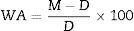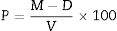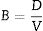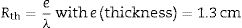This work proposes an effective solution for the recycling of coffee waste by incorporating it (10, 20 and 30wt.%) as a secondary material with clay (Cretaceous deposit of Moroccan Meseta) to produce porous lightweight ceramics (class BIII) whose quality is similar to that of ceramic materials with insulating character. The applied raw materials were characterized in terms of their composition (XRD, XRF, IR), microstructural analysis (polarized microscope) and thermal behavior (TDA/TGA). The ceramic materials resulted after firing at 1150°C, were investigated regarding the phases composition, structural characteristics and physical properties of technological interest. In fact, the thermal treatment at 1150°C gives samples having the following technological characteristics: P=42.81%, d=1.46g/cm3, λ=0.39W/mK, WA=29.25% and F.S=10.75MPa. The combination of XRD and polarized microscope, allowed a better analysis of the mineralogical and structural evolution after sintering at 1150°C.
Este trabajo propone una solución efectiva para el reciclaje de residuos de café incorporándolo (10, 20 y 30% .wt) como material secundario con arcilla (depósito cretácico de la Meseta marroquí) para producir cerámicas ligeras porosas (clase BIII) cuya calidad es similar a la de los materiales cerámicos con carácter aislante. Las materias primas aplicadas se caracterizaron en términos de su composición (XRD, XRF, IR), análisis microestructural (microscopio polarizado) y comportamiento térmico (TDA / TGA). Los materiales cerámicos obtenidos después de la cocción a 1150 ̊ C, fueron investigados sobre la composición de las fases, las características estructurales y las propiedades físicas de interés tecnológico. De hecho, el tratamiento térmico a 1150 ̊ C da muestras que tienen las siguientes características tecnológicas: P = 42.81%, d = 1.46 g / cm3, λ = 0.39 W / m × K, WA = 29.25% y F.S = 10.75 MPa. La combinación de XRD y microscopio polarizado permitió un mejor análisis de la evolución mineralógica y estructural después de la sinterización a 1150 ̊ C.
Ceramic tiles are materials produced by a fast firing process of clay, silica sand, calcite, pyrophyllite, feldspar and additives. This building material, generally used in flooring and also as wall covering and ventilated facades, is endowed with high technological properties such as low thermal conductivity, high mechanical strength and excellent chemical and abrasion resistance. These properties mean that ceramic tiling was actually the material with the highest increase in production and sales over all other ceramic building materials [1]. The ceramic industry in Morocco dates from the 1970s only. A real growth began during the 1990s, especially with the launch of an important national project of construction. The Moroccan ceramic industry includes floor and wall tiles, stoneware, brick and porcelain. Morocco has become known all across North Africa and Europe for its pottery and ceramics. Fes, Meknes, Sale and especially Safi are the main pottery centers of the country, producing about 80% of Morocco's production [2–9].
Raw materials have been deeply involved in such an evolution: the flexibility of current manufacturing cycles enables the use of a very wide range of clays, whose chemical and mineralogical composition, particle size distribution and ceramic properties were reappraised. Currently, an industry-oriented, technological classification of clay raw materials is proposed on the basis of chemical (Fe2O3 content) and mineralogical parameters (amount of phyllosilicates and carbonates) together with particle size (fractions<2μm and >63μm) and plasticity. It firstly discriminates light firing and dark-firing clays according to an iron oxide threshold of 3%. Light-firing clays are distinguished by the amount of kaolinite group minerals and plasticity in “kaolins” (high-grade, low-grade, and raw kaolins, kaolinitic loams) and “plastic clays” (ball clays, pyrophyllitic clays, white bentonites). Dark-firing clays are classified according to their coarse-grained fraction and the amount of carbonates in carbonate-rich types (marly and carbonatic clays), red loams and red clays; these latters are further differentiated by the relative abundance of clay minerals. Such a classification is essential to draw the guidelines for body formulation and to explain the criteria followed in the industrial practice for each category of ceramic tiles [10].
The recycling of by-products and wastes represents an increasingly urgent problem for the immediate future of human kind. Different percentages of fugitive phase with three different average particles were added to the green porcelain compositions [11]. The properties of these stoneware tiles as apparent density, linear shrinkage, modulus of rupture, porosity, roughness and water absorption were studied as function of the added fugitive phase. In another study by J.J. Reinosa et al. [12], Stoneware tiles were produced by the incorporation of galvanic waste to industrial compositions that were processed from kaolinitic clay, feldspar, quartz and recycled domestic glass. The followed procedure allowed to effective immobilization of up to 10wt.% of waste with heavy metals in a porcelain stoneware that satisfy both the mechanical and the chemical standards required to massively commercialize such a product.
Coffee wastes, which are produced in large quantities in Morocco, are an important by-product. However, coffee wastes are particularly rich in alkaline and alkaline-earth metals, and might be adequate to replace the traditional feldspars, which are used in high content as fluxes in clay-based ceramic formulations but are becoming scarce and costly [1]. The Moroccan law 28.00 on waste management defines the valorization of waste as any operation of recycling, reuse, and recovery, use of waste as a source of energy or any other action to obtain raw materials or reusable products from waste recovery in order to reduce or eliminate the negative impact of this waste on the environment. Soluble coffee has seen a significant rise in its production and consumption during the last decades. For instance, in Morocco, the Consumer Protection Association reported an increase of around 3% in coffee consumption between 2014 and 2015 [13].
Numerous studies have been conducted on coffee wastes and their potential development. The chemical composition of exhausted coffee waste generated in a soluble coffee industry was investigated by D. Pujol et al. [14]. The chemical characterization included elemental analysis, mineral composition and ash content, summative composition; acidic functional groups, lipophilic extractives, total polyphenols, condensed tannins determination and FTIR analysis. The fluxing effect of coffee waste additions to an industrial clay-based mixture was evaluated by W. Acchar et al. [15]. Based on the characterization results and the mullite–silica–leucite phase diagram, additions of 5–20wt.% coffee wastes were made to the clay-based mixture and the resulting compositions were evaluated after sintering at temperatures between 1100 and 1200°C. The results obtained show that firing temperatures near 1180°C and ∼10wt.% coffee waste addition lead to linear shrinkage, water absorption and flexural strength values that fall within the range specified by floor tile standards, requiring no significant changes in processing parameters. Coffee waste can thus advantageously replace feldspars in the role of fluxing material, with the potential to reduce not only natural ceramic raw material consumption, but also production and landfill costs as well as waste disposal area requirements.
D. Eliche-Quesada et al. [16] have used different forms of waste in the manufacture of ceramic bricks. Various industrial wastes such as urban sewage sludge, bagasse, and sludge from the brewing industry, olive mill wastewater, and coffee ground residue were blended with clay to produce bricks. The incorporation of coffee grounds and olive mill wastewater of clay was more beneficial, with compressive strength values similar to bricks without waste and a 19% improvement in thermal conductivity. A. Kovalcik et al. [17] have published in 2018 a review paper on the valorization of spent coffee grounds. Recycled glass as a supplementary filler material in spent coffee grounds geopolymers have been studied by Arul Arulrajah et al. [18]. In Italy, Coffee is one of the most frequently consumed drinks. P. Ricciardi et al. [19], have proposed coffee chaff as sound insulation and absorption material for the Italian building industry.
