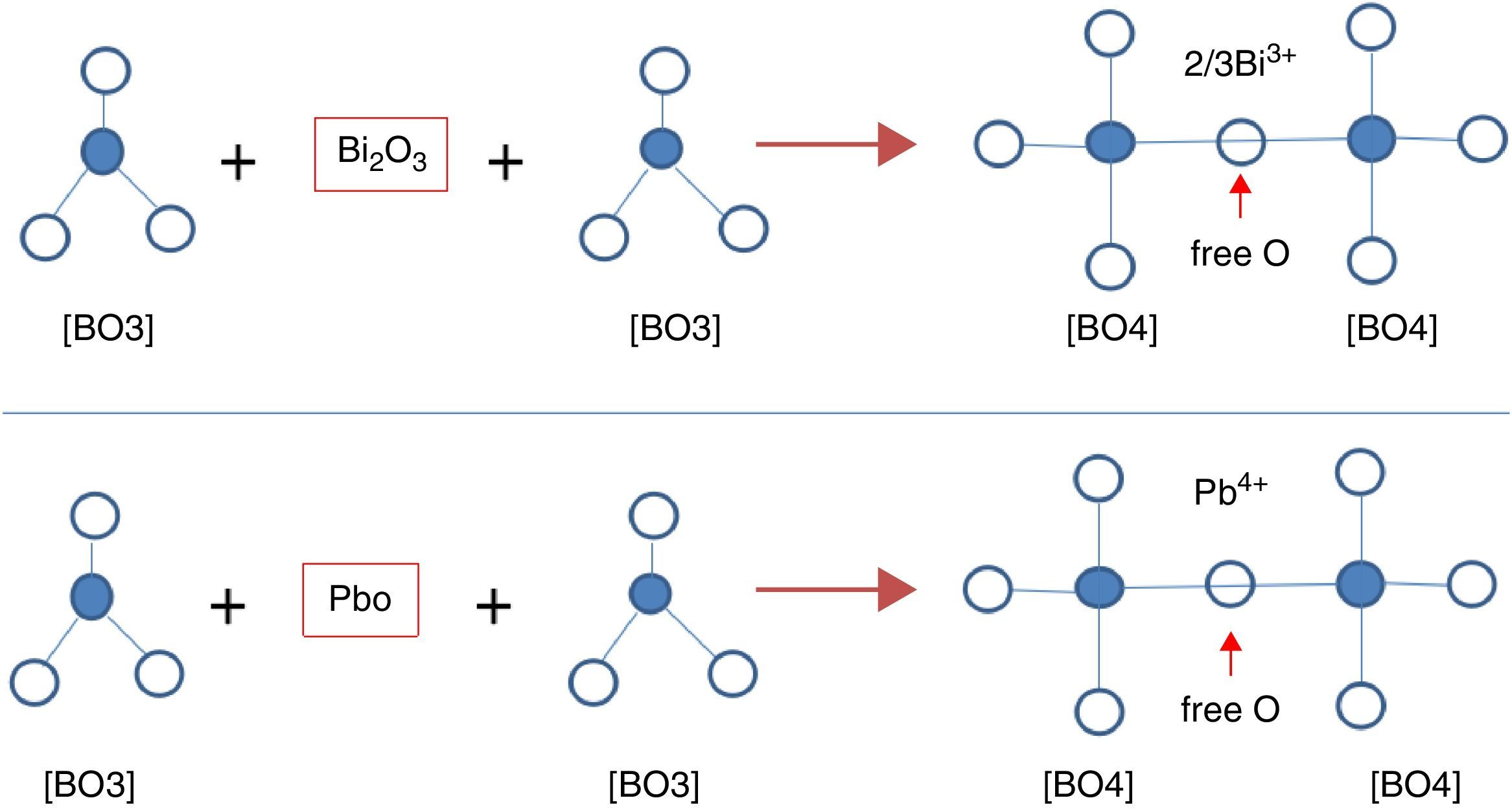
From Table 1 it can be seen that glasses with HMO=80% had a bigger glass forming ability than glasses with HMO=90%. This is due to the increase of B2O3. The role of B2O3 in glass matrix is enhancing the glass structure by decreasing the crystalline trends. When HMO=80%, PbO and Bi2O3 had a bigger glass forming range from 10 to 70%. But when B2O3=30%, PBB31 exhibited poor mechanical property while when B2O3=40% (PBB41), the melts broken to pieces during casting which proved a very poor mechanical property. These phenomena can be explained by B2O3 performance. It is well known that, with the increase of B2O3, the introducing of modifiers like PbO, Bi2O3, will convert the sp2 planar BO3 into more stable sp3 tetrahedral BO4 units in addition to non-bridging oxygens as illustrated in Fig. 5.
According to theoretical relationship between [BO4] with oxides, the number of [BO4] increase sharply and almost linearly (except the Li2O2) with oxides content in glass. Considering the high oxide content (70–90%) in this study, the B2O3 in glass present mainly as [BO4] unit.
Due to structural (shape) mismatch of [BO3] and [PbO4], it is hard to form homogeneous and dissoluble molten which will induce the phase separation during cooling. Oxides transfer the B2O3 structure through offering free oxygen, and change the layered triangle [BO3] to frame tetrahedron [BO4] which can be easily dissolve into the [PbO4] and form homogeneous glassy network, because in layered triangle [BO3] structure, even though the BO bond strength is big (498kJ/mol), the energy between layers are mainly Van Edward force which is very weak, the layered structure is unstable and can be easily broken. While the frame structure [BO4] is more stable, and highly connectivity to [PbO4]. On the other hand, considering the glasses fabricated are transparent and good quality, it can be deduced that B2O3 present in glass mainly in the form of [BO4], not [BO3].
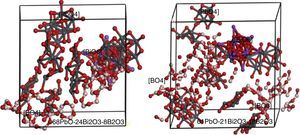
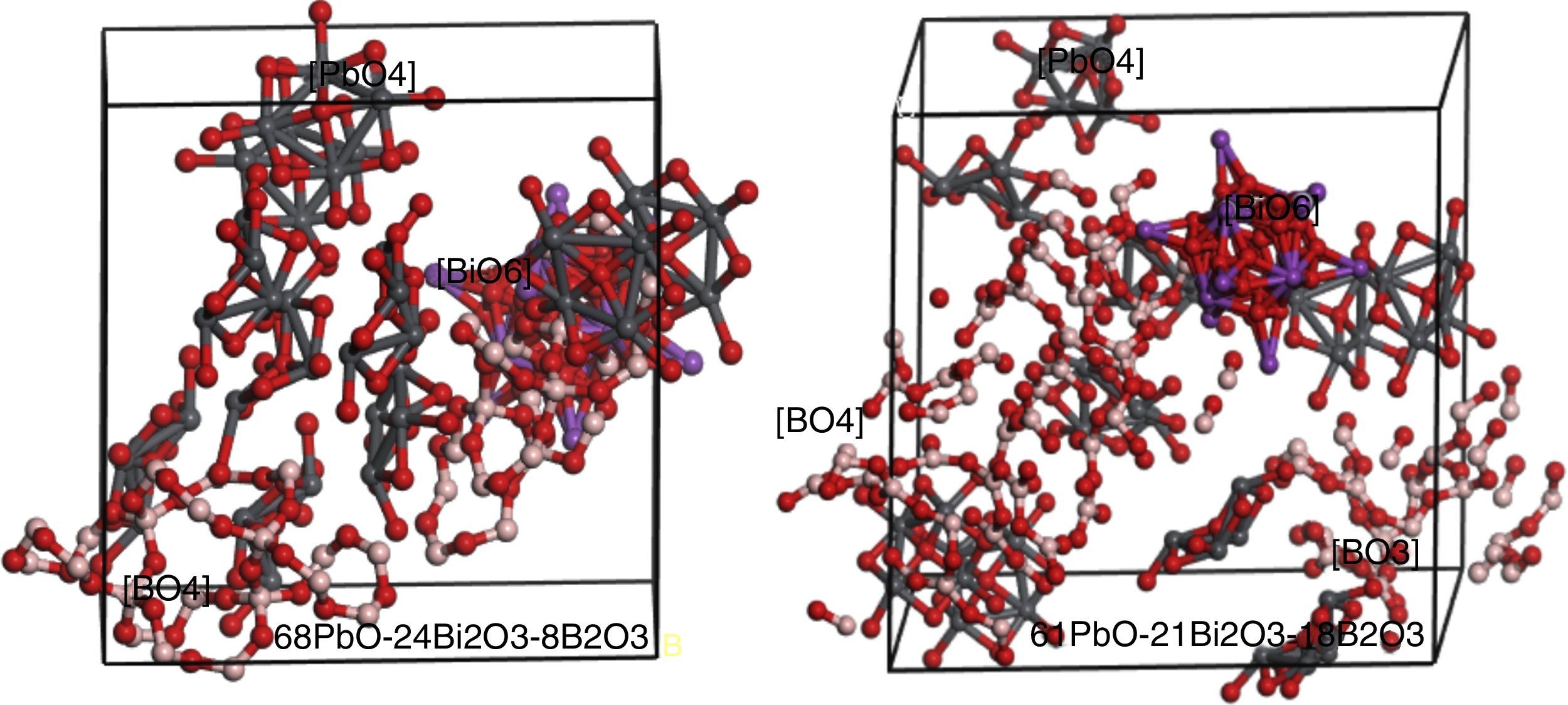
Simulation of PBB system employed Amorphous cell program in Materials Studio simulation software was carried out to prove the assumption. The simulated two PBB systems are based on: 68PbO–24Bi2O3–8B2O3 and 61PbO–21Bi2O3–18B2O3 in mol percent, finally using DMOL3 modulus to optimize the structure (Fig. 6).
