This research focuses on the synthesis and characterization of six ceramic perovskite oxides based on La0.8Sr0.2Ni(1−x)CrxO3 system, with different levels of chromium modification (x=0.0, 0.2, 0.4, 0.6, 0.8 and 1.0), for use as anode material in solid oxide fuel cells (SOFC). The oxides were obtained in two reaction stages, starting from corresponding nitrate salts and citric acid until formation of a solid metal complex consistent with citrate species. The solid metalorganic foams were calcined at 1000°C for 120min under oxygen flow conditions and were characterized by infrared and ultraviolet spectroscopy (FTIR-UV), validating the proposed methodology in terms of purity and chemical composition. The characterization by X-ray diffraction (XRD), scanning electron microscopy (SEM) and X-ray energy dispersive spectroscopy (EDX) confirms the obtention of a homogeneous perovskite structure in all analyzed phases. The evaluation of crystallite size by means of the Debye–Scherrer equation establishes a nanometric prevalence around 3.2–4.4nm along main diffraction signals. The electrical characterization of materials by solid-state impedance spectroscopy allowed identifying a particular behavior depending on the microstructure of solids for potential applications in SOFC devices.
Esta investigación se centra en la síntesis y caracterización de seis óxidos de perovskita cerámicos basados en el sistema La0.8Sr0.2Ni(1-x)CrxO3, con diferentes niveles de modificación de cromo (x=0.0, 0.2, 0.4, 0.6, 0.8 y 1.0), para uso como material de ánodo en pilas de combustible de óxido sólido (SOFC). Los óxidos se obtuvieron en dos etapas de reacción, partiendo de las correspondientes sales de nitrato y ácido cítrico hasta la formación de un complejo metálico sólido consistente con especies de citrato. Las espumas sólidas metal-orgánicas se calcinaron a 1000° C durante 120 minutos bajo condiciones de flujo de oxígeno y se caracterizaron por espectroscopia infrarroja y ultravioleta (FTIR-UV), validando la metodología propuesta en términos de pureza y composición química. La caracterizaron por difracción de rayos X (XRD), microscopía electrónica de barrido (SEM) y espectroscopia de dispersión de energía de rayos X (EDX), confirman la obtención de una estructura perovskita homogénea en todas las fases analizadas. La evaluación del tamaño del cristalito por medio de la ecuación de Debye-Scherrer establece una prevalencia nanométrica alrededor de 3,2 – 4,4nm a lo largo de las principales señales de difracción. La caracterización eléctrica por espectroscopía de impedancia de estado sólido, permitió identificar un comportamiento particular dependiente de la microestructura de los sólidos para potenciales aplicaciones en dispositivos SOFC.
A solid oxide fuel cell (SOFC) is an electrochemical device that converts the chemical energy of a fuel into electricity and heat, under operation conditions around 800–1000°C. These systems are a suitable option for the reduction of oil consumption, allowing the chemical transformation of hydrogen and oxygen into electrical energy, without limitations related with typical thermodynamic cycles (Carnot cycle), achieving high efficiency levels [1].
Within the main SOFC components, the anode is one of the most relevant electrodes, since it allows the main reaction in which the hydrogen oxidation occurs; additionally, it must conduct electrons and ions in a definite asset. The most common material used is metallic ceramic of yttrium-stabilized zirconia, which employs powdered nickel as a contact agent (Ni–YSZ) [2–4]. This material presents a high catalytic activity and conductivity; however, it has low redox stability, low H2S tolerance of the fuel feed [5] and allows the formation of carbon deposits that block the access to the three-phase boundary, reducing the efficiency of the fuel cells [6–9]. So, the current research in the field of synthesis of advanced anodic materials focuses on the synthesis of new materials based on perovskite structure (ABO3), which allow the combination of a high number of transition metals and rare earths as an important source for development of catalytic and conductive properties, mostly concentrated in the lanthanum strontium chromites La1−xSrxCrO3[10–12], which have shown excellent properties in combustion and reforming reactions of light hydrocarbons, such as methane and propane in response to current drawbacks of Ni–YSZ anodes [13,14]. Although typical solid-state reaction processes have been used as a mechanism to obtain this type of materials for anodic applications, the implementation of new or improved synthesis routes has allowed the development of fast methods characterized by optimal reaction rates and low cost as in the case of combustion technique, in which, a self-sustaining exothermic redox reaction from a gel is thermally treated until formation of a solid foam (citric complexes) that reacts with the oxidant source represented by the nitrates, to form the corresponding metal oxides with nanometric sizes. The success of this process focuses on homogeneity and purity of obtained solids due to appropriate mixture of the components in an aqueous medium, followed by an exothermic redox reaction between the fuel and an oxidizer, enabling a rapid reaction that dissipates the heat of combustion and limit the crystal growth process, reducing the possibility of premature local partial sintering among the primary particles [15].
Under the above considerations, this work analyzes the effect of the optimization of a method of chemical synthesis that involves the combustion of citrate species to obtain homogeneous and pure oxide phases based on La0.8Sr0.2Ni(1−x)CrxO3 systems [16–19], with different levels of chromium modification (x=0.0, 0.2, 0.4, 0.6, 0.8, 1.0), to allow the obtention of ceramic materials in the nanometric scale, with structural, morphological and electrical properties of significance for electrocatalytic purposes in the design of SOFC anodes [20–27].
Experimental procedureFor the synthesis of these materials, were used the corresponding nitrates of La(NO3)3·6H2O 99.9%, Sr(NO3)3 99%, Ni(NO3)3 99%, Cr(NO3)3·9H2O 99% and citric acid (C6H8O7·H2O 99.99%), all from Merck, in concentrations of 1.0 mol L−1. The molar relation between the metal nitrates and citrate in each composition was 1.0:1.0 in all cases. The solutions were dosed in stoichiometric quantities and kept under magnetic stirring and reflux at 80°C for 1h until the formation of corresponding hydrogels. The temperature was increased to 250°C until the beginning of a combustion process that promotes the obtention of solid metalorganic precursors, which were moltured and calcined at 1000°C for 120min under oxygen flow conditions to remove the carbonaceous residues from the previous combustion step, allowing consolidation of the crystalline phases. The characterization of obtained solids was performed using infrared and ultraviolet spectroscopy (FTIR-UV) on a Perkin-Elmer FTIR-1000 and a HP 8453 UV-Vis equipment, in order to evaluate the main vibrational modes and specific electronic transitions. The results were analyzed and compared with spectral databases to verify the effectiveness of the proposed synthesis method.
The structural characterization was made by X-ray diffraction (XRD) on a X’pert PRO PANalytical-MPD diffractometer, equipped with an Ultra-fast X’Celerator detector in Bragg–Brentano configuration, using the Cu Kα radiation (λ=1.54186Å) between 20° and 80° 2θ. The obtained data were evaluated using the X’Pert High-Score software and refined by GSAS and PCW software, allowing to validate the formation of crystalline phases and to estimate the crystal size using the Debye–Scherrer equation.
The morphological and textural properties of solids were evaluated by scanning electron microscopy (SEM) on a FEI Quanta 200-r equipment, using uncoated specimens at different magnifications, allowing to establish some surface characteristics derived from synthesis method. The composition of the material was corroborated through analysis by microprobe of energy dispersive X-ray (EDX) in a spectrometer S4 Pioneer Bruker sequential X-ray equipment by scattering of wavelengths. Finally, the electrochemical characterization was performed at room temperature to get an approximation of electrical behavior of solids in an AUTOLAB potentiostat–galvanostat using a modified cell. The tests were performed in pellets of 8mm radius and 2mm thickness, between 100.0mHz and 100.0kHz, all of them compacted at 1.0MPa and sintered at 1100°C for 60min. The geometry of the pellets was corrected with the use of a reference cell and adapted for this purpose to prevent interference in the electrical responses.
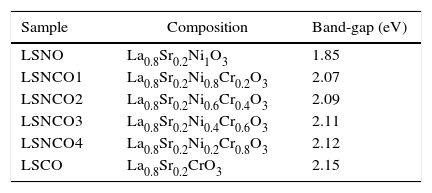
Results and discussionUltraviolet spectroscopy (UV)The samples were characterized and identified as shown in Table 1.