The present paper aims at investigating a particular application of local raw materials (Moroccan red clay) and coffee waste which has been recently studied as a recycled construction material, due to its physical resemblance to sandy soils, to evaluate their potential for the manufacture of porous red paste (class BIII) ceramic production.
Materials and experimental proceduresRaw materialsThe red clay used in this study comes from the region of Safi (Morocco). It is a thin clay of lower Cretaceous age (Hauterivian) very widely used in the region of Safi for the manufacture of pottery. The red clay sample used comes exactly from the “Barrage quarry” (Fig. 1: 32°19′47.60″N; Y: 9°10′26.46″O) where the layer over 20m thick provides a homogeneous and pure red clay [3]. Coffee Waste has several physical and chemical properties. From these properties arise possibilities of valorization and use of this material. In this work the coffee waste was collected from different coffee shops on Casablanca, Morocco. The sample was washed with distilled water, dried in a temperature of 105°C for 24h, crushed and sieved for a particle size of 63μm (Fig. 2).
Our methodological approach includes three phases: Preparation and characterization of the raw material (IR, XRF, XRD, ATD/ATG, polarized microscope, thermal conductivity); Composition of mixtures and firing (different proportions of coffee waste (10, 20, 30wt.%)); and Characterization of the elaborated ceramics (water absorption, porosity, density, shrinkage, XRD, thermal conductivity, flexural strength).
The infrared (IR) spectroscopy is a method frequently used to show vibration of chemical bonds for the raw materials and elaborated ceramics. Fourier transform infrared (FT-IR) spectra were obtained with a Bruker Tensor 27 FTIR spectrometer operating in the range 4000–400cm−1. For this purpose, 1% of clay sample was mixed with 99% of KBr. The different phases were identified using the origin lab 8 software. X-ray diffraction (XRD) analyzes were carried out using a Panalytical, serial number: DY2042. Ash content was determined as per AOAC method 920.93 [20]. Coffee waste (0.5g) were added to a crucible, previously washed and dried with deionized water, and dry ashed at 500°C in a muffle furnace. Sample preparation for ICP-AES analysis was performed as per Szymczycha-Madeja et al. method [21]. Briefly, waste (0.5g) was added to a 30mL polypropylene centrifuge tube. The mixture was ultrasonicated for 15min and subsequently made up to 25mL with H2O. Next, the solution was centrifuged at 7000rpm for 10min. The supernatant was decanted and stored at −20°C until required. The elemental analysis (C, H, N and S) of coffee samples was determined using a PerkinElmer EA2400 series II Elemental Analyzer. N detection limit was 1.20%.
Thermal analyses (DTA-TGA) were realized with a SETARM LABSYS™ EVO 1F apparatus functioning in air at 5C/min. The thermal conductivity λ is the physical quantity that characterizes the ability of a material to conduct heat, it describes the amount of energy passing through a material of unit volume, for a unit change of temperature gradient. The experimental measurements were obtained using the λ-Meter EP500e, the samples were placed between two plates of dimensions 500mm×500mm. The top plate is lowered with a pressure of 2500Pa until the pressure set point is reached. The measurement area is the innermost square of dimensions 200mm×200mm, and the rest is a frame that must be made of a highly insulating material. To take the measurements, the temperatures applied were 10, 25 and 40°C, with a temperature difference between the heating plate and the cold plate of the order of 15°C in all measurements. The microstructural analysis of raw materials and ceramic samples was performed using a binocular microscope OLYMPUS-SZX9 with a camera (DS Camera Head DS-5M). The observation of ceramic samples under a binocular microscope identifies some traces related to the manufacturing technique, the characteristics of the ceramic, and the size and color of inclusions and pores.
After the characterization of the raw materials, the constituents (red clay and coffee waste) underwent crushing operations and wet grinding. The slip was placed in an oven for 24h at 110°C until complete desiccation. The product obtained was dry milled, sieved and we retained the fraction size less than 63μm. This latter was moistened using a sprayer in the limits of 4 and 6% of water. The moistened powder was pressed into molds of 10cm×5cm×1cm using a hydraulic press of 80MPa. The specimens in the form of briquettes were dried in an oven (110°C, 24h) and then heated to a firing temperature up to 1150°C in an experimental furnace Nabertherm GmbH [1] (Fig. 3).
The water absorption, bulk density and apparent porosity were measured according to ASTM C373-88 [22,23], which involves drying the test specimens to constant mass (D), boiling in distilled water for 5h and soaking for an additional 24h at ambient temperature. After impregnation, the mass (S) of each specimen while suspended in water and their saturated mass (M) is determined. The test was carried out on four representative specimens. Water absorption, WA (%), expresses as percent, the relationship of the mass of water absorbed to the mass of the dry specimen as follows:
The Apparent porosity, P (%), expresses as percent the relationship of the open pores of the specimen to its exterior volume. Calculate the Apparent porosity as follows:
The bulk density, B (g/cm3), expresses in grams per cubic centimeter of a specimen is the quotient of its dry mass divided by the exterior volume. Including pores as follows:
The mechanical test of flexural strength measurements using 3R type: RP25 ATF, serial number: 1506 F5H 109. This mechanical test is the most important test to predict the quality of the ceramics. The three-point bending test shows the evolution of stiffness according to the thickness and width of ceramics bricks. A sample to be tested is placed on two supports and one applies in the center of the ceramic brick a force increasing until rupture according to the norm ASTM C674-88 [23].