In 68PbO–24Bi2O3–8B2O3 glass, the B2O3, as a glass former, exists as [BO4] which is highly connected with the [PbO4] network, while [BiO6] as a modifier. In 61PbO–21Bi2O3–18B2O3 glass, the B2O3 exist both as [BO4] and [BO3], it can be observed that the [BO4] unit connected with [PbO4] as network former, while the excessive B2O3 is isolated by the network in the form of [BO3].
In the case of B2O3 content is beyond a certain limit (e.g. 30% in mol.), the excessive B2O3 transfer the form of [BO4] into [BO3] due to the famous boron anomaly. It can be deduced that, with the increase of [BO3] unit, more and more [BO4] units were transferred to [BO3], and PBB glass structure become more and more loose, so accordingly the glass forming ability and glass properties degraded greatly, until no possibility to form PBB glass (e.g. glass with 40% B2O3).
Thermal propertyTable 2 gives properties of PBB glasses.
Composition (mol.%), glass transition temperature (Tg), crystalline temperature (Tx), density (ρ), cutoff wavelength (λ) and Verdet constant (V), refractive index (n), optical absorption (α) at 633nm of glasses.
| Glass code | V (min/Gcm) | ΔT (°C) (Tx−Tg) | Tg (±1°C) | Tx (±1°C) | n ±0.01 | ρ (kg/m3) | a (cm−1) (×10−5) | Q | λ (nm) ±1 |
|---|---|---|---|---|---|---|---|---|---|
| PBB12 | 0.1373 | 80 | 317 | 397 | 2.26027 | 8.29 | 2.3241 | 0.059 | 464 |
| PBB13 | 0.1503 | 89 | 289 | 378 | 2.27451 | 8.31 | 2.15 | 0.071 | 474 |
| PBB14 | 0.1475 | 62 | 327 | 389 | 2.32241 | 8.36 | 2.5610 | 0.053 | 469 |
| PBB15 | 0.1492 | 56 | 330 | 386 | 2.33251 | 8.39 | 2.5640 | 0.051 | 471 |
| PBB16 | 0.1512 | 53 | 332 | 385 | 2.33620 | 8.42 | 2.5613 | 0.057 | 473 |
| PBB17 | 0.1518 | 41 | 330 | 371 | 2.33731 | 8.54 | 2.5662 | 0.051 | 475 |
| PBB21 | 0.1042 | 94 | 303 | 397 | 2.20711 | 8.58 | 2.2136 | 0.041 | 441 |
| PBB22 | 0.1089 | 87 | 305 | 392 | 2.1344 | 7.99 | 2.27 | 0.048 | 449 |
| PBB23 | 0.1109 | 84 | 304 | 388 | 2.16473 | 8.03 | 1.9291 | 0.053 | 450 |
| PBB24 | 0.1194 | 75 | 303 | 378 | 2.1993 | 8.11 | 2.1631 | 0.048 | 452 |
| PBB25 | 0.1252 | 79 | 306 | 385 | 2.2056 | 8.16 | 1.9982 | 0.067 | 454 |
| PBB26 | 0.1264 | 98 | 302 | 400 | 2.2066 | 8.25 | 2.3211 | 0.056 | 455 |
| PBB27 | 0.1299 | 73 | 310 | 383 | 2.1577 | 8.26 | 2.3145 | 0.059 | 457 |
| PBB28 | 0.1329 | 68 | 311 | 379 | 2.1582 | 8.31 | 2.3201 | 0.059 | 459 |
| PBB29 | 0.1351 | 62 | 309 | 371 | 2.1601 | 8.37 | 2.3984 | 0.037 | 460 |
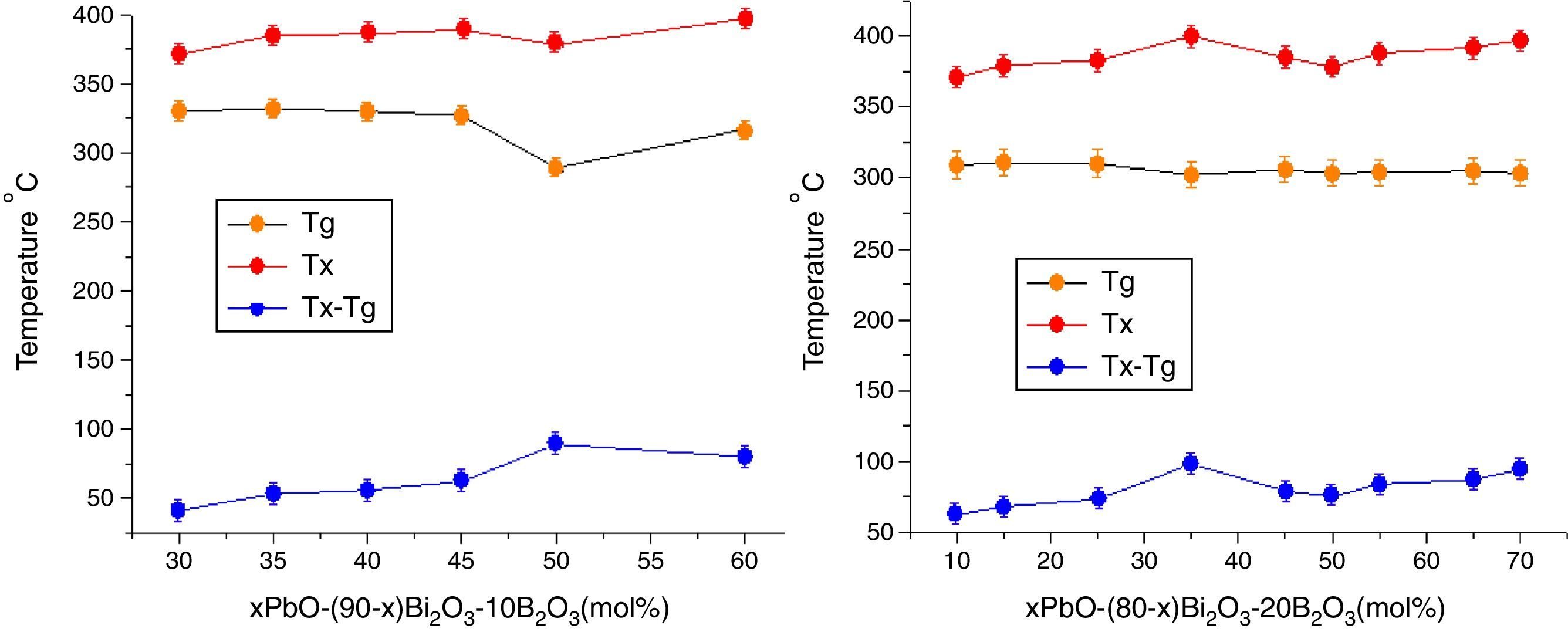
The Tg gives information on both the strength of inter-atomic bonds and the glass network connectivity [21], from Table 2, one can observe that Tg always decreases when increasing of PbO+Bi2O3 content as a whole. This is because the addition of PbO+Bi2O3 weakened the bond between each atom in the network and increased the number of non bridging oxygens, especially in OB linkages. Continuous substitution of B2O3 by PbO+Bi2O3 leaded to a continue decrease in Tg. The substitution of stronger BO bonds (498kJ/mol) by weaker PbO bonds (305kJ/mol) and BiO induces the increase of non bridging oxygen atoms number and a bigger tendency to form BO3 units. As a consequence, the network is soften and weaken with lower thermal stability ΔT=Tg−Tx.
In addition, Pb2+ ions and Bi3+ had lower electrical field intensity (0.27 and 0.23) than B3+ ions (>1.4) [34], so PbO and Bi2O3 exhibited high polarisability, the high polarisability explains why the Tg shifted toward lower temperature [35].
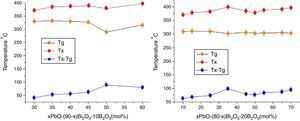
Fig. 7 shows the Tg, Tx changes with the PbO in different HMO contents. Based on same HMO content, substituting of PbO with Bi2O3 leads to a decrease in the molar volume and decrease in Tx because the network starts to be cross-linked due to BO3 (planar trigonal units) to BO4 (tetrahedral units) conversion [36]. This also could be ascribed to the introduction of more free oxygen, as a polarizable component into the glass structure when substituting of PbO with Bi2O3[37,38]. Additionally, introducing more Bi3+ cations, as a glass modifier, would cause structural complexity which could ease the divetrification [39]. So increasing of Bi2O3 at the cost of reducing PbO induced poor thermal stability of glasses. While on the contrary, B2O3 helps to increase the thermal stability within certain limit. For example, the average ΔT of glasses PBB12–PBB17 was lower than that of PBB21–29. Among all glasses, the glass with composition of 35PbO–45Bi2O3–20B2O3 in mol.% showed the highest ΔT.