Samples with corresponding compositions in La0.8Sr0.2Ni(1−x)CrxO3 system and calculated band-gap values obtained by UV analysis.
| Sample | Composition | Band-gap (eV) |
|---|---|---|
| LSNO | La0.8Sr0.2Ni1O3 | 1.85 |
| LSNCO1 | La0.8Sr0.2Ni0.8Cr0.2O3 | 2.07 |
| LSNCO2 | La0.8Sr0.2Ni0.6Cr0.4O3 | 2.09 |
| LSNCO3 | La0.8Sr0.2Ni0.4Cr0.6O3 | 2.11 |
| LSNCO4 | La0.8Sr0.2Ni0.2Cr0.8O3 | 2.12 |
| LSCO | La0.8Sr0.2CrO3 | 2.15 |
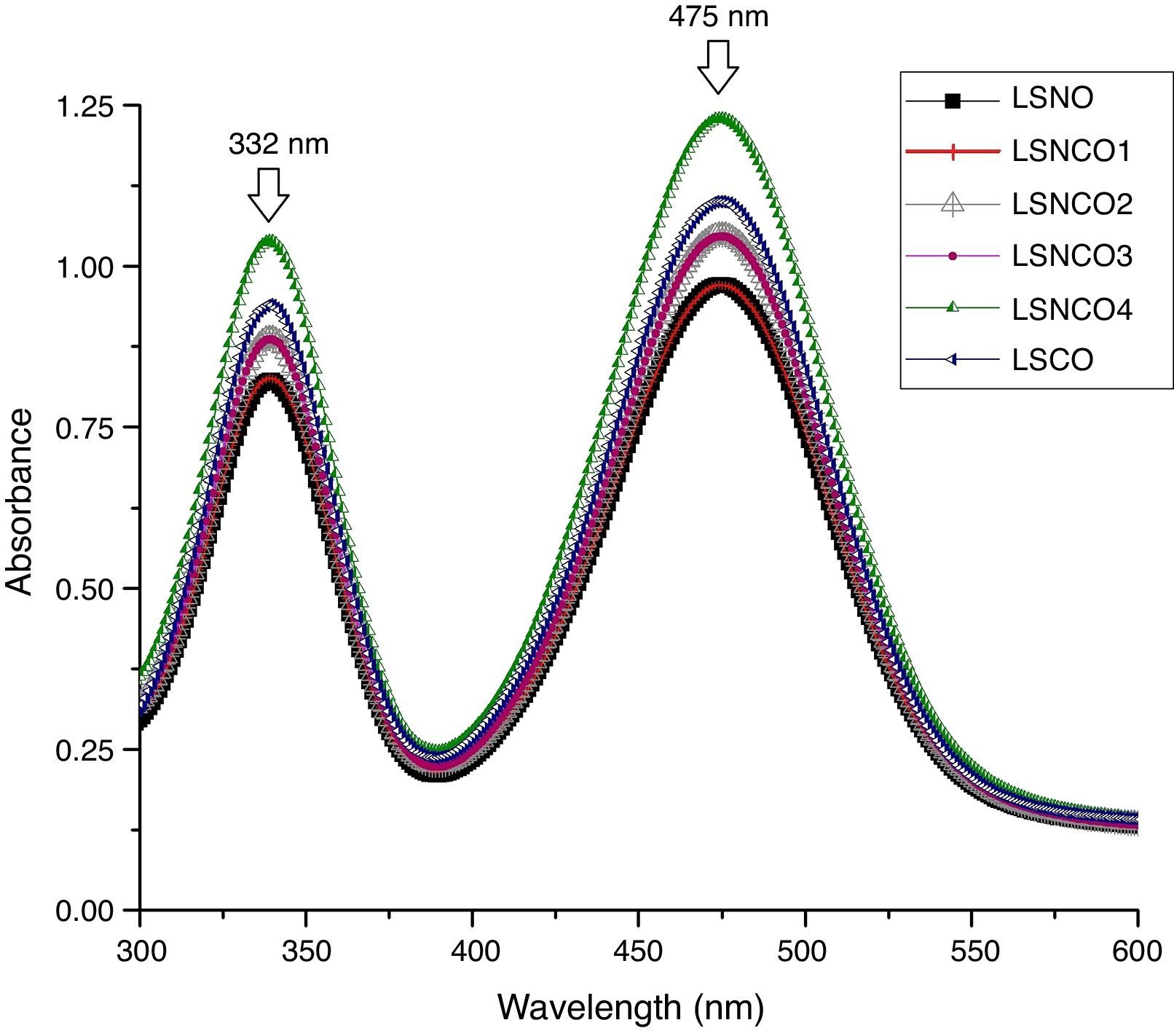
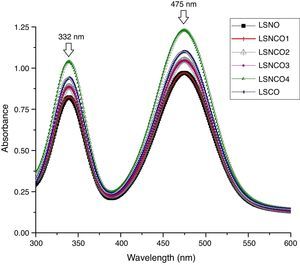
Analysis by UV spectroscopy confirmed the obtention of metal oxide species, associated with electronic transitions located at 332 and 475nm, where the small variation in absorbance is associated with the level of cation modification. In Fig. 1, the highest absorbances are associated with the presence of chromium species, which indicates the formation of corresponding Cr2O3 oxide attributed to its more stable state of oxidation. The binding energy associated with each wavelength, using the Planck equation, display transition energies around 1.85 and 2.15eV for corresponding wavelengths, in which it is possible to identify similar energy values based on electronic transitions related with typical perovskite oxides [28]. A detailed analysis between 200 and 700nm confirms the presence of electronic transitions π→π* of moderate energy corresponding to the region of the far and near UV, while transitions n→π* significantly diminished, belonging to the visible region of the spectrum. Such transitions are characteristic for elements of transition series with d configuration [29–31].
The band-gap values obtained for each system are shown in Table 1, where it is clear that a semiconductor behavior marked for the sample with the highest concentration of nickel cation, with a value of 1.85eV in comparison with the LSCO system, demonstrating that obtained band-gap values are directly proportional to the concentration of chromium cation. These values obtained are in agreement with the studies of Strehlow et al., in which these values are characteristic for semiconducting materials related with perovskite structures [32].