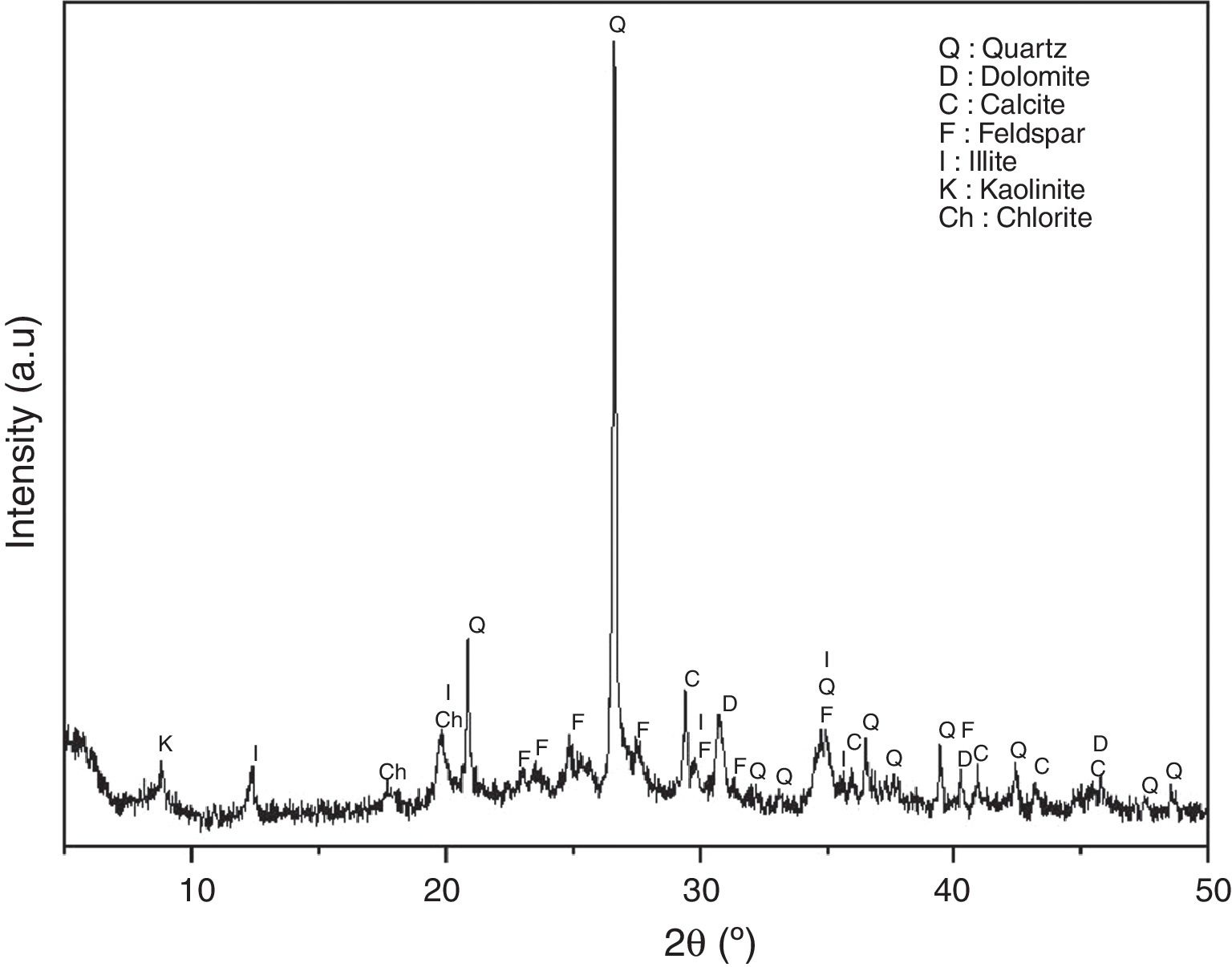
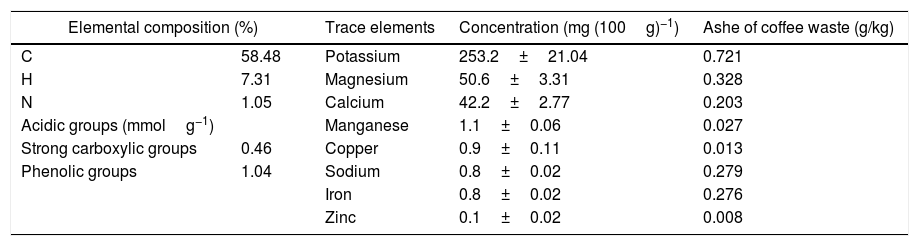
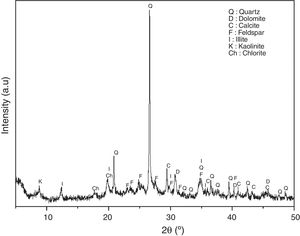
Results and discussionCharacterization of raw materialsIn order to assess the potentiality of clay in the ceramic industry, representative clay was analyzed from a chemical, mineralogical and technological view point. Table 1 shows the chemical composition in terms of oxide contents and the loss on ignition for raw powder clay. This clay shows the expected typical composition, rich in silica (51.6%), alumina (19.2%) and magnesia (7.36%); because of the presence of clay minerals and quartz, they have a decisive influence on the refractoriness and mechanical resistance of the final product [24]. These oxides are accompanied by a significant amount of iron oxide (9.0%), which is responsible for a dark coloring of the fired pieces [9,10]. Moreover, the presence of large amounts of fluxes as well as CaO, Na2O and TiO2 increases the chance to form a considerable amount of liquid phase at a relatively lower firing temperature. The large amount of K2O (2.89%) content in clay samples reflects the abundance of illite (Fig. 4). Elemental analysis of coffee waste is shown in Table 2. The percentage of the different elements is within the range of values reported in the literature for spent coffee [25]. The ash content of the studied waste was 0.9%.
Elemental analysis and acidic groups of coffee wastes.
| Elemental composition (%) | Trace elements | Concentration (mg (100g)−1) | Ashe of coffee waste (g/kg) | |
|---|---|---|---|---|
| C | 58.48 | Potassium | 253.2±21.04 | 0.721 |
| H | 7.31 | Magnesium | 50.6±3.31 | 0.328 |
| N | 1.05 | Calcium | 42.2±2.77 | 0.203 |
| Acidic groups (mmolg−1) | Manganese | 1.1±0.06 | 0.027 | |
| Strong carboxylic groups | 0.46 | Copper | 0.9±0.11 | 0.013 |
| Phenolic groups | 1.04 | Sodium | 0.8±0.02 | 0.279 |
| Iron | 0.8±0.02 | 0.276 | ||
| Zinc | 0.1±0.02 | 0.008 | ||
The grain size distribution of this clay is predominately fine particles (59.44%<0.01mm), which explains its average plasticity index. The plasticity of a clay is a fundamental technological parameter that influences the characteristics of ceramic materials. It was carried out on the fraction less than 100μm and consists in varying the water content of the material in order to evaluate its consistency. In this study, we have opted for the “Atterberg limits” method, which aims to define the transition thresholds between the solid and plastic state (plasticity limit: WP) and from the plastic state to the liquid state (liquidity limit: WL). The interval between these two limits defines the field of plasticity (plasticity index: IP). The plasticity index of the studied clay is of the order of 18% (field of little plastic clays). According to many previous studies, the plasticity of clays depends on the nature of their clay minerals and is inversely proportional to the size of the grains [26]. Clays with high particle size generally show low plasticity. Indeed, the specific surface of large particles reduces the amount of water adsorbed, resulting in a decrease in plasticity. The results in Fig. 4 show the predominance of quartz (Q), dolomite (D), calcite (C), feldspar (F), illite (I), kaolinite (K) and chlorite (ch) presenting favorable properties for ceramic use.
