This study synthesized and characterized amorphous mesoporous silica from palm kernel shell ash. Sol–gel synthesis technique was used to produce the amorphous mesoporous silica from palm kernel shell ash. Silica is an affordable material with numerous applications including glass production, electronics and polymer materials, drug delivery and energy storage. It has been manufactured from different agricultural bio-resources with limited research on palm kernel shell ash (PKSA). The synthesized amorphous mesoporous silica was characterized using X-ray fluorescence (XRF), X-ray diffraction (XRD), Fourier transform infrared (FTIR) techniques, scanning electron microscope (SEM) and energy dispersive X-ray (EDX). The XRD patterns reveal the amorphous nature of the extracted amorphous silica, EDX confirmed the presence of SiO2, while FTIR analysis shows the existence of silanol and siloxane groups. Thermal gravimetric analysis (TGA) indicate presence of physically adsorbed and chemically bound water. Silica yield after synthesis was 96.83%. This success means decreases in environmental contamination caused by indiscriminate disposal of palm kernel shell (PKS) and amorphous silica for advanced material applications including glass and ceramics, drug delivery.
Este estudio sintetizó y caracterizó la sílice mesoporosa amorfa a partir de la cáscara del núcleo de palma. Se usó la técnica de síntesis de sol-gel para producir la sílice mesoporosa amorfa de la cáscara del núcleo de palma. La sílice es un material asequible con numerosas aplicaciones que incluyen la producción de vidrio, electrónica y materiales de polímeros, la administración de medicamentos y el almacenamiento de energía. Se ha fabricado a partir de diferentes recursos biológicos agrícolas, con una investigación limitada sobre la cáscara del núcleo de palma (PKSA). La sílice mesoporosa amorfa sintetizada se caracterizó utilizando técnicas de fluorescencia de rayos X (XRF), difracción de rayos X (XRD), técnica de infrarrojos por transformada de Fourier (FTIR), microscopio electrónico de barrido (SEM) y rayos X de energía dispersiva (EDX). Los patrones de XRD revelan la naturaleza amorfa de la sílice mesoporosa amorfa extraída, EDX confirmó la presencia de SiO2, mientras que el análisis FTIR muestra la existencia de grupos de silanol y siloxano. El análisis gravimétrico térmico (TGA) indica la presencia de agua adsorbida físicamente y unida químicamente. El rendimiento de sílice después de la síntesis fue del 96.83%. Este éxito significa una disminución de la contaminación ambiental causada por la eliminación indiscriminada de la envoltura del núcleo de palma (PKS) y la sílice mesoporosa amorfa para aplicaciones avanzadas de materiales que incluyen vidrio y cerámica, suministro de medicamentos.
The need to manage waste and reduce environmental pollution has led to wastes being employed as substitute to traditional materials [1]. Palm kernel is useful plant but some parts constitute agricultural wastes. Palm kernel commonly known as Elaeis guineensis from the palm oil trees are found in enormous quantity in Asia and Africa, especially in Nigeria [2]. They are the stiff endocarp of palm fruit that borders the seed and is alternatively recognized as oil palm shell [3]. Pam kernel shell (PKS) is gotten as a remaining superfluous in the removal of the seed in the nut once palm oil has been extracted from the mesocarp of palm oil pod [4]. Many researchers and scientists have used PKS to substitute conventional normal weight aggregates (NWA) as lightweight aggregates (LWA) in motorway construction, essential elements and other applications ranging from biomass, energy storage, bio-fertilizer, to super-capacitor electrode [5–11].
Silica gels are a stiff three-dimensional linkage of colloidal silica. Amorphous mesoporous silica gels are categorized based on the synthesis process as an aqua gel, xerogel and aerogel. Amorphous mesoporous silica [12] has been effectively extracted from several agrarian bio-resources such as sugar-cane [13–16], rice husk ash [17–21], corn cob ash [22–26], bagasse ash [27], clay [28], coffee husk ash and wheat husk ash [29–31]. Techniques such as sol–gel, gasification and acid leaching [26–33] have been investigated to produce pure amorphous silica.