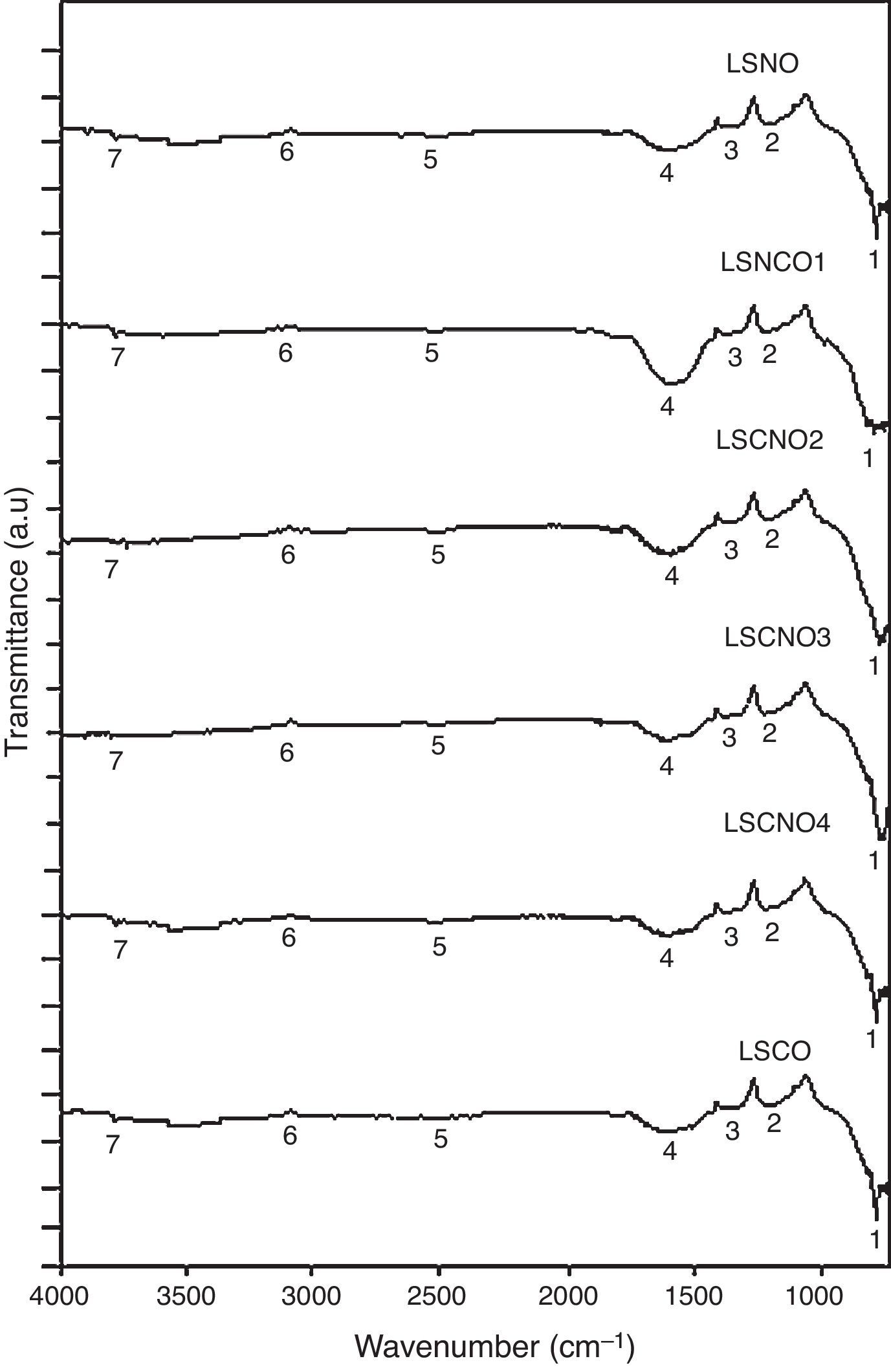
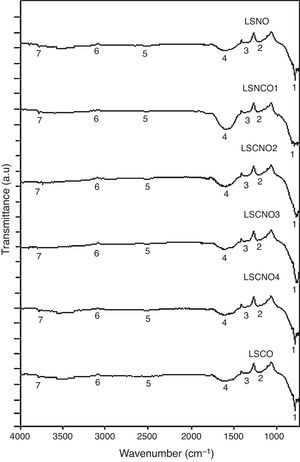
Infrared spectroscopy (FTIR)The infrared spectroscopy analysis (FTIR) allowed to evaluate and identify the effectiveness of synthesis process. The performed analysis using the SDBS databases and is shown in Fig. 2, with the six samples exhibiting a high degree of correspondence between characteristic vibrational bands related with metaloxygen bonds, validating the proposed synthesis method.
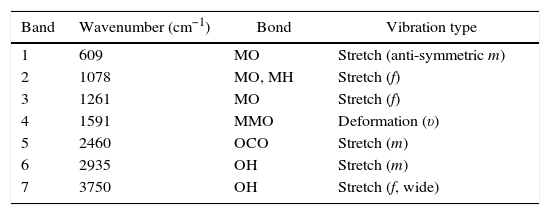
A detailed analysis of the spectra shows the presence of characteristic bands, located at 3750cm−1 and related with vibrational modes of OH link associated with hydroxyl-bonded species. The signal located at 2935cm−1 is characteristic of vibrational mode OH group linked by hydrogen bonds. The signal located at 2460cm−1 represents the vibrational modes associated with the presence of occluded CO2, by the effect of thermal treatments applied on the obtention of ceramics. The signal located at 1855cm−1 can be identified with vibrations associated with MO bond.
Moreover, the signal located around 1591cm−1 may be associated with asymmetric vibrational modes of metal oxides in configuration MOM, which can be moved toward lower wavenumbers associated with the same vibrational modes but in configuration MMO. The signals located at 1261cm−1 and 1078cm−1 correspond with the vibrational mode of CO bond, derived from environmental CO2. Finally, signals located at low wavenumber around 1000cm−1 corresponding with vibrational signals related with metal cations of La3+, Sr2+, Ni3+ and Cr3+ with hydroxyl groups, demonstrating a clear evidence of the interaction between metal cations and oxygen as shown in Table 2[30].
Transmittance signals obtained from metalorganic precursors, with corresponding bonds and type of vibrations.
| Band | Wavenumber (cm−1) | Bond | Vibration type |
|---|---|---|---|
| 1 | 609 | MO | Stretch (anti-symmetric m) |
| 2 | 1078 | MO, MH | Stretch (f) |
| 3 | 1261 | MO | Stretch (f) |
| 4 | 1591 | MMO | Deformation (υ) |
| 5 | 2460 | OCO | Stretch (m) |
| 6 | 2935 | OH | Stretch (m) |
| 7 | 3750 | OH | Stretch (f, wide) |
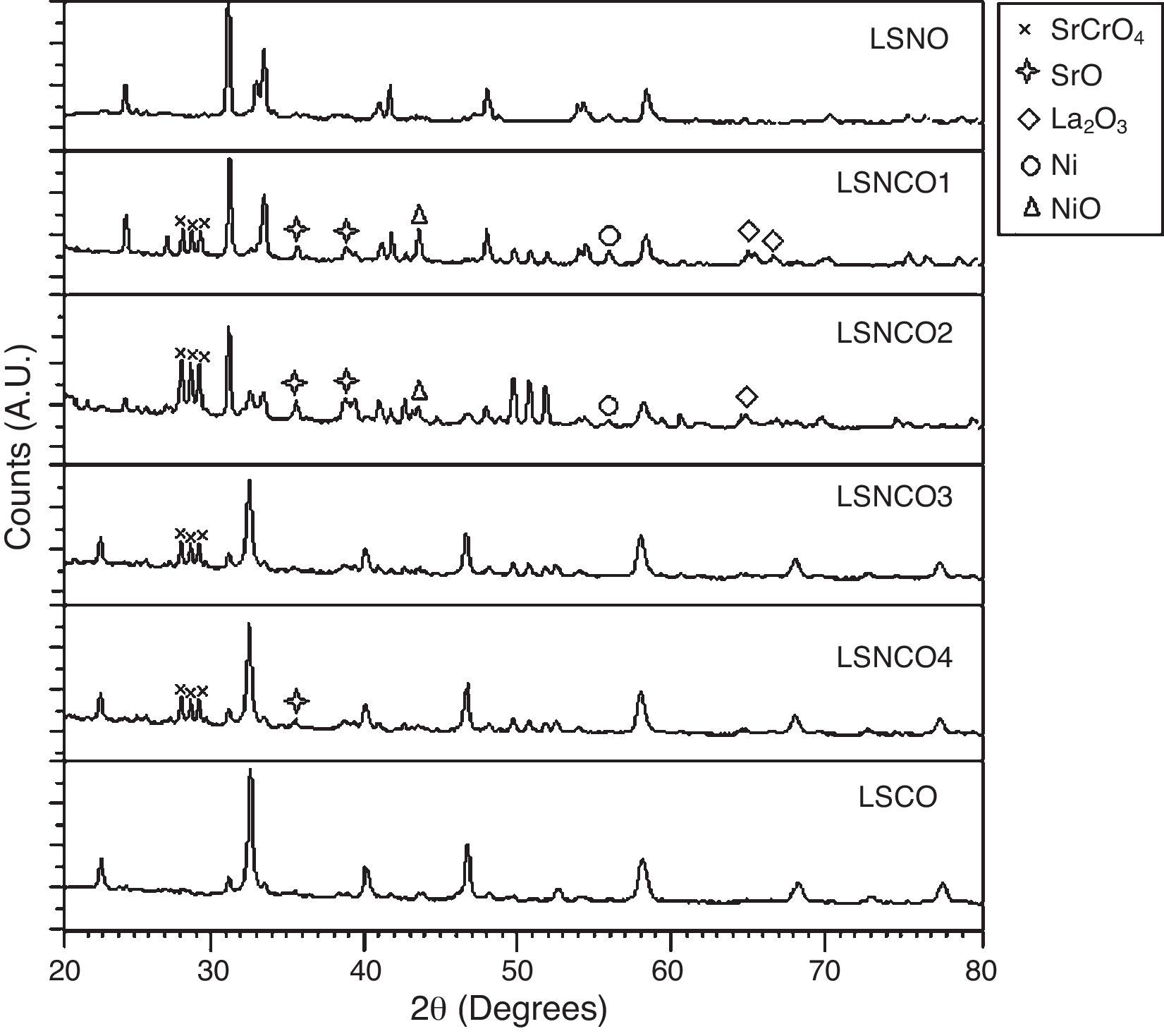
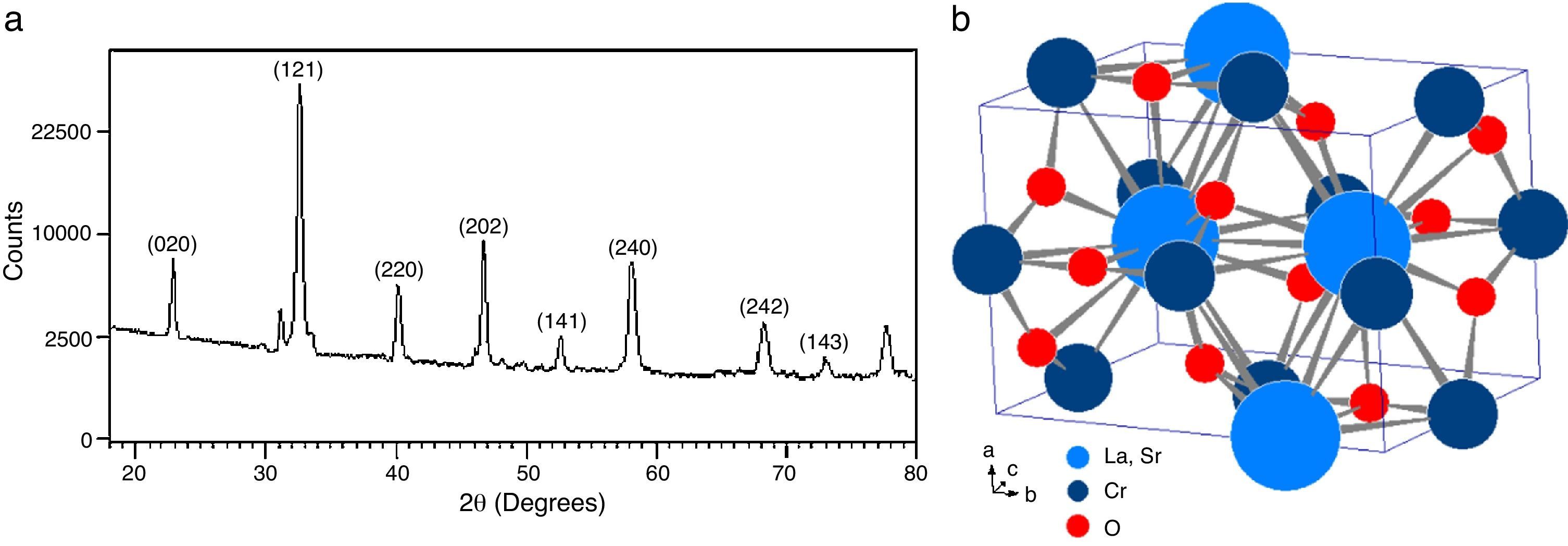
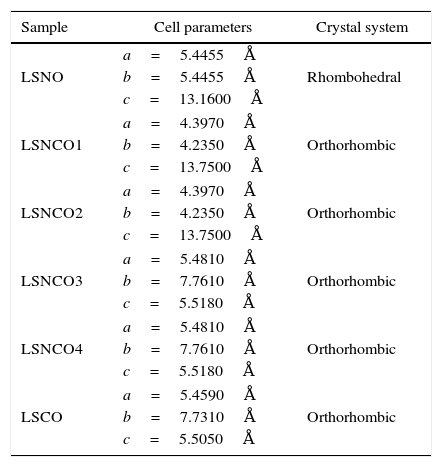
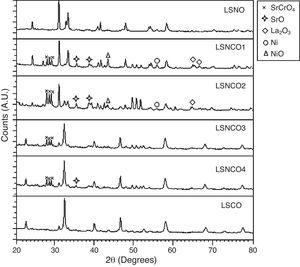
The analysis of the calcined oxides was performed using X’Pert High Score software. The results confirm that progressive insertion of chromium cation in the structure causes a change in the structural parameters of ceramic, allowing evolution from a rhombohedral arrangement related with the LSNO sample until an orthorhombic structure associated with the LSCO sample. This could explain the change in conformation of X-ray