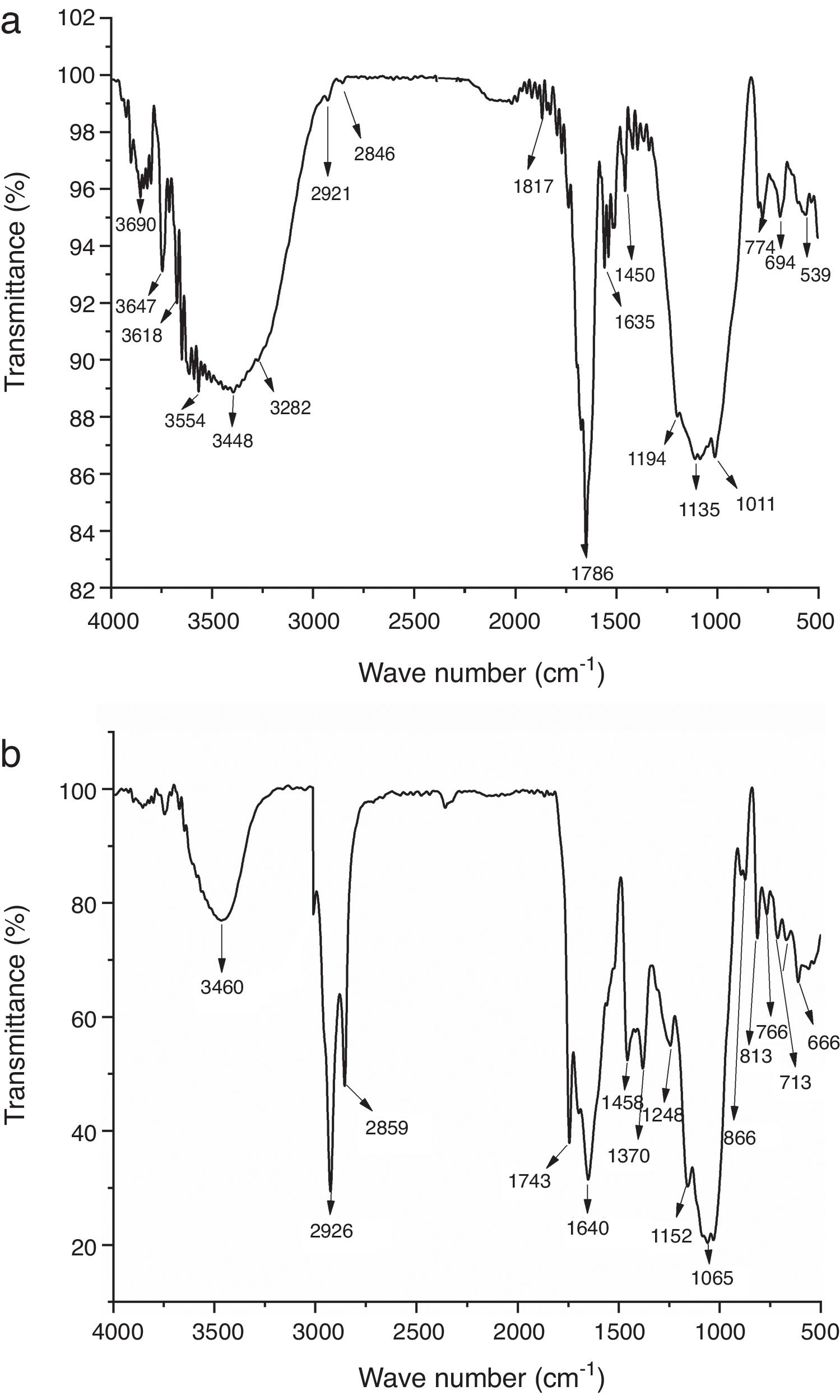
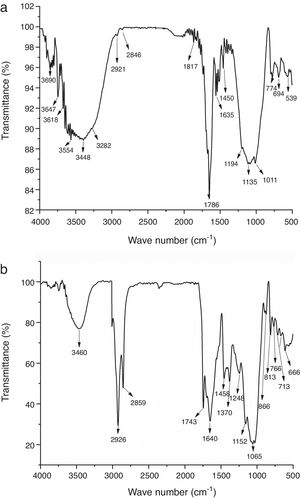
Infrared spectroscopy is adapted to spectral measurements in the range of 4000cm−1 to 450cm−1. The IR spectra of the raw materials are shown in Fig. 5. The results of the IR spectra for clay show the existence of low organic matter content (bands between 2846 and 2921cm−1) [27]. The bands located at 539 and 1450cm−1 are attributed to deformation vibration of SiOMg and CO bond vibration in carbonates respectively. The band observed at 3618cm−1 is attributed to mica, also they showed peaks due to SiO stretch and SiOSi stretch between 1135 and 1194cm−1. The pattern shows the presence of sepiolite characterized by the band SiOMg at around 3554cm−1, the water characterized by the band OH between 1817 and 1635cm−1 and iron hydroxide at 3282cm−1[28]. The FTIR spectra obtained for the coffee sample are shown in Fig. 5b. The broad band at about 3460cm−1 included many vibration modes mainly attributed to OH groups with a minor contribution of NH functional groups. The presence of methyl and methylene groups is confirmed by the two sharp peaks at 2926cm−1 and 2859cm−1 attributed to asymmetric and symmetric stretching of CH bonds in aliphatic chains. These peaks have been previously identified in roasted coffee and attributed to the presence of caffeine. The sharp band at 1743cm−1 is associated to the carbonyl vibration (CO) in aliphatic esters or in triglycerides. Therefore, this band can be attributed to lipids. The band at 1640cm−1 is due to CC vibration of lipids. The band at 1458cm−1 corresponds to CH bending of CH3 groups. The bands of coffee spectrum at 1065, 1152, 1248, 1370cm−1 could be attributed to chlorogenic acids which are a large family of esters formed by quinic acid and certaintrans-cinnamic acids. Other peaks detected between 666 and 866cm−1 represented the presence of aromatics groups [14].