This study produced amorphous mesoporous silica from agricultural bio-resources of PKSA via the sol–gel method. It has a combined advantage of reducing disposal as well as pollution problems and making valued amorphous silica at lesser cost. The sol–gel method in comparison with other extraction methods has higher silica yield, low energy consumption and densification at lower temperature [17–33]. PKSA as a source of silica has higher net calorific value (LHV), indicating large energy value and higher ash content compared to other biomass like coconut shell, wheat husk and rice husk [31–33]. The prepared amorphous mesoporous silica from palm kernel shell ash (PKSA) have been characterized by means of XRD, XRF, SEM/EDX, FTIR and TGA techniques.
Materials and methodsSilica synthesisFig. 1 shows the PKS collected from South West Nigeria, washed and dried in open air. Combustion of PKS was done for 3h at 750°C with a heating speed of 10°C/min and allows cooling in the muffle furnace. Amorphous silica was extracted from the PKSA, shown in Fig. 2, using the sol–gel technique as hitherto reported by Okoronkwo et al. [25]. About 200ml ratio of 2N NaOH was added to 50g PKSA samples and heated for 2h using a hot plate with continuous stirring to dissolve the silica present in the ash and produce a silicate solution. Ashless filter paper was used to filter the solutions and 100ml boiled distilled water was used to wash the residue. After cooling, the filtrate was titrated with 2N HCl to pH in the range of 7.5–8.5 with continual stirring and nurtured for 24h to allow gel development. The gel was softly broken after ageing and centrifuged for 4min at 4000rpm. While the supernatant was castoff, the aqua gels were placed inside an oven to dry at 80°C for 24h to yield, silica xerogels.
Moisture content of silica gelsThe silica gels moisture content was determined using an air oven technique [34]. 1g of extracted silica was heated in aluminium humidity pans at 110°C for 1h. The samples were thereafter cool in a desiccator and weighed. Percentage (%) weight loss was recorded as the moisture content of extracted silica.
CharacterizationXRD of PKSA and extracted silica was scanned using GBC EMMA X-Ray diffractometer having Cu Kα emission at 25kV acceleration voltage and 400μA current from 2θ 15° to 55° at a speed of 4.00°/min. The quantitative investigation of chemical constituents of PKSA and silica extract was carried out by means of X-ray fluorescence (XRF) Phillip Magix Pro XRF instruments. Morphology was observed with a SEM (Zeiss Ultra Plus) and EDX at secondary electron image (SEI) and high vacuum (HV) mode with 20kV accelerating voltage. FTIR spectra were recorded using Tianjin GangDong FTIR 650 in the range of 4000–450cm−1. A small quantity of sample (1wt.%) was scrupulously mixed with ground KBr in an agate mortar and a disc was prepared in vacuum maintaining a pressure of 33kg/cm2. The spectrogram of the sample is observed on computer monitor and a graphic representation of the spectra was taken. Thermal gravimetric analysis (TGA) was evaluated using Perkin-Elmer Pyris 6 TGA analyzer. Approximately 10mg weight was heated from 30 to 1000°C with a heating rate of 5°C/min in nitrogen atmosphere.
Results and discussionsThe extracted silica content of washed xerogel was 54.35% and percentage moisture was 2.68%.