Refractive indexRefractive index of glass is sensitive to network structural changes [40], so it is generally influenced by the bond polarizabilities of its constituents. In the case of conventional glasses, it has been observed that both the linear and nonlinear refractive indices increase with the concentration of non-bridging oxygen bonds that are induced by the addition of network modifiers. However, this is not the case for glasses with a significant fraction of heavy metal cations with ns2 electron pairs (Pb2+, Bi3+). In this case, the concentration of heavy metal cations, and in particular their high polarizability and big atomic mass, are main factors determining refractive index values.
From Table 2, the refractive indices of PBB12–PBB17 as a whole are higher than that of PBB21–PBB29, and they increased with the Bi2O3 content at the cost of PbO. This is because with the incorporation of Pb2+ (r=0.132nm, α=1.315nm3) and Bi3+ (r=0.103nm, α=1.508nm3), when light passes through the glass, more energy was required to distort electron cloud of Pb2+ and Bi3+, so a great part of the energy is converted into the vibration energy outside the nucleus field, and the propagation speed is greatly influenced, eventually leading to the improvement of refractive index [11].
Beside the polarizability, the phenomenon of ‘Boron anomaly’ also influences the refractive index. It is related to the boron ion co-ordination number changes from 3 to 4, when PbO or Bi2O3 are introduced [19,21,28], that is why even at the same HMO contents, the refractive index varied when PbO played different roles.
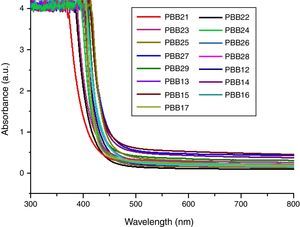
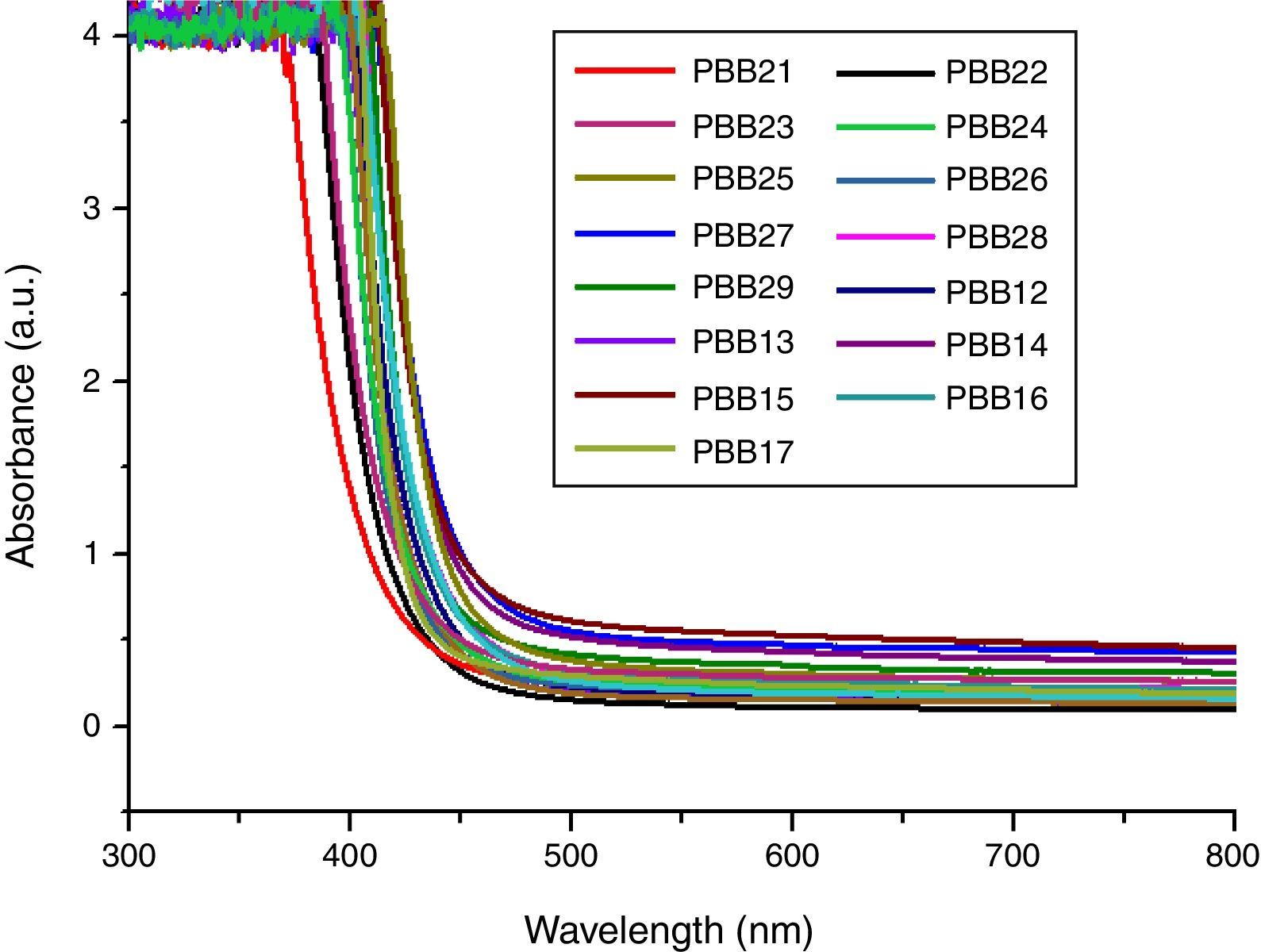
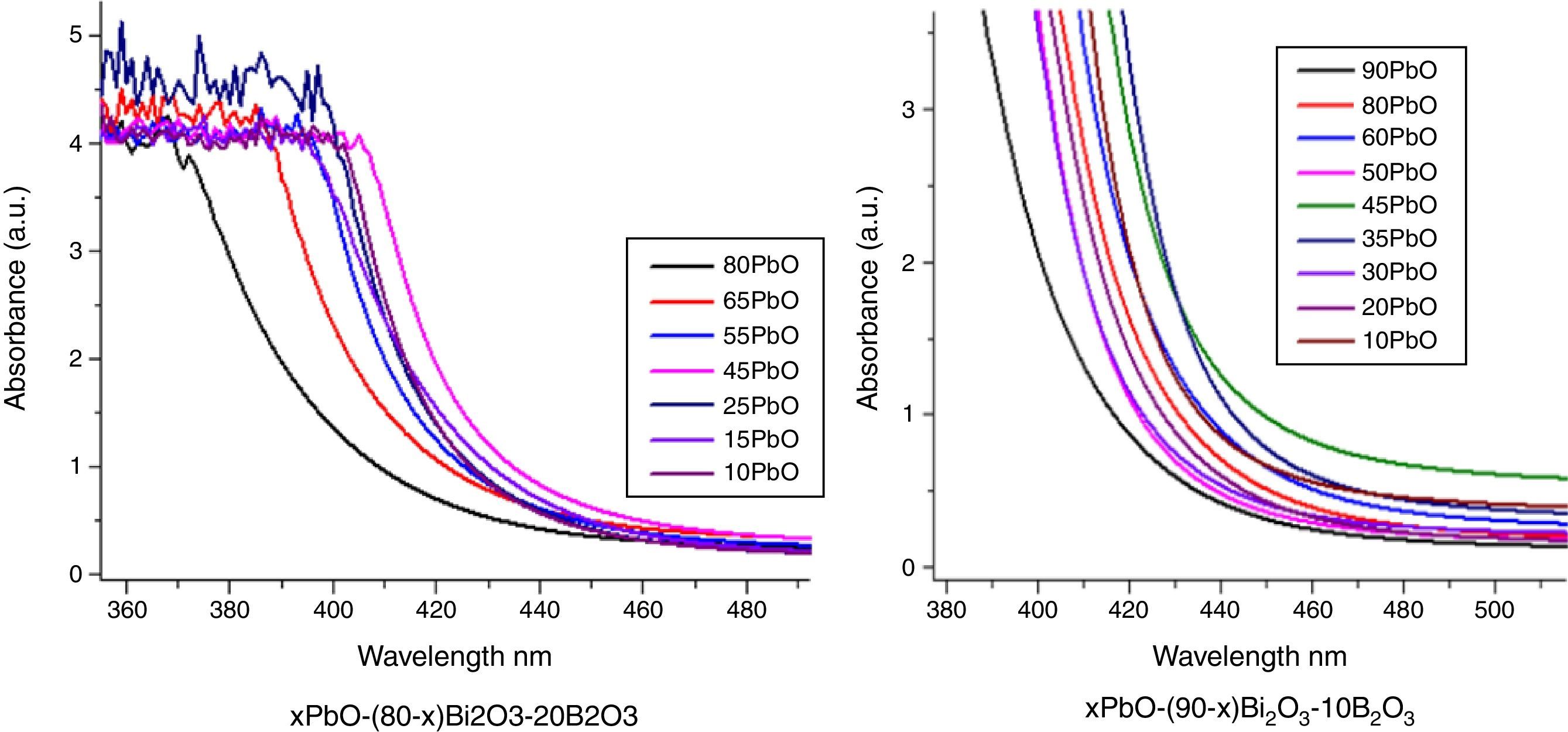
Optical absorption and cutoff wavelengthAs addressed in previous section, when light pass the glass, a great part of the light energy is converted into the vibration energy outside the nucleus field for electron cloud distortion, so the optical absorption inside the glass would increase. From Table 2, the optical absorption of glasses with HMO=90% were higher than glasses with HMO=80%. Another reason for the absorption is the color changed at different compositions; for example, the color of glass with 90% HMO were dark yellow, while colors for glasses with HMO=80% were light yellow. Even based on same HMO content, PbO-rich-glasses were yellow, while Bi2O3-rich-glasses were brown, and the brownish color intensified on prolonged stirring of the melts [18]. Fig. 8 shows the UV–vis spectra and cutoff of PBB glasses.