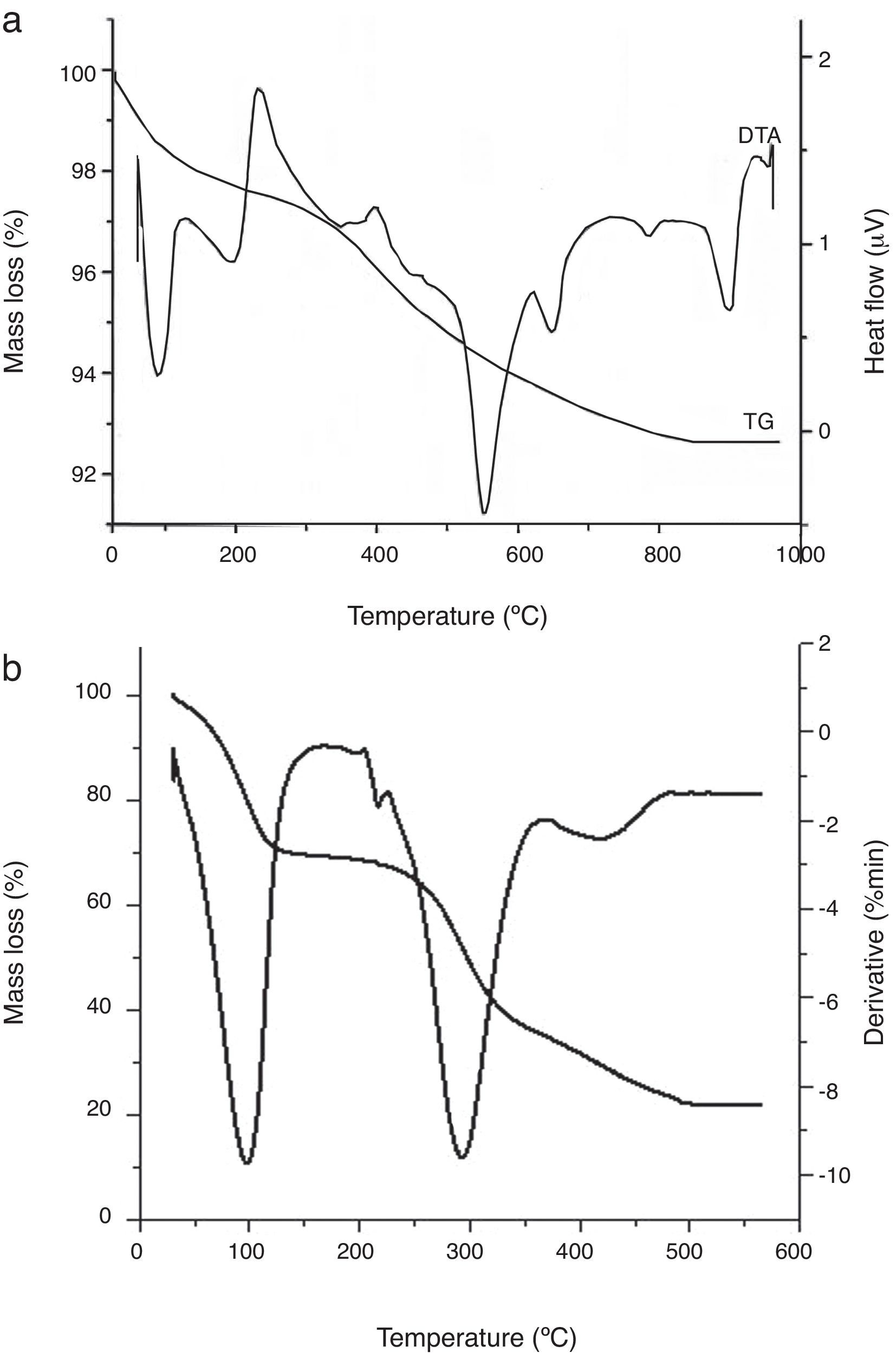
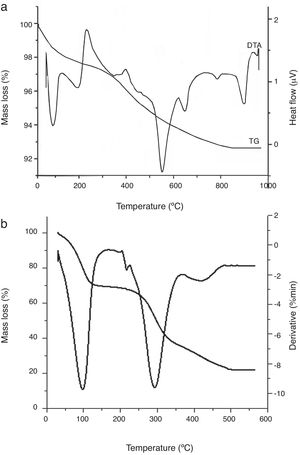
The TGA-DTA curves of the red clay and coffee waste are shown in Fig. 6. The first peak corresponds to the elimination of the moisture and interlayer water. The second peak at around 200°C can be attributed to the decomposition of organic matter and dehydration of illite. The endothermic peaks at 550 and 650°C can be attributed to dehydroxylation of kaolinite which transforms into metakaolinite and illite respectively. The metakaolinite particles hold the platelet shape of the kaolinite. The endothermic peaks at 780 and 880°C can be attributed to decomposition of dolomite and calcite respectively. Porous oxides are formed during the decomposition of dolomite with a variation in molar volumes ranging from 62.94cm3mol−1 for CaMg(CO3)2 to 16.92cm3mol−1 for CaO and 11.26cm3mol−1 for MgO. After calcination, the resulting oxides have lower molar volumes, larger surfaces and greater porosity than carbonates. Carbonate calcinations result in the formation of an oxide having a pseudo-array of the normal cubic network of the oxide. The thermal decomposition of dolomite is as follows [29–31]:
From 800°C onwards, a highly endothermic calcite decomposition reaction occurs in which calcium carbonate decomposes into calcium oxide and carbon dioxide when heated. It is a calcination reaction: CaCO3
CaO+CO2.Finally, there is a small exothermic peak at 940°C. It is likely related to the formation of spinel structure and/or primary mullite. TGA plot of coffee waste is illustrated in Fig. 6b. The thermal treatment of the raw material show two steps: (1) below 400°C, the mass loss is due to the main volatile matter and tars elimination. Above 400°C, a plateau region is reached and the material is almost totally carbonized. According to H. Laksaci et al. [32], the pyrolysis of lignocellulosic materials gives rise to three phases: char, oils (tars) and gases. A rudimentary porosity is obtained on the char fraction as a consequence of the release of non-carbon elements such as hydrogen, oxygen and nitrogen in the form of gases and tars, leaving a rigid carbon skeleton formed by aromatic structures.