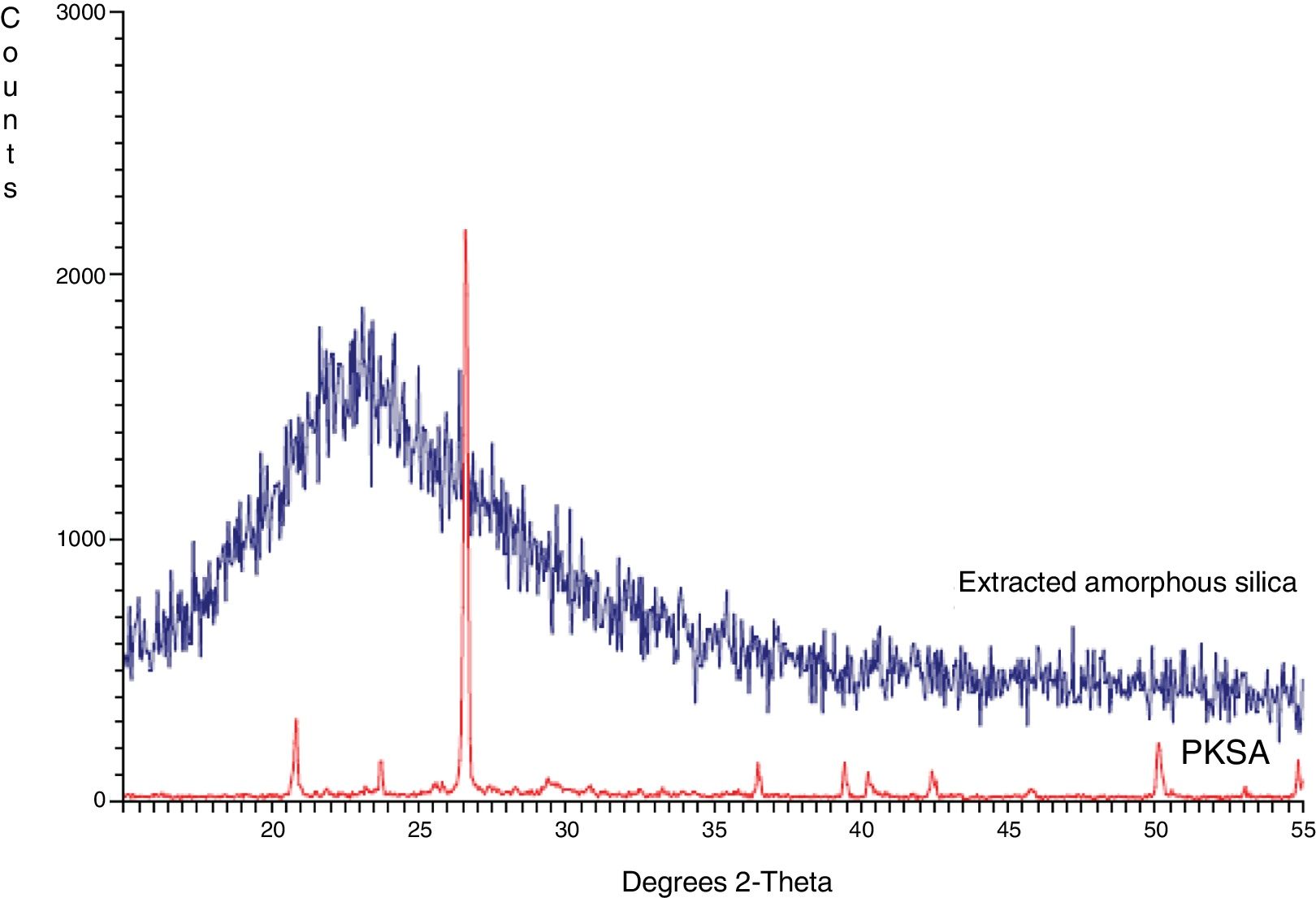
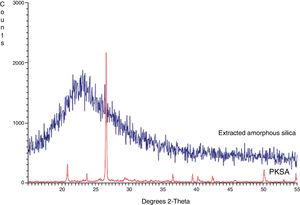
Structural propertiesThe XRD pattern of PKSA and extracted amorphous silica are shown in Fig. 3. The XRD pattern for the ash shows the present quartz at theta=24, and 43° (SiO2 PDF Card #331161). The broad XRD array of extracted silica at theta=22.5° shown in Fig. 4. The observed pattern is distinctive of amorphous solid, thereby confirming the formation of amorphous silica phase identification of this peak gave hexagonal symmetry of quartz silica [JCPDS value (47–1144)]. This is in concordance with similar XRD patterns of amorphous silica obtained from corn [22–26].