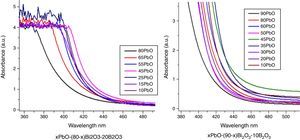
It can be seen that all glasses exhibited a steep cutoff and this makes them an appealing candidate in optical switches [41] and shielding applications. In addition, the cutoff shifted to longer wavelengths with the increase of PbO+Bi2O3 contents. For the same HMO content, cutoff of glasses shifted to shorter wavelength with the substitution of Bi2O3 with PbO as shown in Fig. 9. There are 3 reasons for the cutoff shifts with different compositions:
- 1.
The increase in molecular mass of oxides can induce the red-shift of glass cutoff which agreed well with [42]. From Table 1, when HMO=90%, glass PBB17 had the highest Bi2O3 (60%), because the molecular mass of Bi2O3 is 465.96g/mol, while mol. masses for PbO and B2O3 are 223.2g/mol and 69.62g/mol respectively, and PBB17 had the biggest red-shift compared with other glasses. In these glasses, the Bi2O3 contributed more to the UV cutoff red-shift than PbO.
- 2.
From the point of polarizability, the Bi2O3 induced more red-shift of UV cutoff than PbO [43]. Due to the fact that lead oxide offers only one atom per molecule, whereas bismuth oxide contributes two bismuth cations. Bi3+ cation possesses an extremely low electronic polarizability (0.002Å3) (while for Pb2+ (4.9Å3) and its unit field strength is very large which affecting strongly the electron charge density of the surrounding oxygen ions. So Bi3+ cations have a high polarizability (1.508Å3) [40] and small cation oxygen field strength. Therefore, glasses with more Bi2O3 had higher oxide ion polarizability and this induced more red-shift.
- 3.
From the point of glass energy band gap. The shift in cutoff could also be explained by the change of band gap for different constituents in glass systems: the band gap Eg=hc/λ, where h and c are constants, λ is the cutoff wavelength. The Eg for B2O3 is 8eV, Eg for PbO is 2.73eV and Eg for Bi2O3 is 2.76eV. The decrease of Eg in the glass can move the UV cutoff to longer wavelength. So the increase of HMO red-shifted the UV cutoff, at the same HMO content, PbO-rich glasses has shorter cutoff wavelength than Bi2O3-rich glasses.
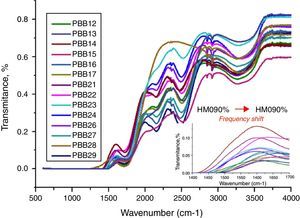
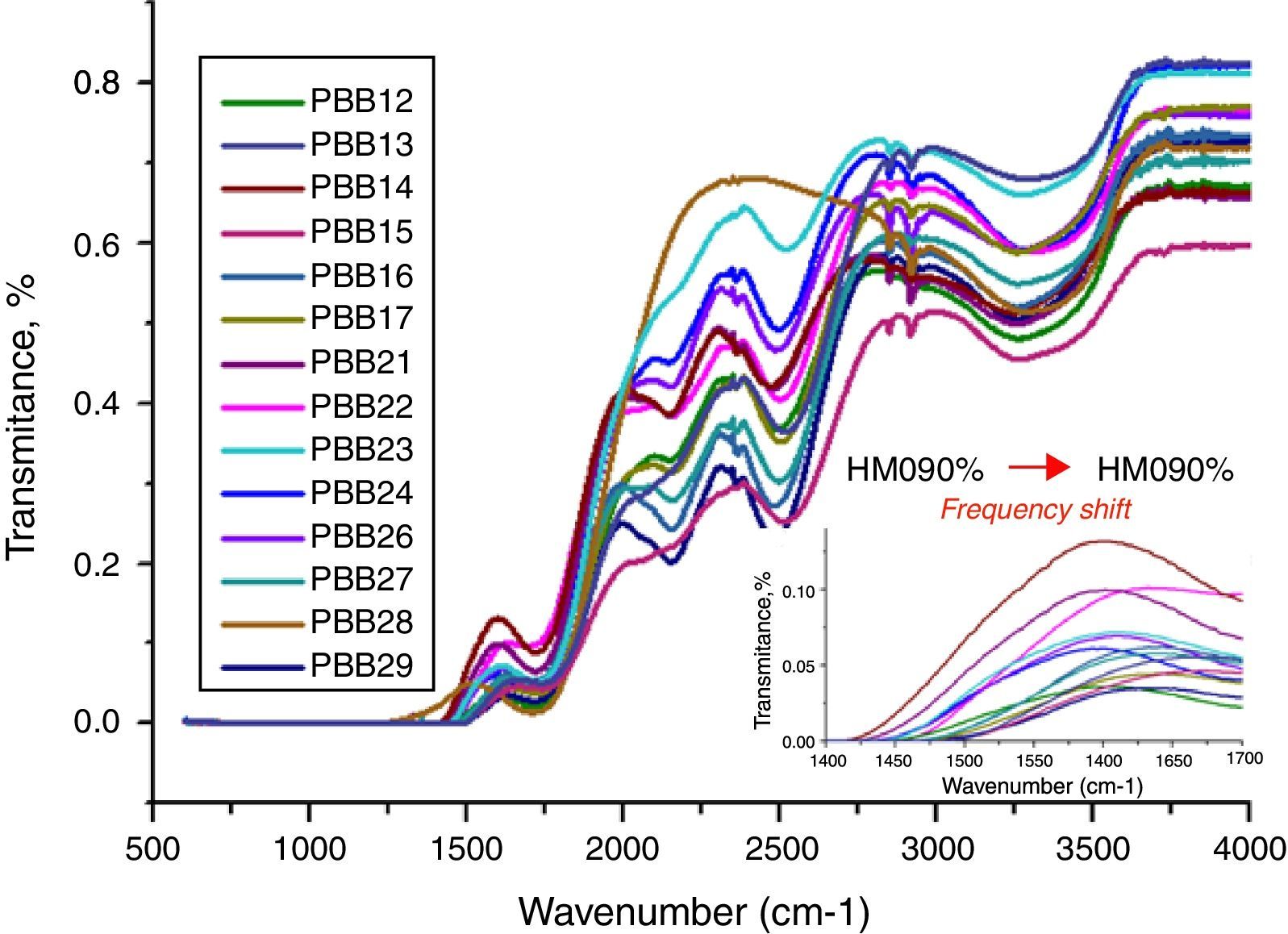
The FT-IR spectra of glasses recorded in the range of 1500–4000cm−1 are displayed in Fig. 10. The different roles of PbO induced difference of FT-IR transmission. Normally PbO acts as a glass modifier at low content and enters the glass as Pb2+ ions, the PbO bonds strongly ionic and the cations enter the network in an interstitial manner. Every added oxygen atom of PbO is used to convert two B2O3 units to two BO4 units. For example, in glasses PBB16, PBB17, PBB26, PBB27 and PBB29, PbO entered the glass network by breaking up the Bi\O\Bi and B\O\B bonds and introduced coordinate defects known as dangling bonds along with non-bridging oxygen ions (Bi\O\…Pb2+…\O\Bi), which in turn neutralize the negative charge of non-bridging oxygen ions by forming BiO6 and BO4 units [26]. Therefore, Pb2+ acted as a glass forming agent and was incorporated in the form of PbO4 units [32].
Pb2+ fulfills a dual role in glass structure depending on its concentration. With the PbO content increased, a considerable portion acted as a double bridge between adjacent Bi2O3 and form –Bi\O\Pb\O\Bi – besides PbO4 and BiO3 units. As can be seen from the structure of PbO in Fig. 3, Pb2+ ions are likely to form compact pyramidal PbO2 units. These pyramidal units reduce the availability of ionic charge balance, and reduce the formation rate of tetrahedral [BO4] units. As for Bi2O3, due to their high polarizability and asymmetric octahedra [BiO6], Bi3+ in glass may exist in six and eight-coordination [10].