diffraction patterns, as shown in Fig. 3, and it is consistent with previous works [33,34], where it is possible to evaluate the cation chromium effect in the crystal structure. According to Oliveira et al., it is well known that substituting a trivalent metal ion at site A for a bivalent metallic cation as Sr promotes a modification on structure of perovskite, with corresponding presence of SrCrO4 secondary phases assisted by the simultaneous change in the concentration of chromium cation. However, in the case of pure compositions of La0.8Sr0.2NiO3 and La0.8Sr0.2CrO3, the effect disappears and a pure structure is possible only under null concentration of a second cation in the B position [35].
In Fig. 3, for increasing values of chromium cation, the signals show the formation of secondary phases of SrCrO4, which is related with low kinetic reaction rates and the simultaneous effect between strontium and chromium cations, which finally disappears when the pure phase of La0.8Sr0.2CrO3 is consolidated [36]. The signals of LSNCO 1–4 solids, around 57° 2θ, confirm the presence of nickel oxide and is associated with the signal present at 44° 2θ, as has been shown in previous works by Gallego et al. [37]. According to Montenegro and Rodríguez [38], the signals located at 34 and 39° 2θ in the intermediate compounds of nickel and chromium indicate the presence of strontium oxide (II) (SrO), which does not show evidence of integration to the main crystalline phase, even after the thermal treatment developed at 1000°C under oxygen flow (50mLmin−1) for 120min [39,40].
According to Sakai and Waernhus et al. [41,42], the strontium chromate is characterized by its high resistivity and low catalytic activity. Reason why, the formation of this compund is related directly with the interaction and mobility between nickel and chromium cations in the stabilization process of crystalline phases, where strontium cation shows an important role in the stabilization of the perovskite structure. Therefore, vacancies and mobility of ions during thermal treatments cause deficiencies in the A site of the structure, which provoke that B site elements to form binary oxides; this is the reason why the crystal phases of pure LSNO and LSCO solids remain stable along the synthesized series.
Table 3 shows the cell parameters and the crystal characteristics obtained for each sample, which displays the concentration of chromium cation and the systematic distortion of rhombohedral crystalline structure related with LSNO sample until stabilization of an orthorhombic arrangement of LSCO oxide, in coherence with the perovskite structure and the type and size of atomic bonding of atoms [43].
Cell parameters and crystal systems obtained for perovskite samples.