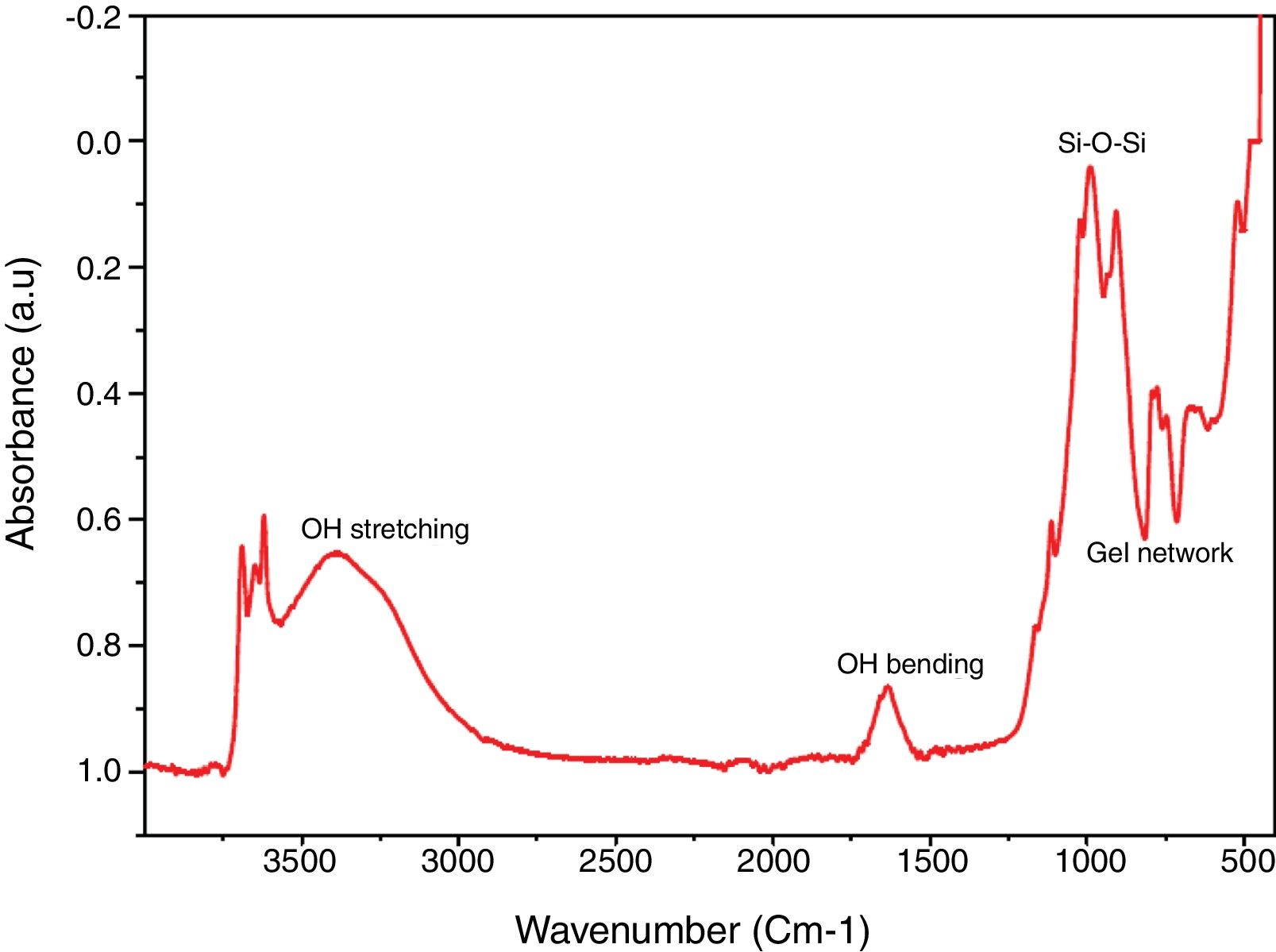
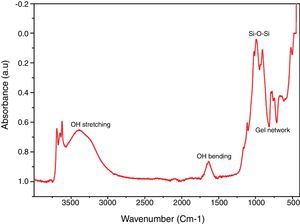
Chemical propertiesFTIR spectral identified the key chemical compound existing in the silica as revealed in Fig. 4. Band 770–795cm−1 (Al–Mg–OH) (free silica and or quartz admixtures) and 740–755cm−1 (Si–O–Al) was assigned to symmetric stretching vibration network of gel network [35–37]. Band 1050–1170cm−1 was owed to symmetric stretching vibration network of Si–O–Si [38] and broad band at 1633–1645cm−1 and 3390–3445cm−1 is due to O–H bending and stretching vibration from Si–OH silanol groups and are owing to adsorbed H2O molecules on the samples surface [39,17].