The IR spectra of pure B2O3 gives two absorption band at wave-numbers 1300–1700 and 720cm−1, but from Fig. 11, the frequency from 500 to 1500cm−1 is linear. This is due to the low B2O3 content in these glasses in which B2O3 did not act as main network former. So the absorption band at 720cm−1 was covered and cannot be observed. However, bands from 1500cm−1 to 1700cm−1 are attributed to the bending vibration and stretching vibration of BOB in [BO3] triangles [44,45]. Glasses with 20% B2O3 content had higher intensity in these bands than glasses with 10% B2O3. In addition, with increase of HMO content, the frequency of [BO4] unit in glass with 20% B2O3 shifted to a lower frequency (from 1500cm−1→1450cm−1) (Fig. 2 onset). This was due to the formation of bridging bonds of PbOB and BiOB. Since the stretching force constant of PbO bonding is substantially lower than that of the B–O, with the increase of HMO content, the stretching frequency of PbOB might tend to be lower, and PbOB bonds became dominant in glass network. It can be presumed that the increasing polarization of Pb2+ and Bi3+ contributed to the formation of PbOB rings and their chains [46].
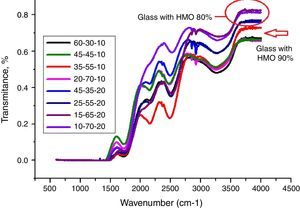
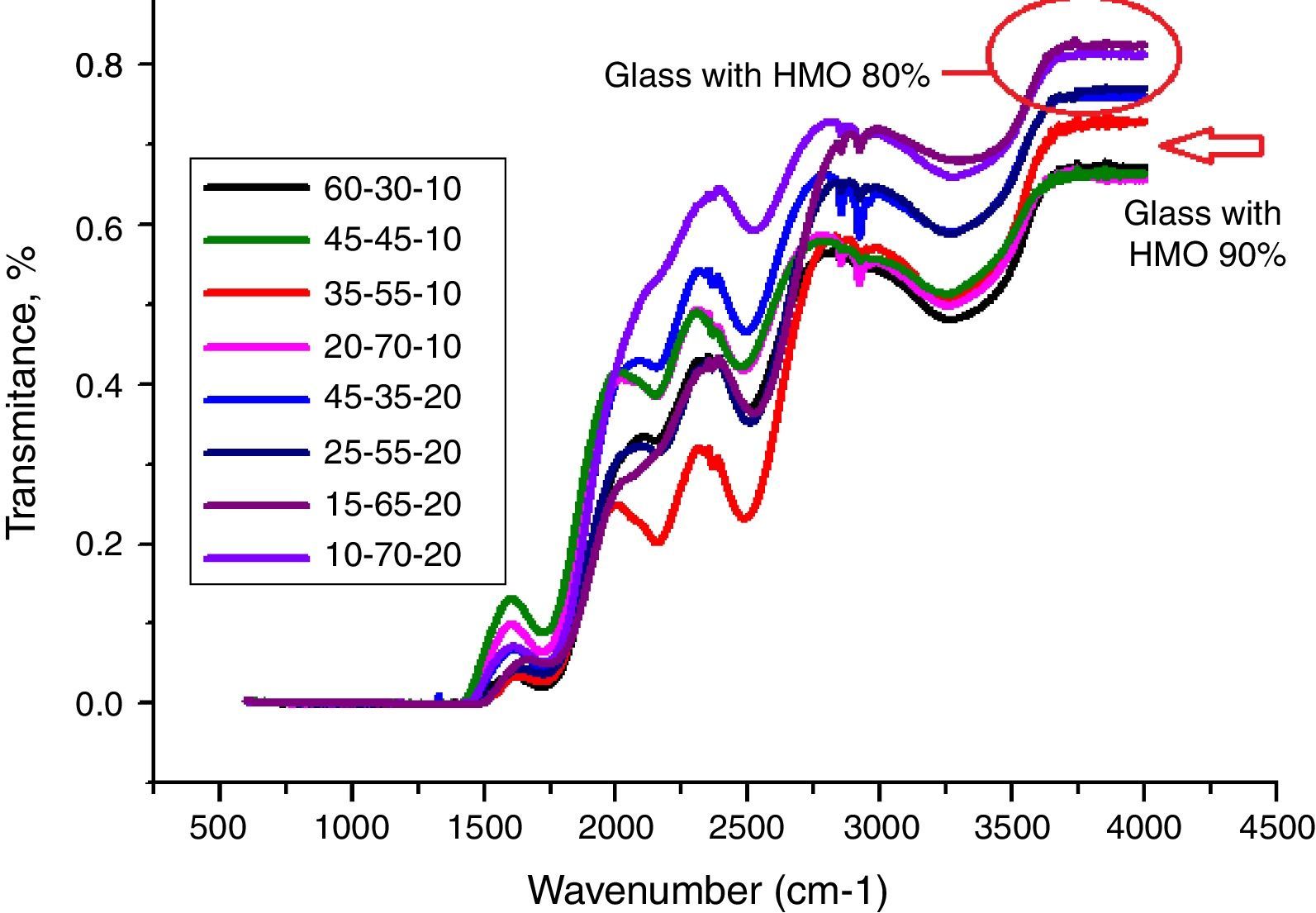
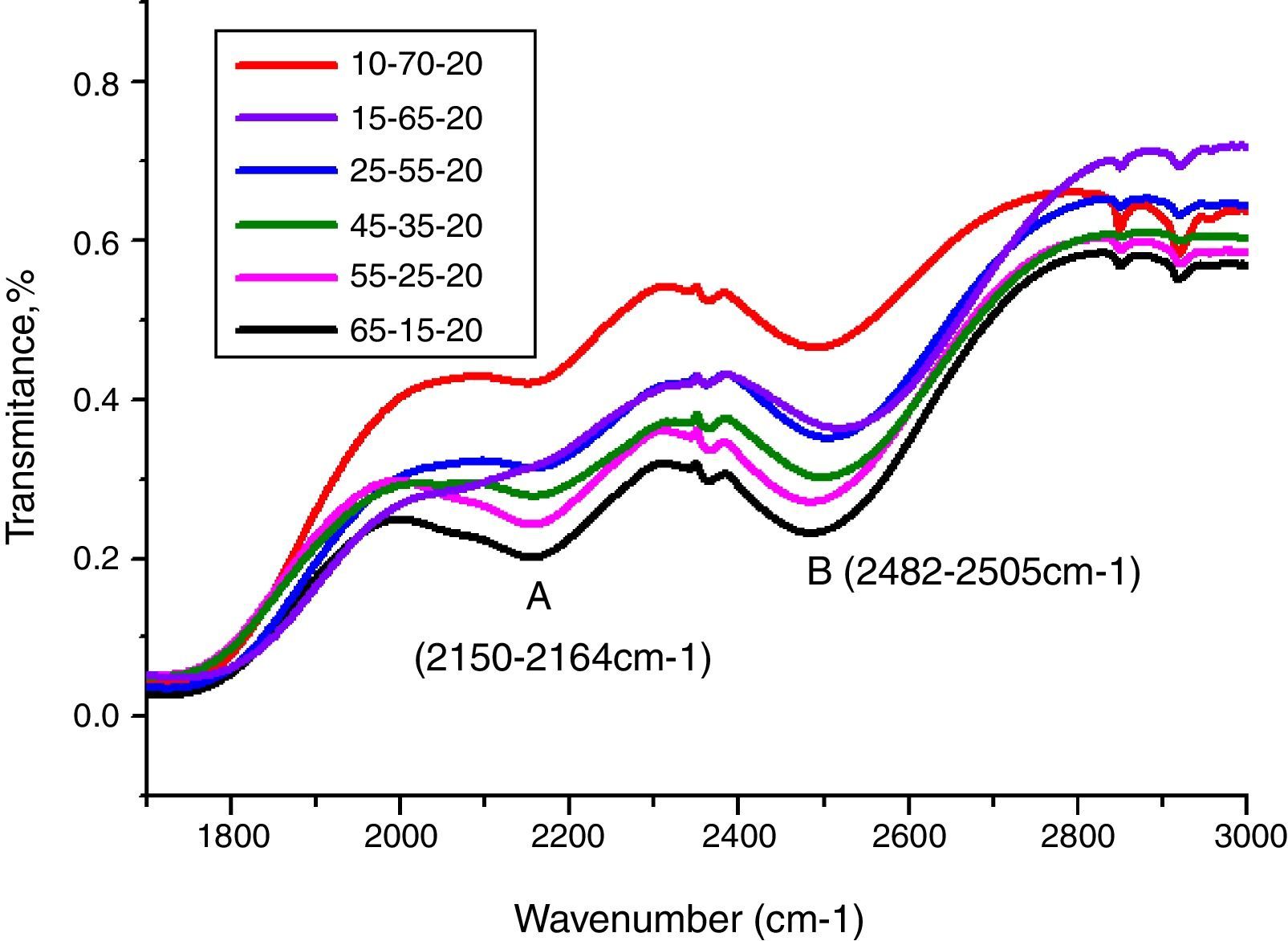
From Fig. 11, HMO glasses had high transmission (up to more than 80%) in the far-infrared region. The transmissions of glasses with HMO 80% are higher than glass with 90% HMO as shown in Fig. 12. PBB13 glass has the highest transmission (82.39%) in the range of 3500–4000cm−1. Peaks located around 3450cm−1 are assigned to OH absorption [45] because these glasses were fabricated in air.