| Sample | Cell parameters | Crystal system |
|---|---|---|
| LSNO | a=5.4455Å | Rhombohedral |
| b=5.4455Å | ||
| c=13.1600Å | ||
| LSNCO1 | a=4.3970Å | Orthorhombic |
| b=4.2350Å | ||
| c=13.7500Å | ||
| LSNCO2 | a=4.3970Å | Orthorhombic |
| b=4.2350Å | ||
| c=13.7500Å | ||
| LSNCO3 | a=5.4810Å | Orthorhombic |
| b=7.7610Å | ||
| c=5.5180Å | ||
| LSNCO4 | a=5.4810Å | Orthorhombic |
| b=7.7610Å | ||
| c=5.5180Å | ||
| LSCO | a=5.4590Å | Orthorhombic |
| b=7.7310Å | ||
| c=5.5050Å | ||
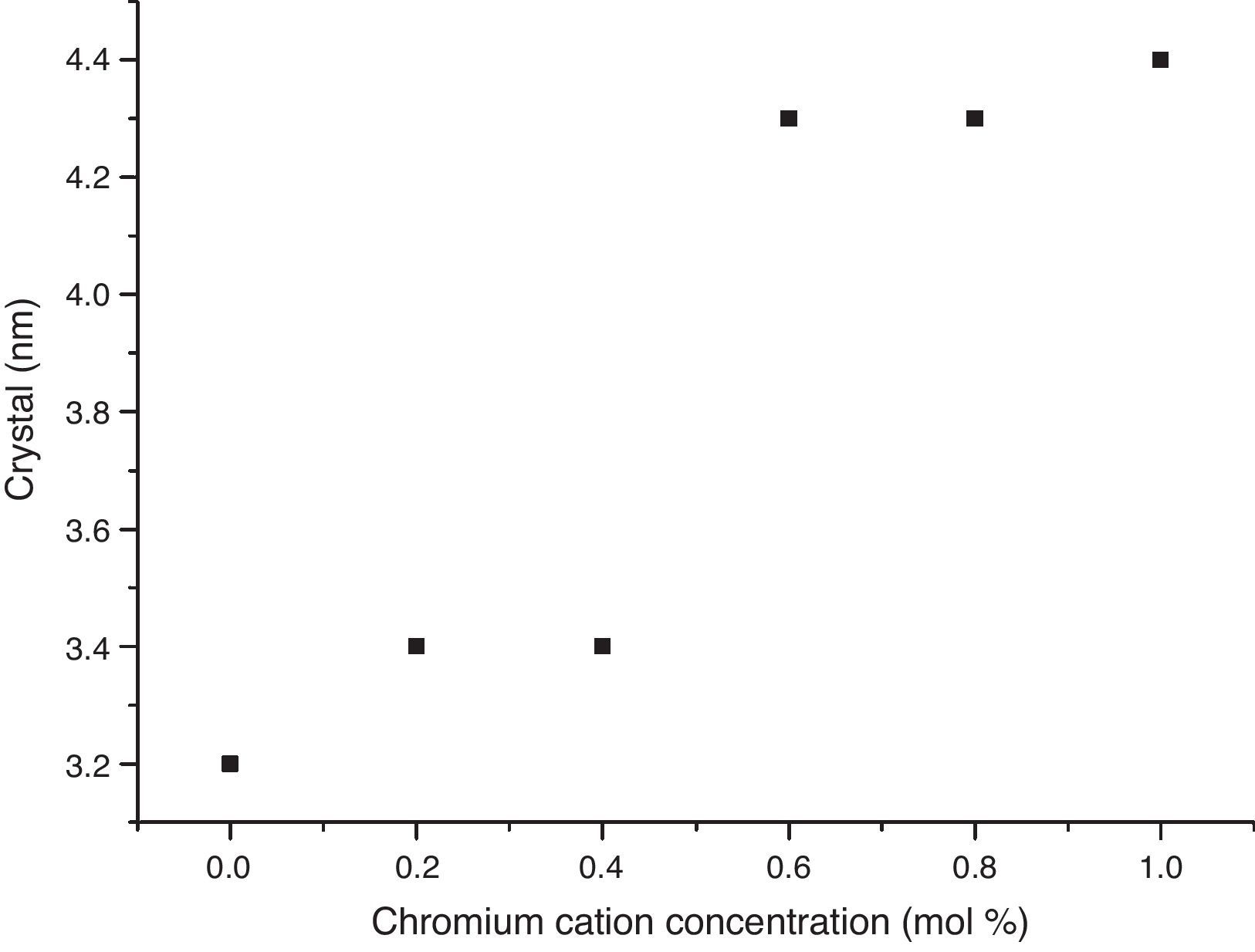
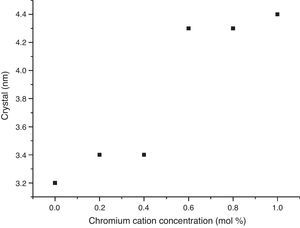
The crystal sizes of all samples were calculated using the Debye–Scherrer equation, using the intensity of highest signals in experimental X-ray patterns, whose data are shown in Fig. 4, in which the effect of the cation concentration is clear, as well as the consolidation of crystallites in the nanometric scale.
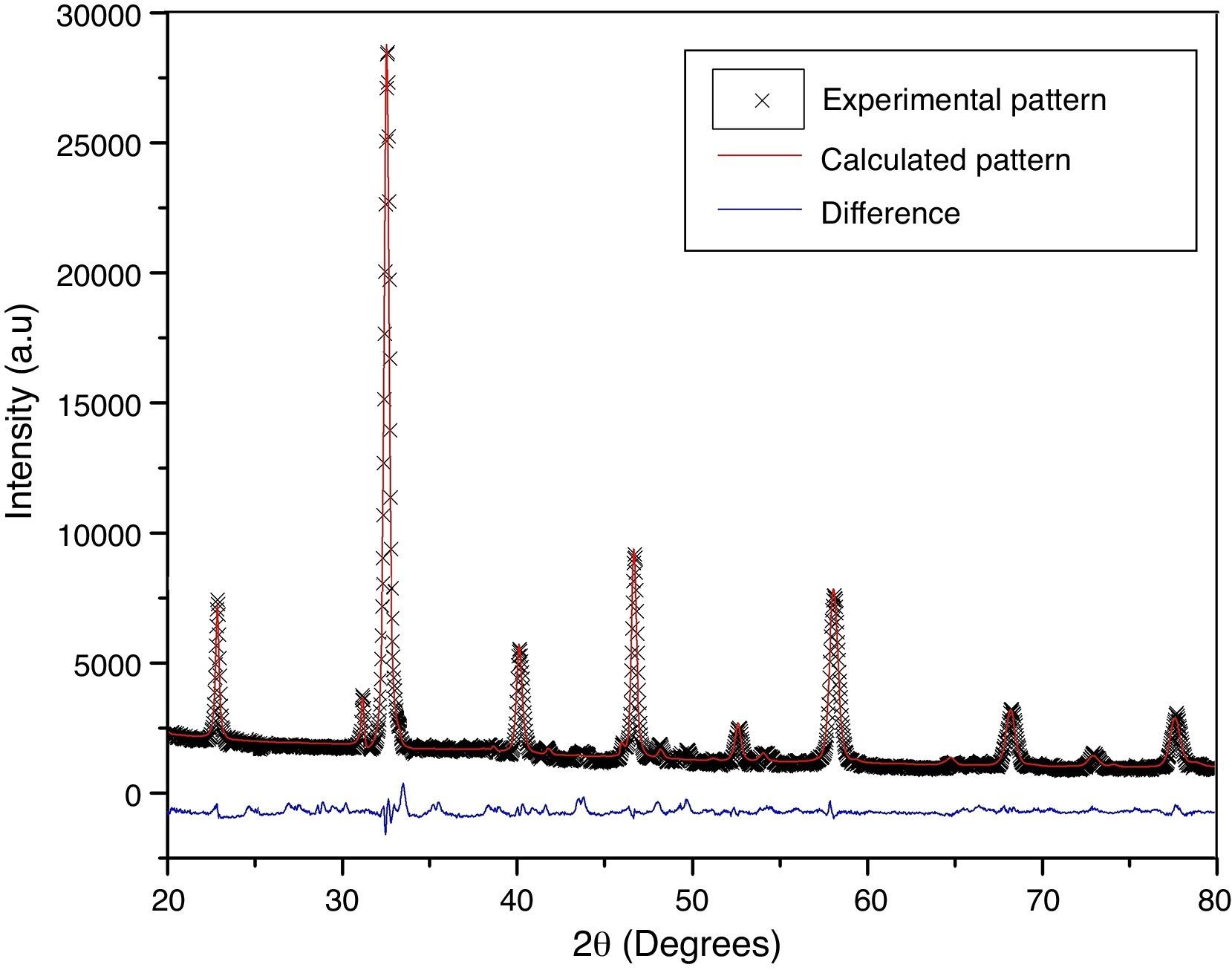
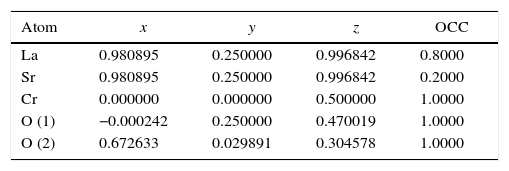
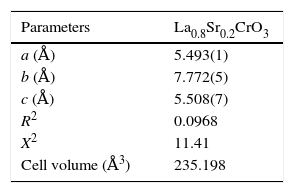
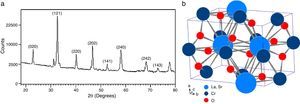
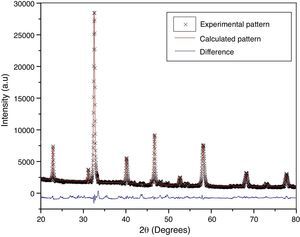
According to X-ray diffraction results of Fig. 5a and b, the LSCO system offered the best characteristics related with crystallinity in comparison with obtained systems. A more detailed analysis is shown in Fig. 6 using the Rietveld refinement and PCW software, confirming an excellent correlation with La0.8Sr0.2CrO3 reference structure of ICSD databases. The red line in Fig. 6 corresponds with the theoretical model provided by the GSAS software. The crosses represent the experimental pattern, and the blue line, the difference between theoretical and experimental patterns, confirming the effectiveness of the proposed synthesis method for the obtention of a chromite crystalline phase, the oxide displayed a perovskite structure with a Pnma (62) space group.