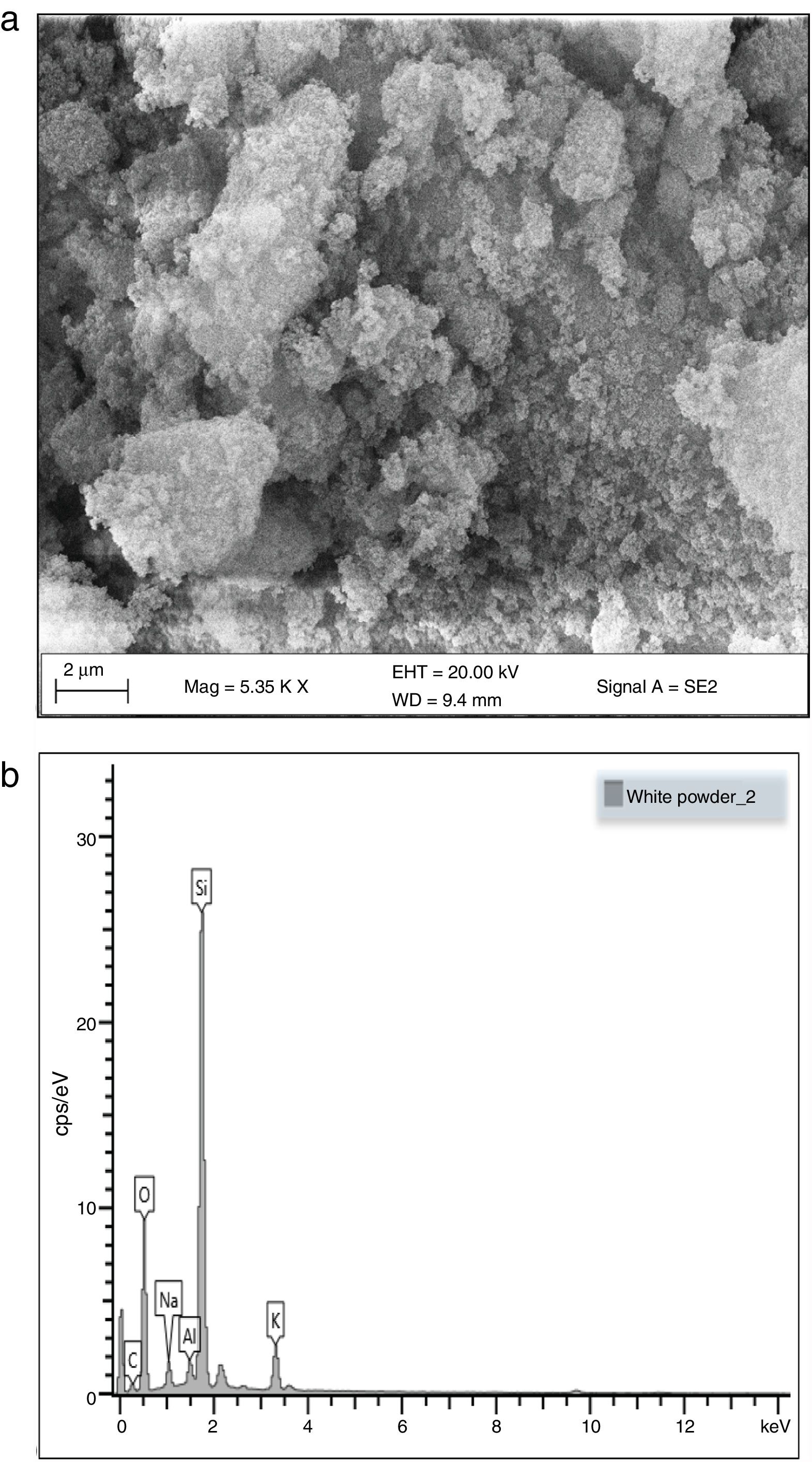
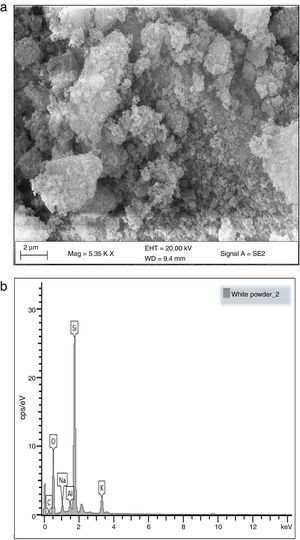
Morphological and elemental analysisFig. 5a and b shows the SEM micrographs of the silica extracted from PKSA respectively. Fig. 4a shows the surface texture and morphology of the amorphous silica extracted from the PKSA with silica–silica agglomeration. The micrograph is observed to have agglomeration of silica with irregular particle shapes, which varies in sizes and are widely distributed. EDX spectral shows strong intensity of Si and O, which confirms silica (SiO2) as the predominant element in the sample. The presence of other elements such as Na, K is a result of the substrate used for the SEM/EDX analysis.