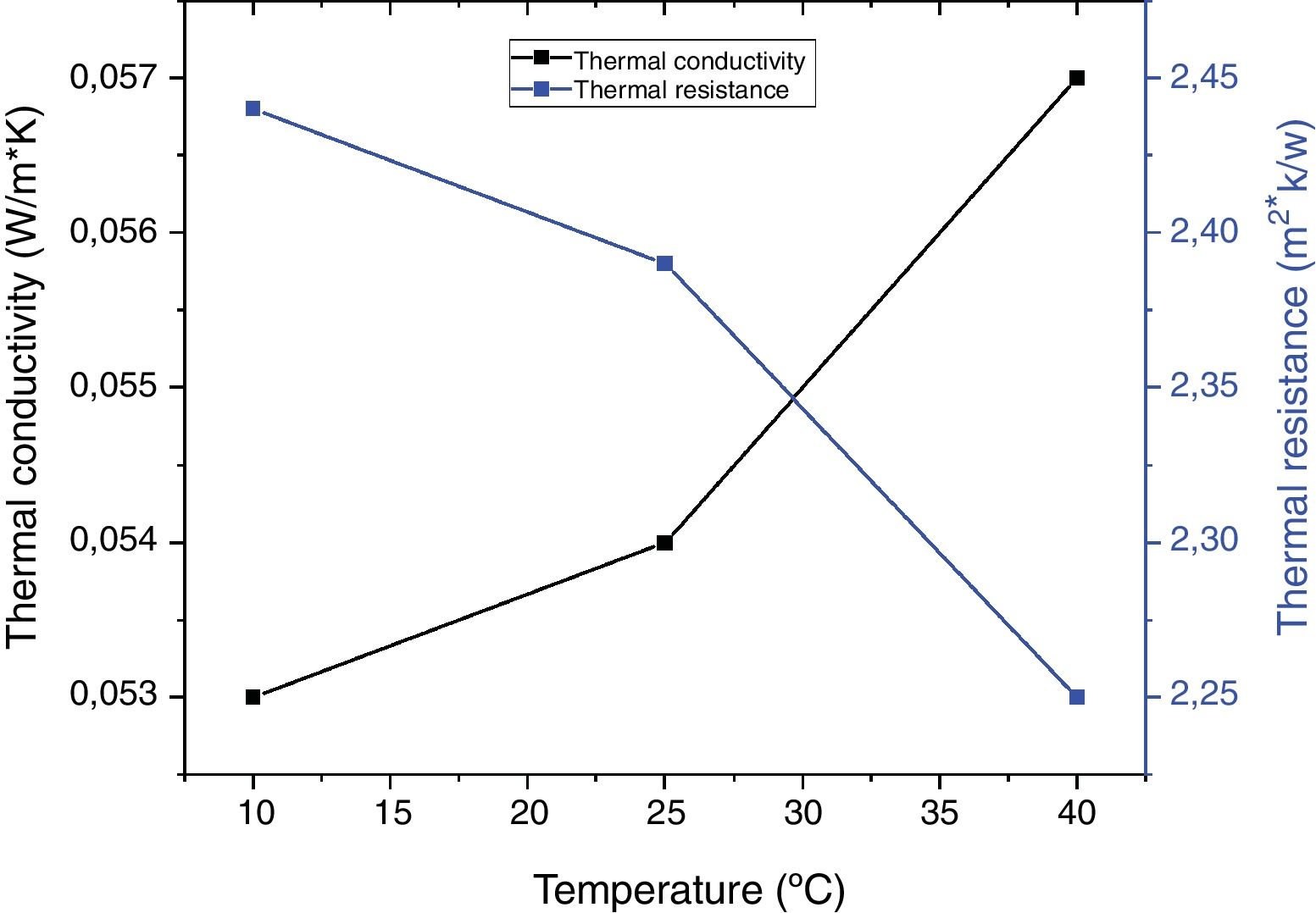
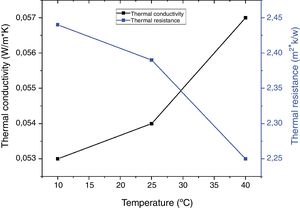
Fig. 7 shows the evolution of thermal conductivity and thermal resistance of coffee wastes according to temperature, in which, the thermal conductivity of a material increases mainly with temperature, whereas, we found an excellent insulation performance with λ<0.060W/mK (10°C: λ=0.053W/mK; 25°C: λ=0.054W/mK and 40°C: λ=0.057W/mK). On the other hand, the thermal resistances Rth decrease as a function of temperature variation (Rth=2.44m2K/W), Despite the fact that the thermal conductivity λ decreases inversely with increasing thermal resistance according to the following relation:
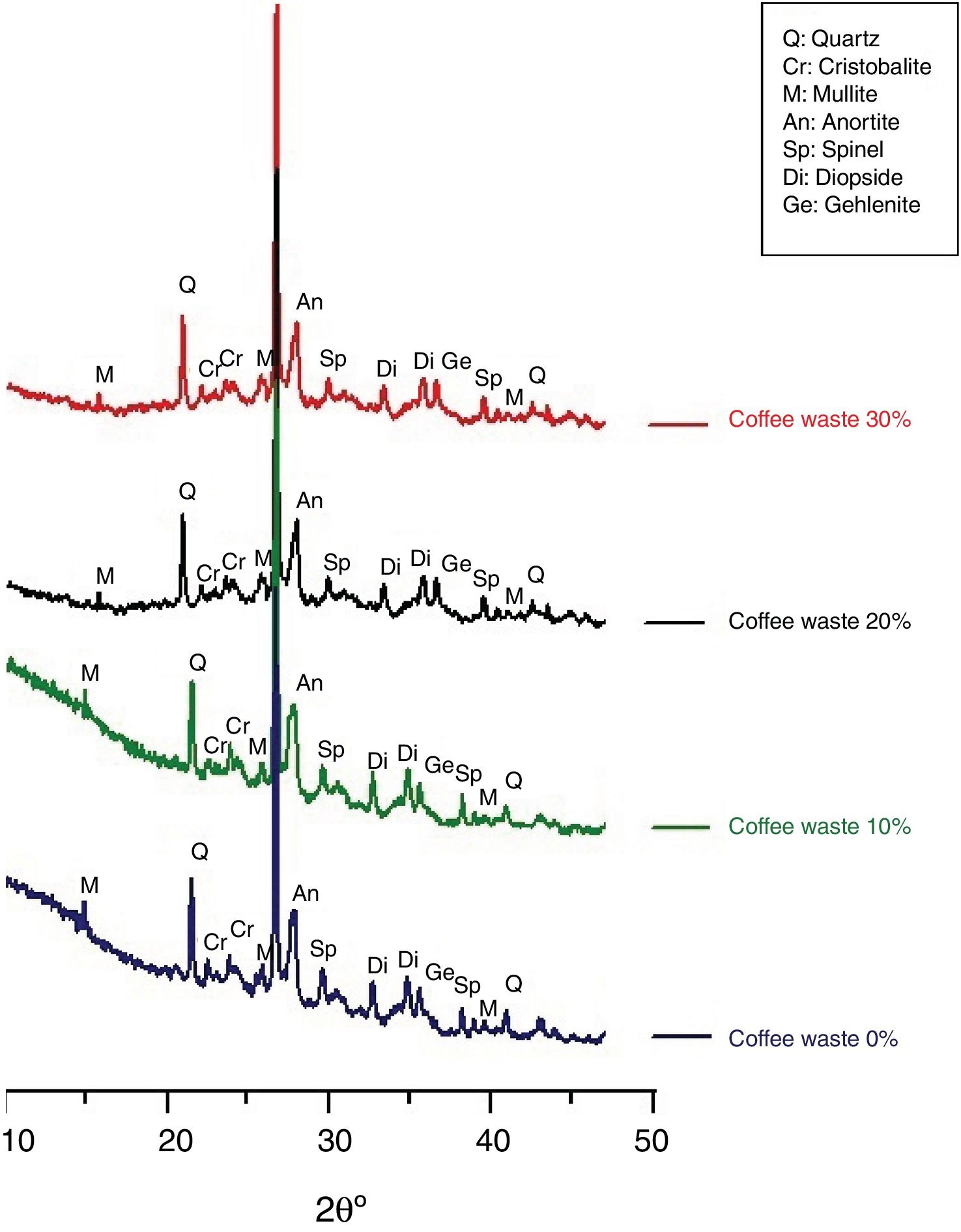
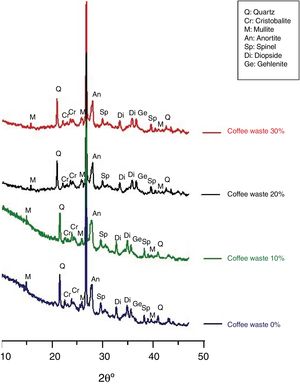
Characterization of ceramicsThe X-ray diffractogramme of the sample (clay and coffee waste) after firing at 1150°C for 2.5h is given in Fig. 8. Quartz was the only minerals that resisted up to 1150°C. New phases such as diopsite, gehlinite, cristobalite, spinel and mullite appeared. These minerals began to crystallize after the destruction of silicate (chlorite, kaolinite, illite), k-feldspar, calcite and dolomite, initially present in the clay and continued to increase until 1150°C. Then, calcite and dolomite decomposed to (CaO) and then will react with other components (sheet silicate decomposed) to generate neoformed calcium minerals (Ca-silicates), such as gehlenite Ca2Al[AlSiO7] and diopsite CaMgSi2O6. Moreover, the feldspar (plagioclase) reflections appear higher than in the raw material, suggesting the formation of anortite (CaAl2Si2O8) caused by the multistage solid state reactions between the clay matrix and carbonate. The cristobalite appeared in the firing products of clay with large amounts of quartz. Also spinel MgAl2O4 is considered as the result of the multistage solid state reactions between the clay matrix and carbonate (dolomite). MgO reacted with Al forming Mg–Al. The mullite appeared at high temperature, which could be related to the presence of sufficient illite. After the destruction of illite by hydroxylation, they reacted with quartz, and modified into mullite [8,9].