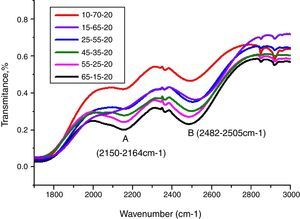
Peaks around 2160cm−1 (A) and 2500cm−1 (B) in Fig. 12 are caused by the PbO bond vibration [29,46]. With increase PbO at the expense of Bi2O3, these two bands occurred obvious broader in shape and stronger in intensity which indicated the electrostatic fields of the strongly polarizing Pb2+ ions are enhanced by the vibration between PbO bonds.
Verdet constant and figure of meritIt is known that the Verdet constant of a magnetic–optical material is related to the electron shell structure of the atoms in glass. If the ions have the electron structure same with inert gas, the applied field can induce the Zeeman splitting on the ion energy levels. Basing on classical electromagnetism theory [19,32], Bacquerel has proposed the relationship between Verdet constant and diamagnetic properties of the materials in Eq. (1):
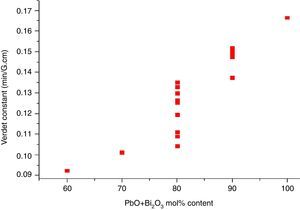
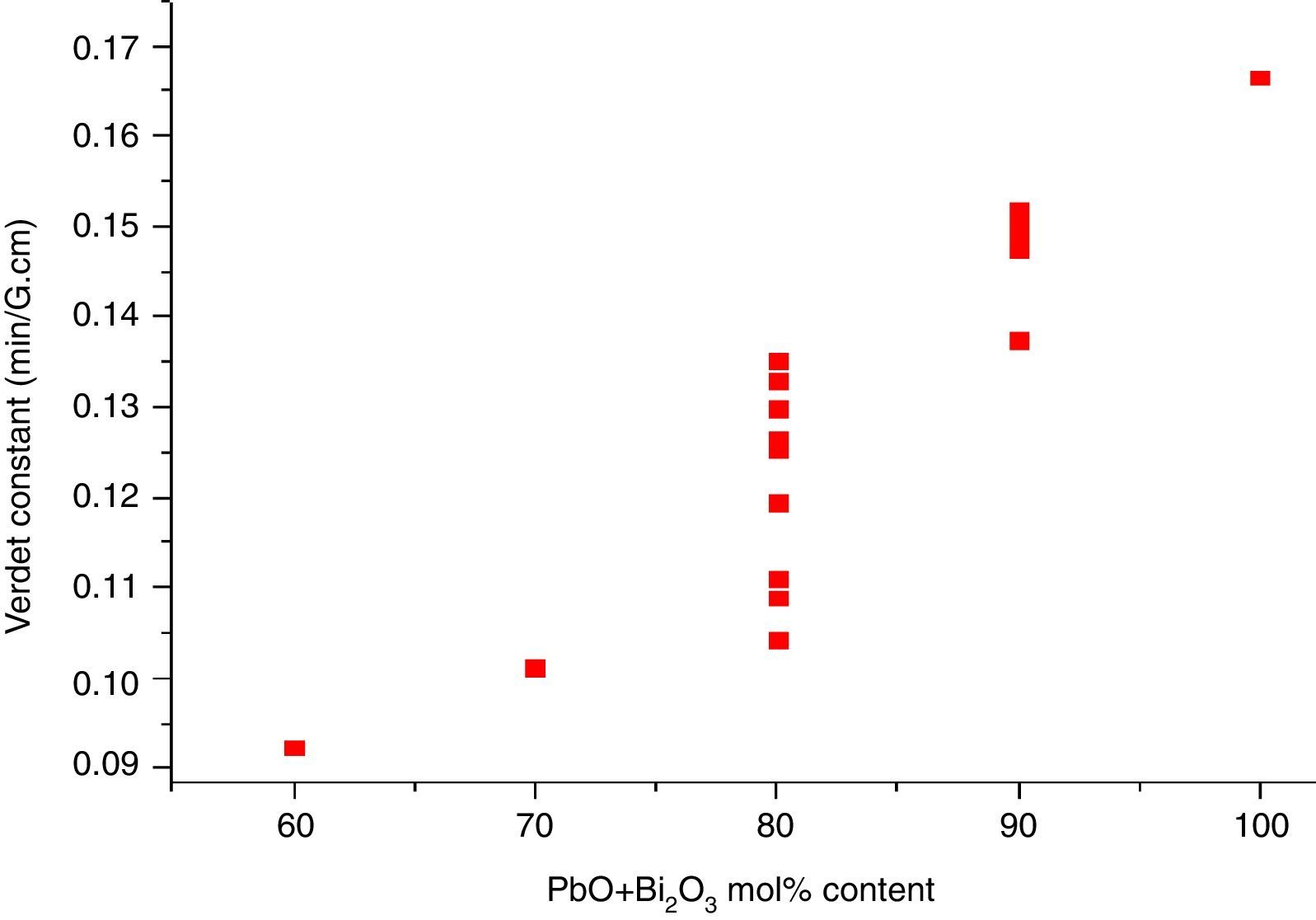
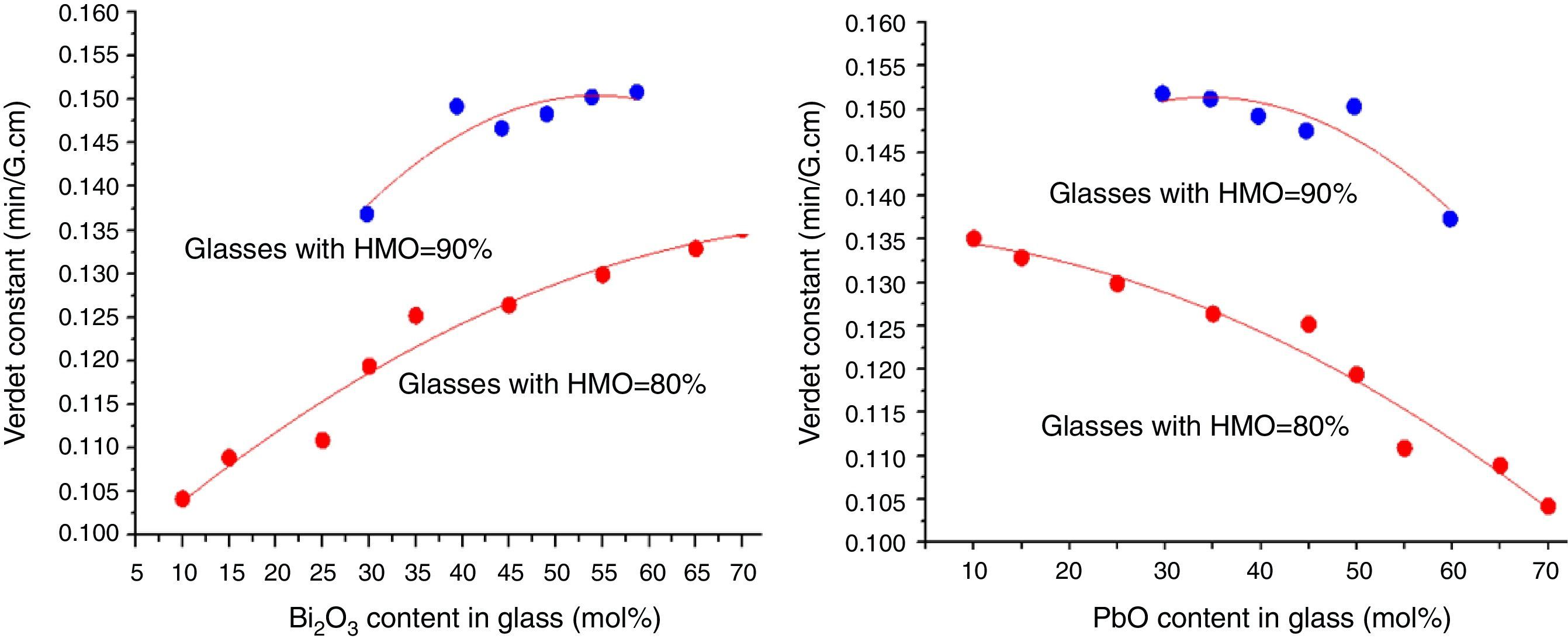
where e and m are the charge and mass of the free electron, c is the light speed, dn/dλ is the dispersion, Vd is the Verdet constant. From Eq. (1), the Verdet constant increased with the dispersion of the medium. In fact, the Pb2+, Bi3+ ions exhibited high dispersion ability through the increase of non-bridging oxygen and structural distortion etc., so glasses with higher HMO content had higher Verdet constant as shown in Fig. 13.On the other hand, the dispersion is proportional to the cation polarizability of glass [18], so the Verdet constant (V) can be expressed by the polarizability (α) in Eq. (2):
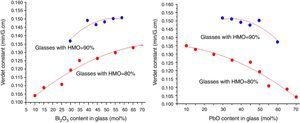
where r is the distance between the atoms or molecules, I is the first ionization energy of atom and α is the polarizability constant expressed in units of m3. The polarizability α represents the polarizability state of an average oxide cation in the glass matrix and its ability to attract electron density to surrounding ions. High oxide cation polarizability means strong electron attract ability of the oxide ion and vice versa. From Eq. (2), Verdet constant is proportional to the cation polarizability of glass. Substituting PbO with Bi2O3 in the borate glasses increases refractive index and dispersion [33]. Bi2O3 has bigger polarizability (1.508Å3) than PbO [47]. This is due to the fact that PbO offers only one atom per molecule, whereas Bi2O3 contributes two bismuth cations per molecule. Additionally, Bi3+ cation possesses an extremely low unit field strength which strongly affects the electron charge density of the surrounding oxygen ions. So Bi3+ cation has very high polarizability (1.508Å3) and a strongly polarized lone pair in the valence shell. These characteristics are responsible for the decrease of polarizing effect of these cations on the oxide ion and increase of non-oxygen bridge, and eventually contributed to the increase of Verdet constant. The Verdet constants for glasses with different Bi2O3 and PbO contents are shown in Fig. 14.Basing on the quantum theory [19], Verdet constant of diamagnetic material is also related to the ion carriers with energy level splitting possibility (Eq. (3)):
where Vd is the Verdet constant, N is the carriers in per unit volume, v is the frequency of the incident wave, vn is the frequency of electrons migration, and An is the parameters correlative with migration intensity. Eq. (3) shows that the Verdet constant is related to the carriers’ concentration N. In case of this study, the Pb2+ and Bi3+ ions are the carriers. Therefore glasses containing 90% HMO had higher Verdet constant than glasses with 80% HMO.On the other hand, based on same HMO content, when substituting PbO with Bi2O3, the carriers per unit volume (or carriers’ concentration) increases because PbO contributes only one Pb2+ per molecule, whereas Bi2O3 contributes two Bi3+. Additionally, the electronic mass of Bi3+ is higher than Pb2+ but ionic radius of Bi3+ is smaller (1.20Å) than Pb2+ (1.32Å), these factors induce the decrease of molecule volume. Due to their more carriers’ number, less molecule volume, higher carriers’ concentration, Bi3+-rich glasses exhibited higher Verdet constant than Pb2+-rich glasses.