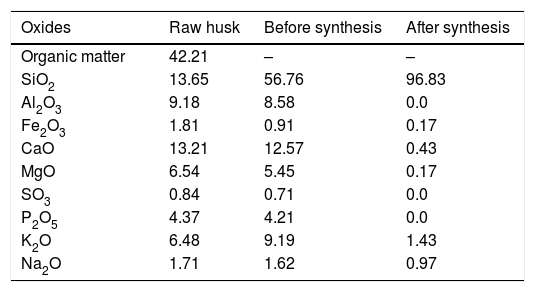
Table 1 shows the elemental composition of PKSA and extracted silica in percentage. The highest quantity of elements present in the ash was silicon dioxide (SiO2, 56.76%) which is similar to other customary siliceous pozzolans (e.g. corn cob ash or rice husk ash). The occurrence of calcium oxide is noticeable (CaO, 12.57%). Potassium (expressed as K2O, 9.19%) which is an essential plant nutrient is the third element by percentage and is characteristic of several biomass ashes [40]. Also present are other important compounds, such as Al2O3, Fe2O3, MgO, SO3, and P2O5. High value of these compounds might be due to the soil content and type of fertilizer used, which differs from location to location. Silica yield after synthesis was 96.83% with minor mineral content as impurities. These impurities; might be owed to entrapment of metal ions in the samples, which might not have been filtered out by washing [25].
The chemical composition of raw husk, PKSA and extracted silica in percentage.
| Oxides | Raw husk | Before synthesis | After synthesis |
|---|---|---|---|
| Organic matter | 42.21 | – | – |
| SiO2 | 13.65 | 56.76 | 96.83 |
| Al2O3 | 9.18 | 8.58 | 0.0 |
| Fe2O3 | 1.81 | 0.91 | 0.17 |
| CaO | 13.21 | 12.57 | 0.43 |
| MgO | 6.54 | 5.45 | 0.17 |
| SO3 | 0.84 | 0.71 | 0.0 |
| P2O5 | 4.37 | 4.21 | 0.0 |
| K2O | 6.48 | 9.19 | 1.43 |
| Na2O | 1.71 | 1.62 | 0.97 |
Fig. 6 shows the pictorial representation of the extracted amorphous mesoporous silica from palm kernel shell ash using the sol–gel technique.
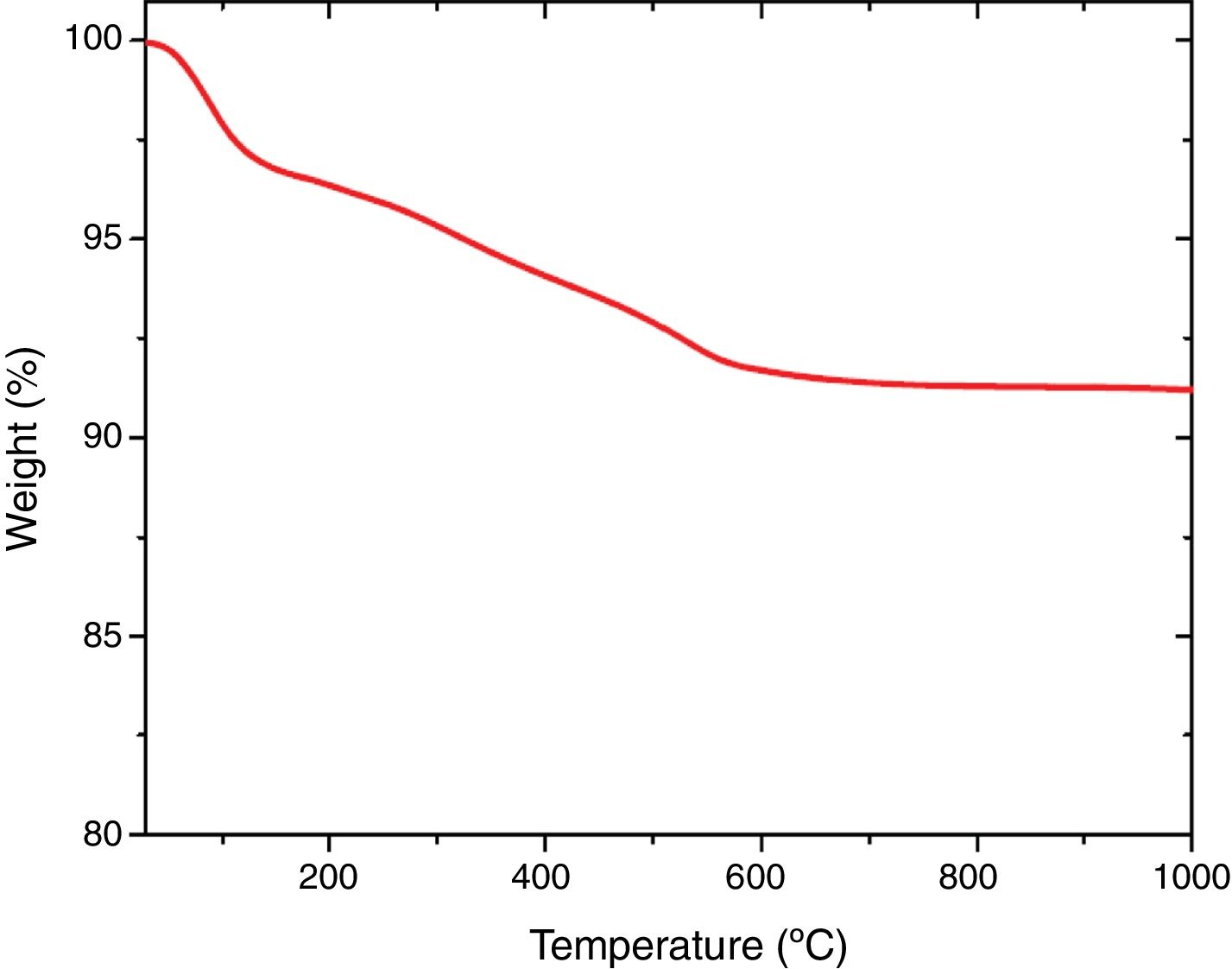
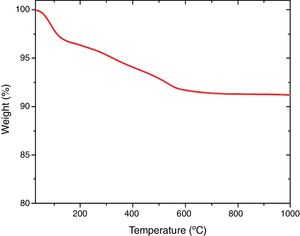