Tables 4 and 5 summarize the statistical parameters, atomic positions, and cell volume obtained from Rietveld refinement.
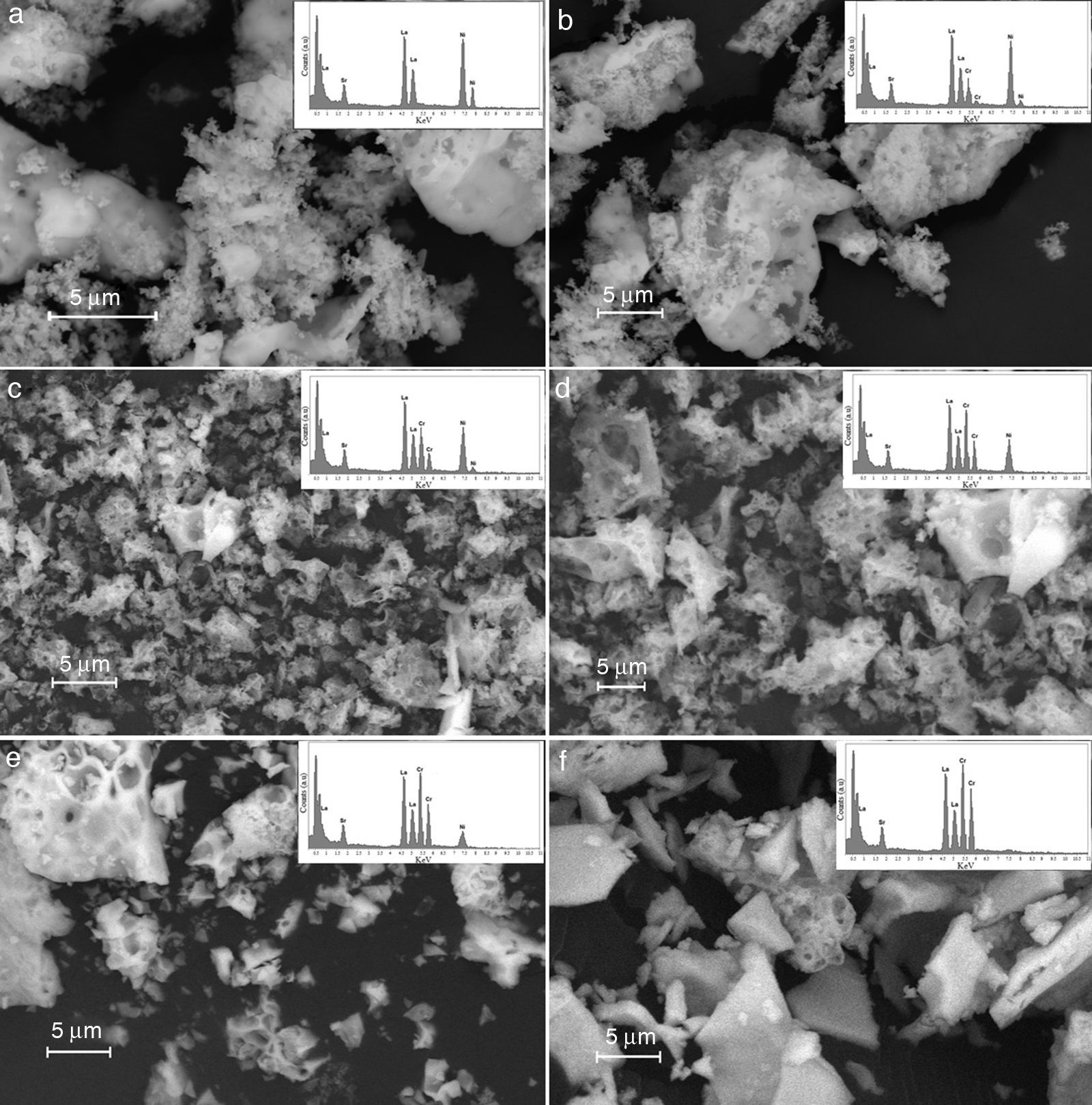
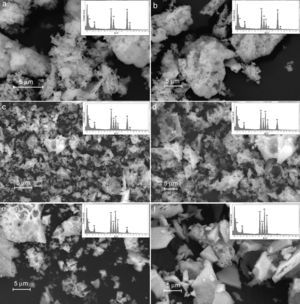
Results derived from scanning electron microscopy are shown in Fig. 7, and the results corroborate the obtention of homogeneous materials, with textural and morphological characteristics derived from synthesis method, with some degree of heterogeneity and porosity consistent with properties for anodic materials in SOFC technologies [44].
From the point of view of microstructure, obtained oxides are sufficiently heterogeneous to facilitate the migration and the transport of gas to active phase sites. This is the reason why the proposed methodology may achieve the maximum efficiency of materials. Thus, the materials with a high degree of densification and crystallization may limit the mass transport, whereas materials with a high concentration of surface defects can generate low mechanical strength for electrocatalytic applications related with the migration of gas species [45].
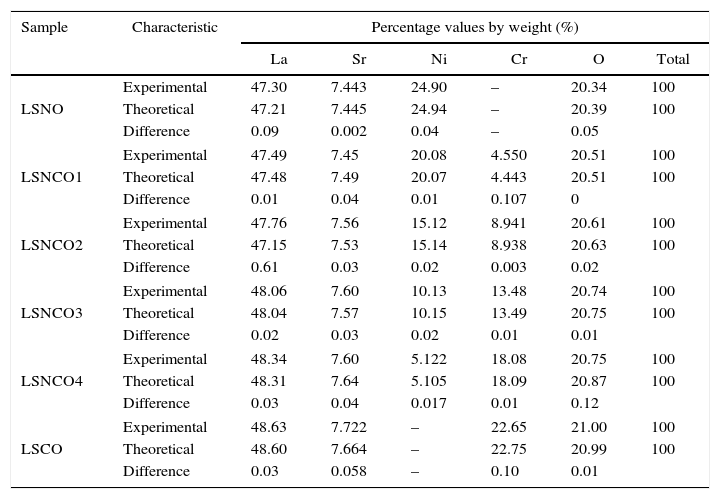
The Fig. 7 shows the scanning electron microscopy images and its corresponding EDX spectrums, showing that the cation contents correspond to the proposed content as shown in Table 6. The obtained values do not exceed a difference of 0.107% w/w); which means that the loss of cations during the synthesis process and the posterior thermal treatments to consolidate the crystalline phases were succesfully avoided.