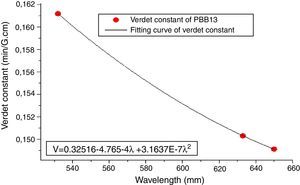
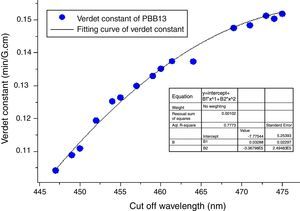
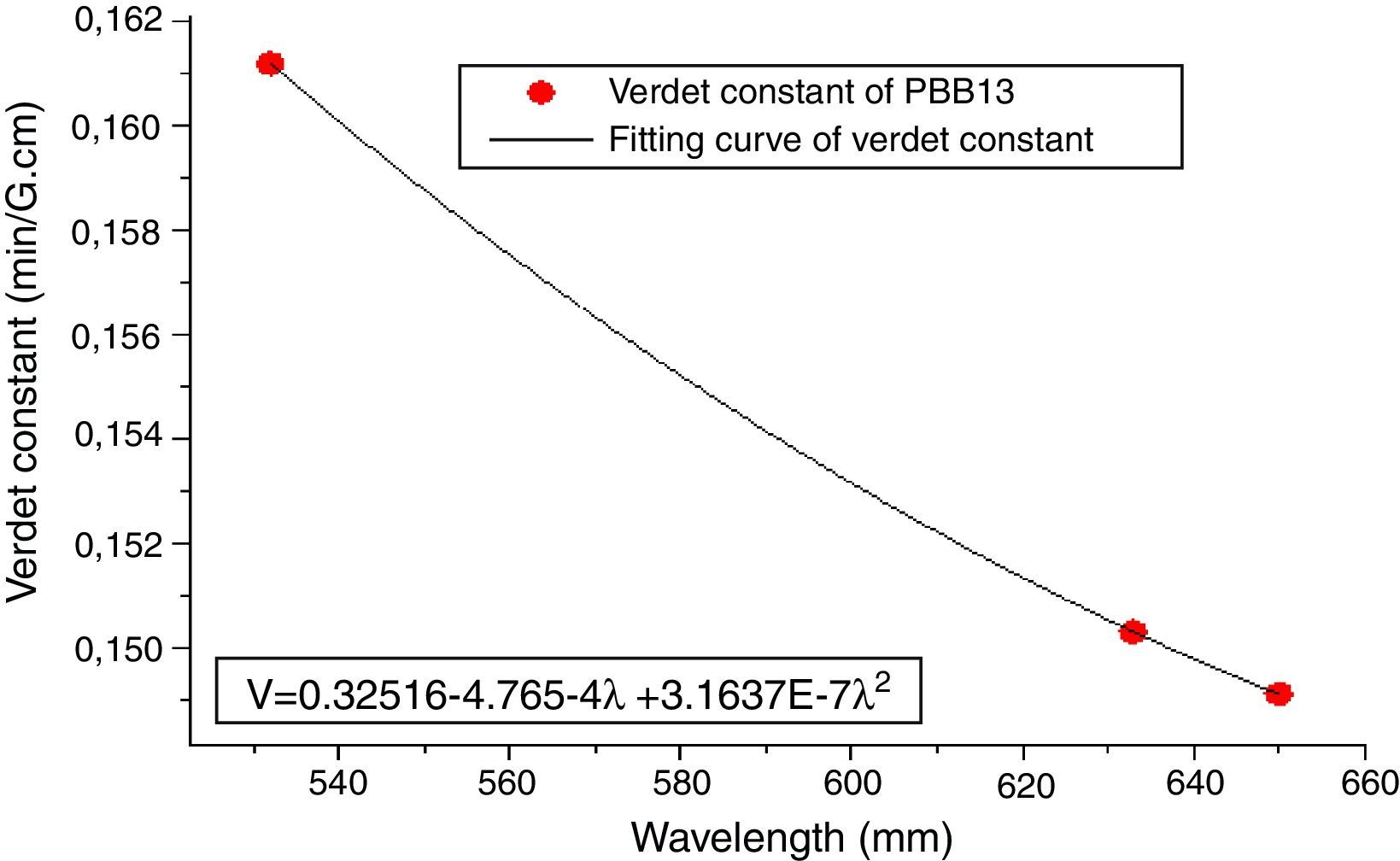
From Eqs. (1) and (3), it can be noticed that the Verdet constant depends on the incident wavelength (frequency). In this study, the Verdet constants of glass PBB13 were measured at three wavelengths (532nm, 633nm and 650nm) as shown in Fig. 15.
It can be seen from Fig. 13, the Verdet constant decreased with the increasing of wavelength, and a fitting curve was made on base of the data, this agreed well with Eq. (1) and other reports on Verdet constants at different wavelengths [29].
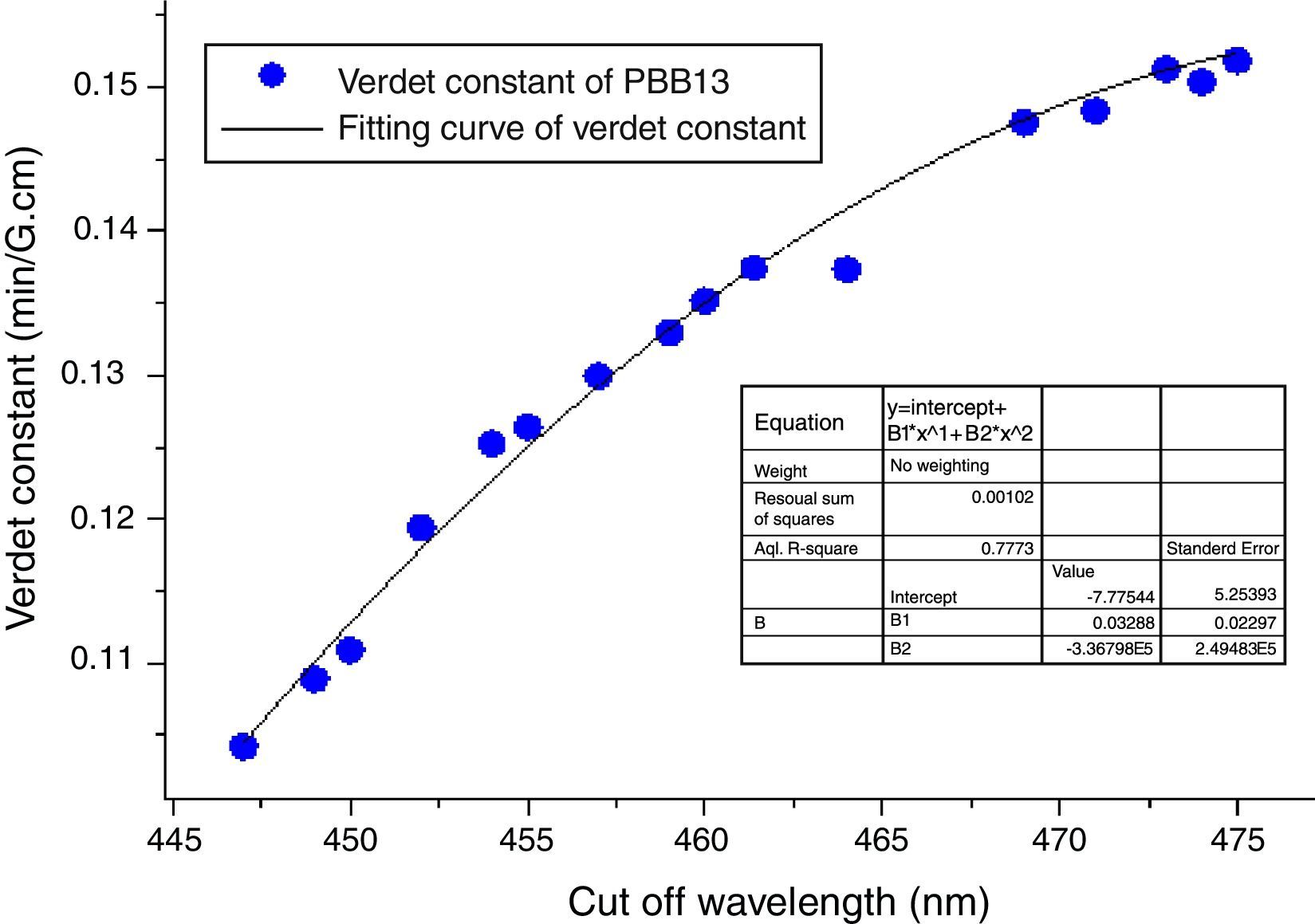
Also from Eq. (3), the Verdet constant of a diamagnetic material has little temperature-dependence which makes PBB glass great advantageous over currently used crystal and paramagnetic counterparts. As addressed previously, the incorporation of HMO weaken the bridged OB bonds and increase the non-oxygen bridge and induced an increase of structural disorder. So as a consequence the energy required for breaking down the glass network decreased (Eg) which leads to shift of cutoff to longer wavelength. Fig. 16 plots the Verdet constant of glasses with different cutoff wavelengths.