Thermal analysisThe results of thermal gravimetric analysis (TGA) are shown by Fig. 6. Two step weight losses were observed. The physically adsorbed water was attributed to the weight loss up to 150°C (TGA step 1), while chemically bound water from the sol–gel production method was attributed to the weight loss from 150 to 600°C [41,42]. Above 600°C, no further weight loss was observed indicating thermal stability of extracted mesoporous silica (Fig. 7).
ConclusionThis work has shown that 56.65% amorphous silica yield, 2.68% moisture and negligible inorganic impurities can be produced from palm kernel shell ash using the sol–gel technique. XRD investigation has revealed the presence of silica in the ash and amorphous nature of extracted silica. SiO2 presence in the samples was established by EDX result. Agglomeration of silica–silica of varied sizes and shapes was observed from the SEM analysis. Key chemical groups existing in the samples were indicated by FTIR data. FTIR analysis shows the existence of silanol and siloxane groups. An after synthesis yield of 96.83% silica was obtained. Thermal gravimetric analysis (TGA) indicate thermal stable mesoporous silica with presence of physically adsorbed and chemically bound water. This result will help in combating the environmental nuisance of palm kernel shell by converting it into useful product. It will also be a cheap source of silica as an industrial raw material for drug delivery, glass production.
Conflict of interestNone declared.
Authors would like to acknowledge the financial support from NRF of South Africa and the PDRF support from the University of Johannesburg.