Quantitative results (%, w/w) of the LSNO, LSNCO1, LSNCO2, LSNCO3, LSNCO4 and LSCO samples obtained by EDX technique.
| Sample | Characteristic | Percentage values by weight (%) | |||||
|---|---|---|---|---|---|---|---|
| La | Sr | Ni | Cr | O | Total | ||
| LSNO | Experimental | 47.30 | 7.443 | 24.90 | – | 20.34 | 100 |
| Theoretical | 47.21 | 7.445 | 24.94 | – | 20.39 | 100 | |
| Difference | 0.09 | 0.002 | 0.04 | – | 0.05 | ||
| LSNCO1 | Experimental | 47.49 | 7.45 | 20.08 | 4.550 | 20.51 | 100 |
| Theoretical | 47.48 | 7.49 | 20.07 | 4.443 | 20.51 | 100 | |
| Difference | 0.01 | 0.04 | 0.01 | 0.107 | 0 | ||
| LSNCO2 | Experimental | 47.76 | 7.56 | 15.12 | 8.941 | 20.61 | 100 |
| Theoretical | 47.15 | 7.53 | 15.14 | 8.938 | 20.63 | 100 | |
| Difference | 0.61 | 0.03 | 0.02 | 0.003 | 0.02 | ||
| LSNCO3 | Experimental | 48.06 | 7.60 | 10.13 | 13.48 | 20.74 | 100 |
| Theoretical | 48.04 | 7.57 | 10.15 | 13.49 | 20.75 | 100 | |
| Difference | 0.02 | 0.03 | 0.02 | 0.01 | 0.01 | ||
| LSNCO4 | Experimental | 48.34 | 7.60 | 5.122 | 18.08 | 20.75 | 100 |
| Theoretical | 48.31 | 7.64 | 5.105 | 18.09 | 20.87 | 100 | |
| Difference | 0.03 | 0.04 | 0.017 | 0.01 | 0.12 | ||
| LSCO | Experimental | 48.63 | 7.722 | – | 22.65 | 21.00 | 100 |
| Theoretical | 48.60 | 7.664 | – | 22.75 | 20.99 | 100 | |
| Difference | 0.03 | 0.058 | – | 0.10 | 0.01 | ||
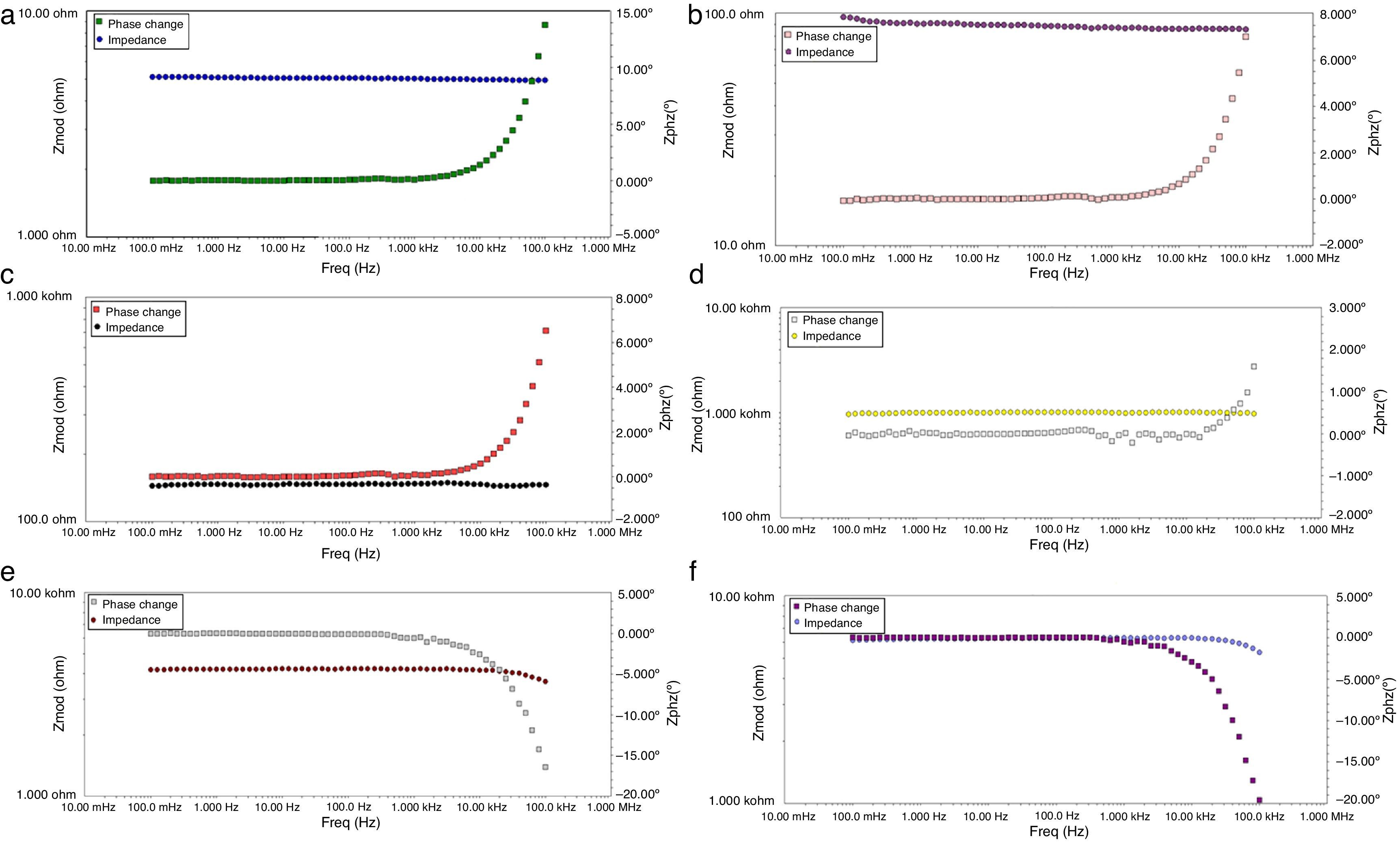
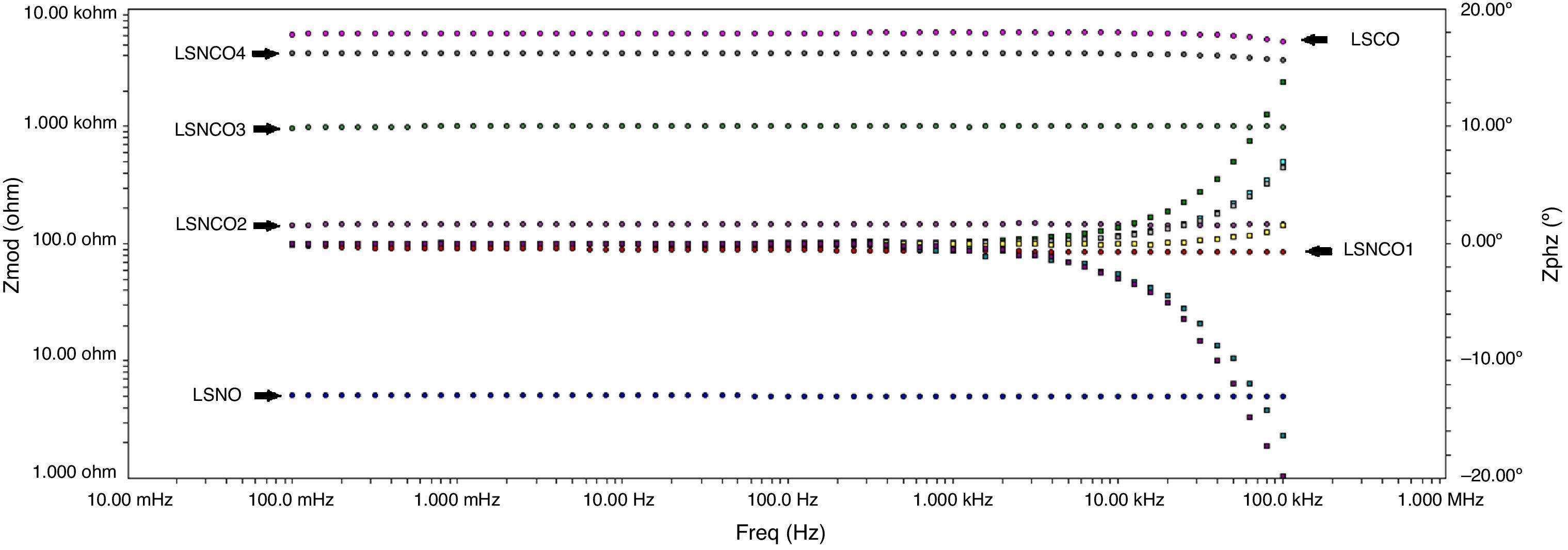
Figs. 8 and 9 correspond with the impedance analysis of the six oxides, with obtained results at room temperature in a modified solid-state cell. Two curves are shown for each sample: the impedance vs frequency and the phase vs frequency change.