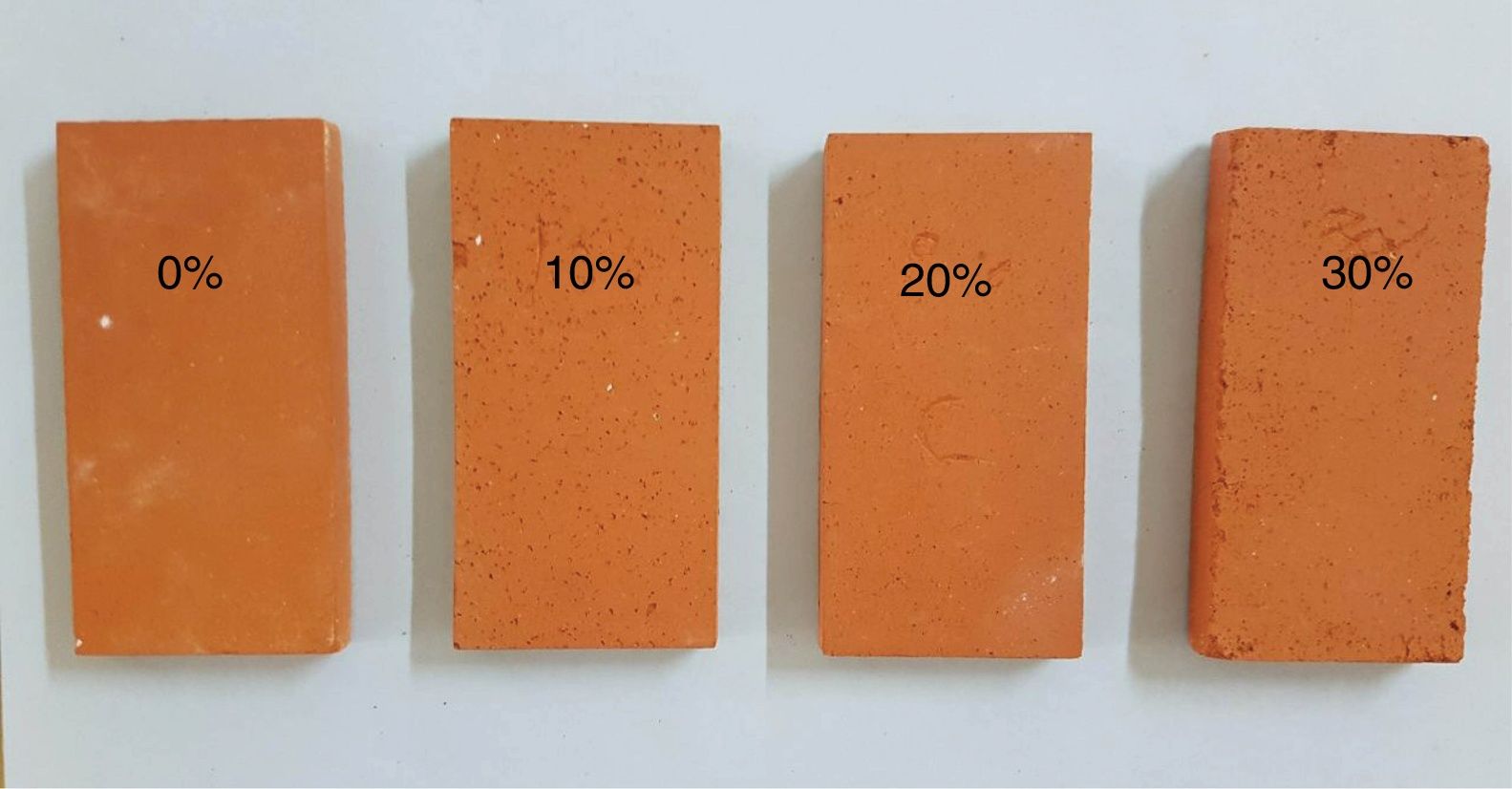
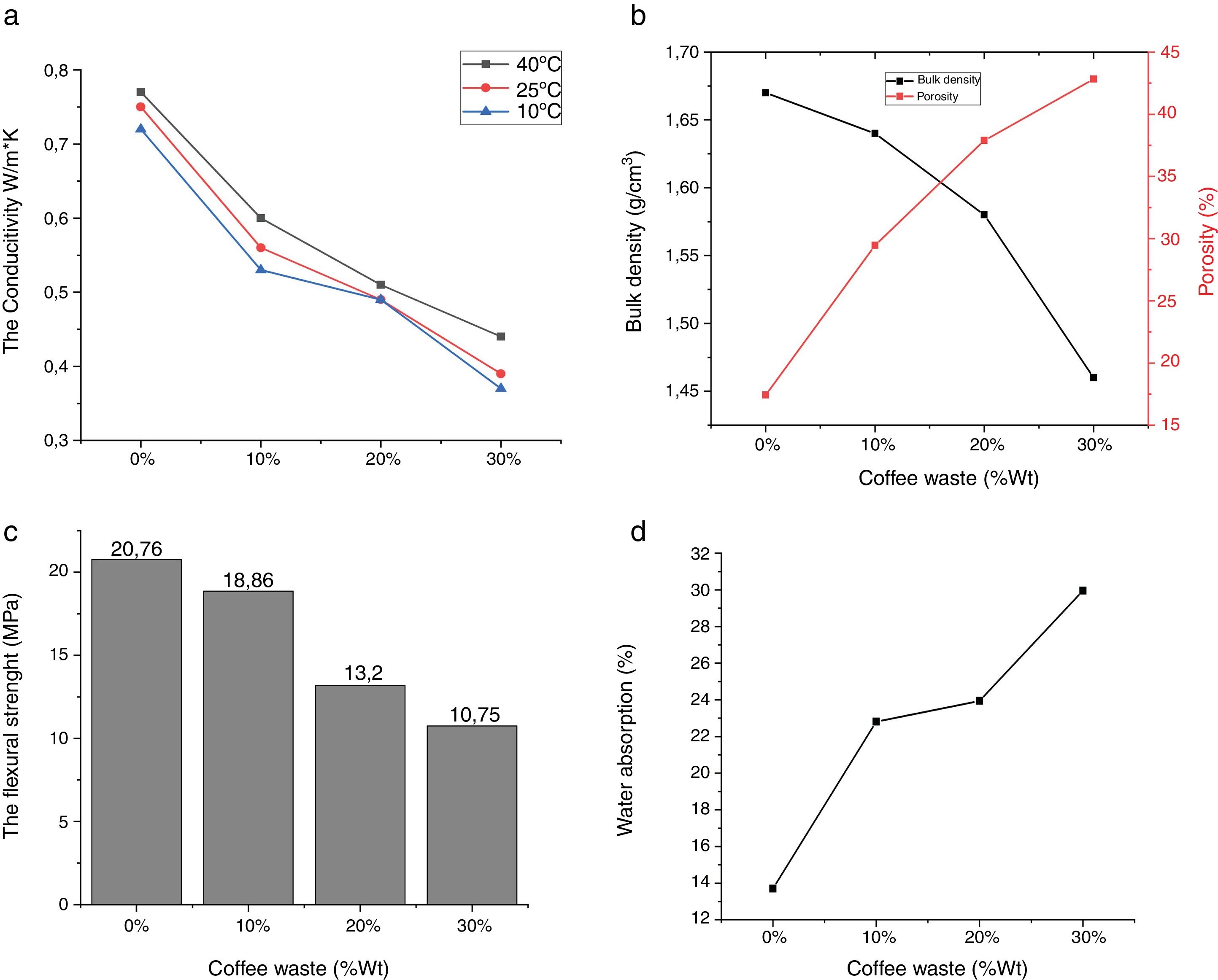
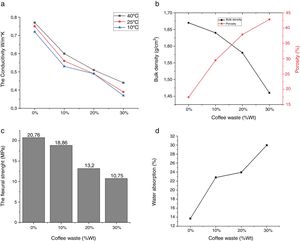
Experimental results of the samples with coffee waste additives are given in Fig. 9. It was observed that the color of the fired samples does not change with increasing the amount of coffee waste addition. The apparent porosity values of fired samples ranged from 17.43 to 42.81%. Bulk density of the product with 0% and 30% of coffee waste showed a reduction of 12.57%. Ceramic tiles are classified by the ISO 13006 technical standard on the basis of water absorption. The addition of coffee wastes gives a porous ceramics (Class BIII, B for pressing) [10]. This porosity is extremely helpful in reducing thermal conductivity of the sample by resisting convective heat transport. Movement of air is restricted in this case as opposed to be a completely vacant closed pore in which heat transfer would be freely carried out by air molecules [33]. The thermal conductivity values indicated a decrease of up to 53.42% which is encouraging for higher energy saving potential in