A fitting curve was normalized with the function of V=−7.77544+0.03289λ−3.38796E−5λ2. It can be seen that the higher cutoff, the higher Verdet constant of glasses. The factors for example Eg, HMO content, cation polarizability, dispersion, electronic mass and radii etc., influenced both cutoff and Verdet constant of glass. From this study, it can be concluded that the increase of HMO content and the decrease of energy gap can improve the Verdet constant and cutoff red-shift of glass. This conclusion agreed well with Ruan et al. [32] for chalcogenide glass.
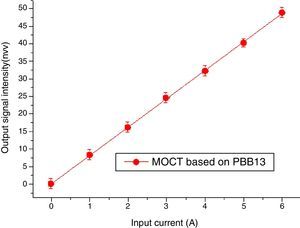
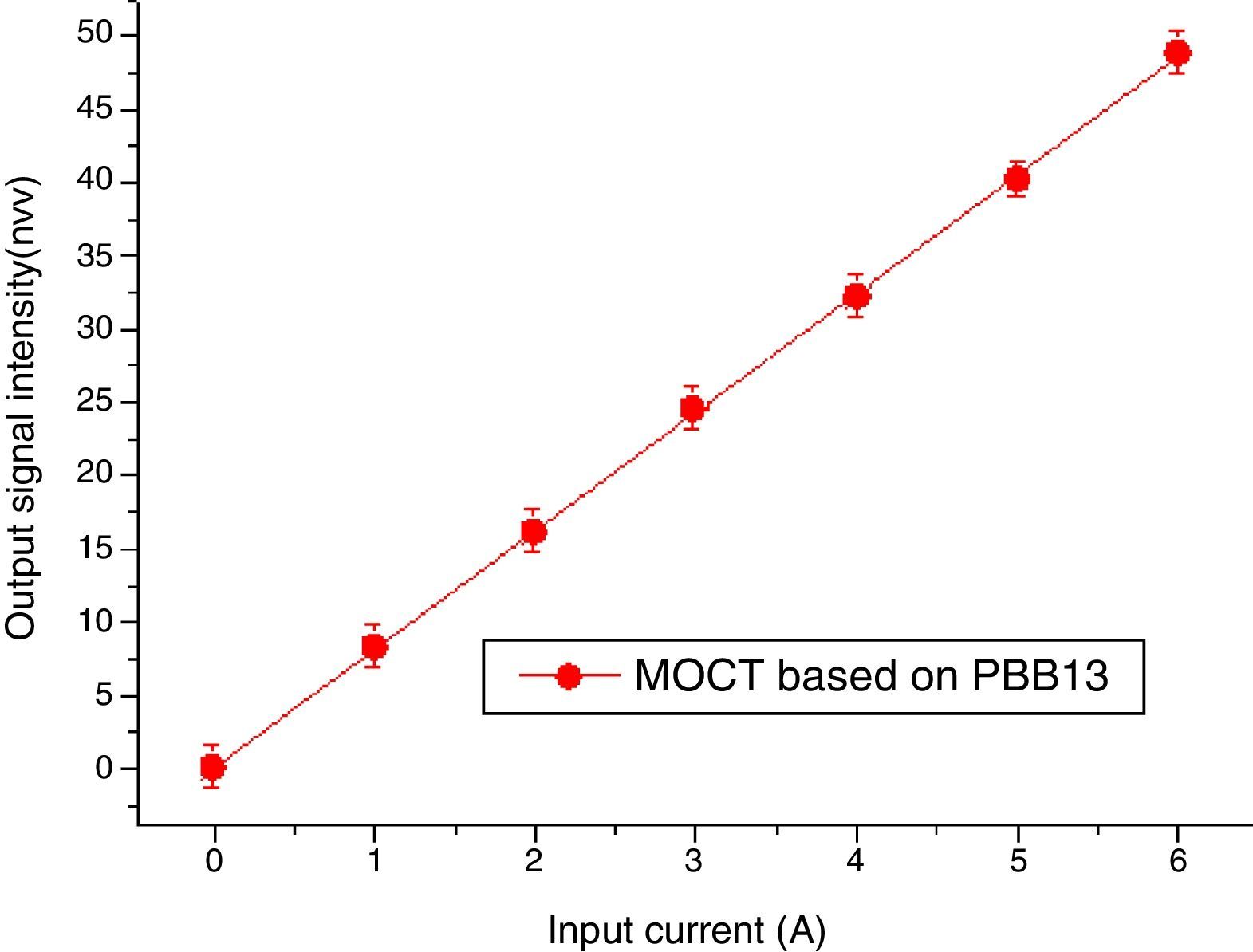
Magneto-optical current sensorThe sensitivity of MOCT prototype based on glass with good thermal stability, high Verdet constant and high figure of merit was computed to be 8.31nW/A, detailed descriptions about the calculation of sensitive can be found in [29].
Data of MOCT sensitivity has been plotted in Fig. 17. The X axis represents current flowing through the conductor ring, the Y axis represents signal intensity collected by the photodetector. Each measurement was repeated for 6 times, their mean value and their least mean squared curve-fit was computed. It is clear from the mean value that the response of the current sensor exhibits a high degree of current linearity. The fitting equation is: I=0.06429+8.902A, the best fit is represented by a red line. The vertical error bars denote the uncertainties associated to each current measurement. From Fig. 17, one can confirm that good metrological properties of the constructed MOCTs were obtained.
Table 3 reports the sensitivity comparison of prototype in this study and optical current transducers (OCTs) from literature. Among influence factors, the material's Verdet constant (Faraday rotation effect) is the most important. From Table 3, it can be seen that even though the MOCT sensitivity based on PBB13 is smaller than flint SF2, it is still higher than PBBGe, TZN and others two glasses [49,50] based MOCT. The results proved ternary PBB glass has good potential for MOCT applications. Note that the sensitivity measurement is related laser power, sensing materials type, sample length and optical setup configuration etc. Which cannot be detailed from literatures, so Table 3 gives readers not a strict technical comparison on MOCT sensitivity.
ConclusionTernary PBB glasses with 80–90% HMO were prepared and characterized in terms of their glass forming ability, thermal stability, UV-vis spectra, FT-IR spectra and magneto-optical effects. Through the investigation on substitution of PbO with Bi2O3, it is found that PbO-rich glasses had a bigger glass forming range and better thermal stability than Bi2O3-rich glasses, Bi2O3 has substantial influence on glass devitrification. However due to its higher cation polarizability, Bi2O3 contributed much more to high refractive index, high Verdet constant and cutoff red-shift than PbO. The role of B2O3 depends on concentration. Among fabricated glasses, PBB13 exhibited the best thermal, magneto-optical performance and was applied on MOCT. High MOCT sensitivity proved the ternary PBB glasses are ideal candidate for magneto-optical devices.
Thanks to the National Natural Science Foundation of China NSFC (No. 61428502 <tel:61428502> ) </tel:61428502>
“studies on hybrid magneto-optic waveguide constructed with Fe:TiO2-SiO2 film on glass waveguide chips”.





![Evolution of [BO3] to [BO4] in PBB glass. Evolution of [BO3] to [BO4] in PBB glass.](https://static.elsevier.es/multimedia/03663175/0000005600000001/v1_201702110054/S0366317516300693/v1_201702110054/en/main.assets/thumbnail/gr5.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)