A meticulous analysis of the impedance curves with respect to frequency identified as LSNO, LSNCO1, LSNCO2, LSNCO3, LSNCO4 and LSNO, respectively, shows low values of resistance for LSNO, LSNCO1, LSNCO2 and LSNCO3 samples, with a high concentration of nickel, associated with an electrical semiconducting behavior due to the nickel incidence at B sites of the perovskite. According with Alvarado et al. [46], the preferential formation of ions in oxides with a high content of nickel is strongly linked with the change of ionic radii of the elements that are replaced in the perovskite structure. Hashimoto et al. [47] have shown that the electrical conductivity increases with the nickel content and this behavior is related with the formation of load carriers during synthesis process; additionally, it has been found that elements with d-orbital partially filled allowed the transfer of charge under a semiconducting regime, in accordance with the reports of Gómez-Garcia et al. [48].
On the other hand, for LSNCO4 and LSCO oxides, the obtained impedance signals are related with an opposite behavior associated with an ionic conductivity effect, in which the value of electrical conductivity decreases notably and the charge transferring are fully identified with high electrical resistivity values [49]. Zheng et al. [50] have observed that a decrease in the electrical conductivity with respect to increase in concentration of chromium is related with development of charge carriers polaron type, which permit to understand the interactions between electrons and atoms in a solid material. In this form, the obtained resistivity values show dependence with respect to development of grain boundaries, usually characterized by promoting a high resistivity to electrical conduction as has been established by Gómez-Cuaspud et al. [33].
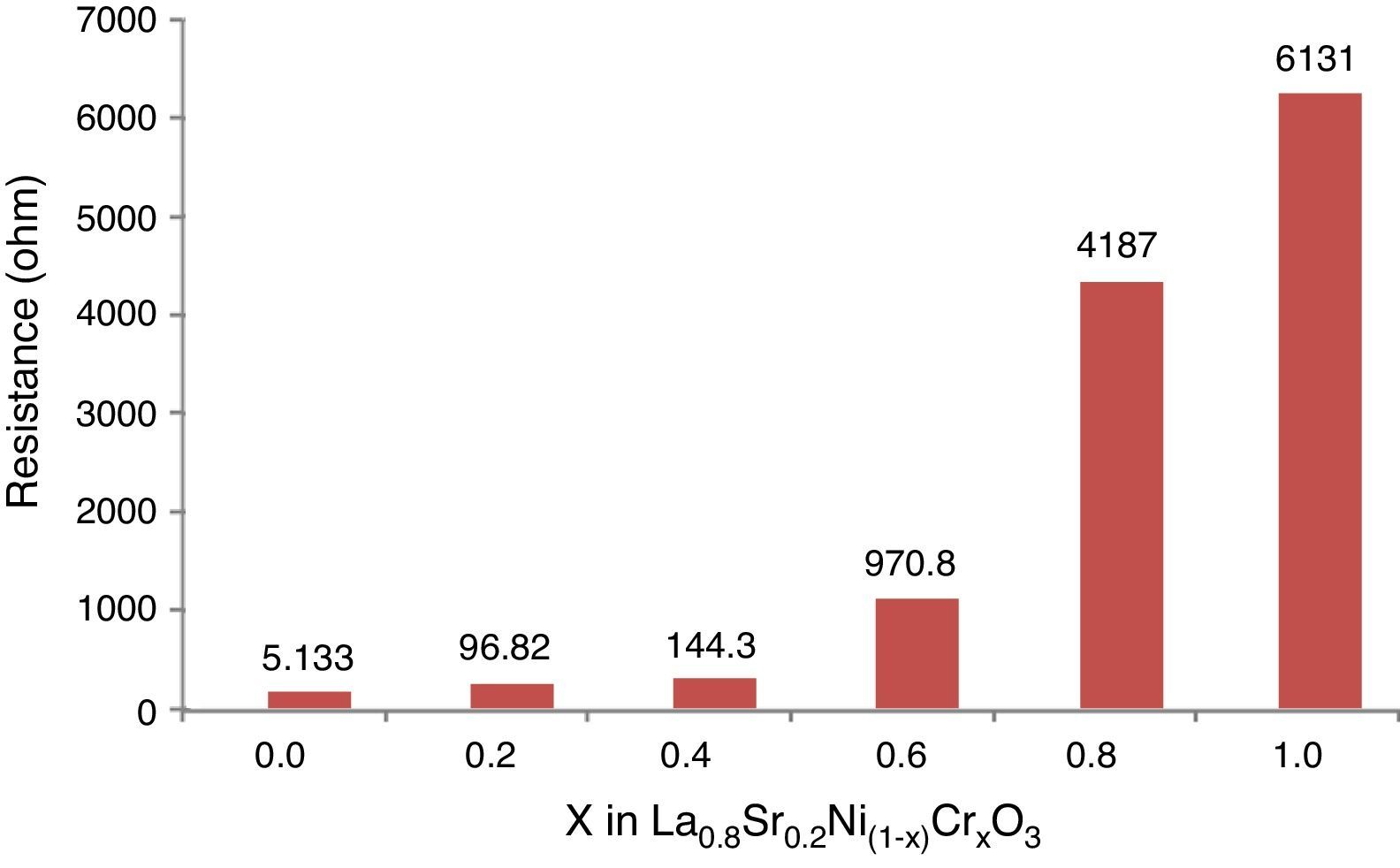
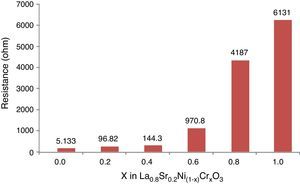
The Fig. 10 shows the resistance values obtained for each system with an exponential behavior, where the increase in resistance is proportional to chromium concentration, causing variations in resistance between 5.13 and 613Ω range. These results are explained according to several authors, by the nature of modification cation that favors major structural changes, influencing significantly the conductive properties of oxides [46–50]. According to previous studies and the present work [51,52], the lanthanum chromites, modified with nickel, are characterized by a mixed ionic and electronic behavior that improves the oxidation of hydrogen fuel in the surface of the catalyst as an important alternative in design of materials for solid oxide fuel cells.
ConclusionsThe spectroscopic analysis performed by means of FTIR and UV techniques on organometallic precursors of La0.8Sr0.2Ni(1−x)CrxO3 along proposed compositions indicated the effectiveness of proposed methodology for the obtention of homogeneous soluble citrate species. Such compounds, contribute to consolidation of homogeneous and nanometric scale phases with a range of textural, morphological and surface properties of relevance in design of anodic electrocatalysts for solid oxide fuel cells.
The structural analyses performed through X-ray diffraction (XRD) showed the obtention of crystalline phases according to proposed methodology with nanometric sizes between 3.2 and 4.4nm, showing the effect of chromium cation in the shrinkage of the unit cell along concentration increases and stabilizes the orthorhombic crystal group system. The analysis by energy dispersive of X-ray spectroscopy shows that the cation contents correspond with the proposed values without evidence of species volatilization.
The Rietveld refinement from X-ray analysis for LSCO system after thermal treatment at 1000°C for 120min confirms the presence of a perovskite crystalline phase, with Pnma (62) space group, orthorhombic structure and preferential orientation along (121) facet with cell parameters: a=5.493Å, b=7.772Å, and c=5.508Å, in concordance with PDF card reference structures.
The scanning electron microscopy (SEM) analysis confirmed a series of textural and surface properties for the obtention of heterogeneous solids with high surface defects concentration for eventual electrocatalytic activity. The results derived from impedance spectroscopy at room temperature permit to identify the best ceramic composition in terms of resistance and conductivity with a strong correlation between nickel concentration and conductivity, in accordance with previous works. On the contrary, the LSNCO4 and LSCO samples with a low nickel content showed a pronounced decrease in electrical conductivity and it is related to the increase of the resistance of materials in accordance with an exponential behavior. Current results indicate that there is no presence of constant phase elements (capacitance) or inductive effects in the mainline of each sample.