residential applications. It can clearly be seen that thermal conductivities of the samples are closely related with their densities and porosities. The relatively good mechanical properties of ceramic products without coffee waste are essentially due to shaping direction of the sample, the shape of the pores and the crystalline/vitreous phase ration. The presence of a crystalline phase (mullite and spinel) in the ceramic matrix provides the piece with a good fired mechanical strength. Depending on the increase in the coffee waste addition and porosity content, the resistance values of the samples progressively decreased from 20.76 to 10.75MPa.
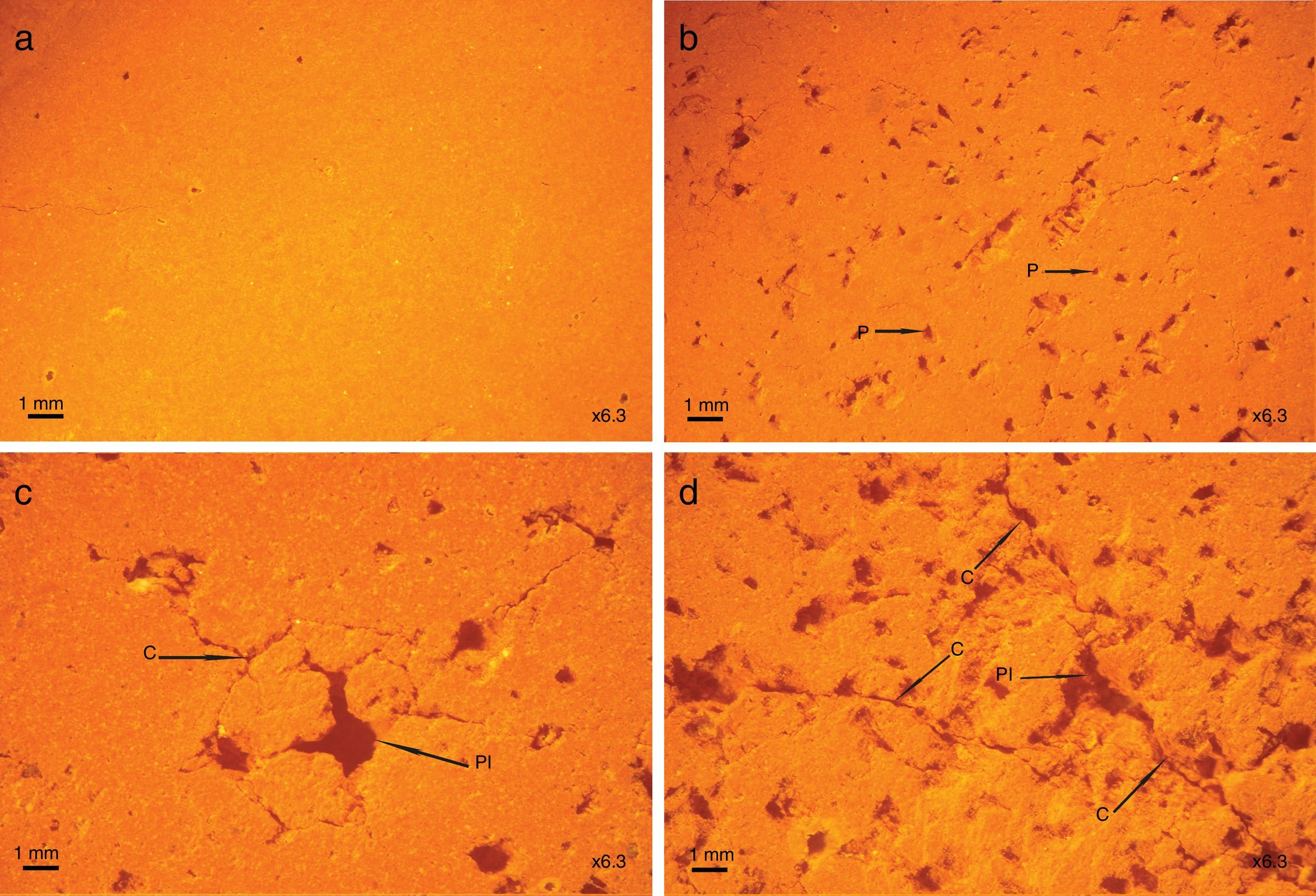
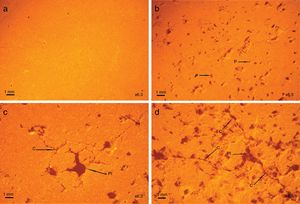
Fig. 10 shows the evolution of the porosity and cracks as a function of coffee waste content. The briquette containing 0% of coffee waste (Fig. 10a) has a homogeneous and dense structure. The addition of 10% of waste (Fig. 10b) makes it possible to obtain a porosity whose size is between 0.1 and 0.2mm. At 20% of waste (Fig. 10c), the pore size reaches 1mm. The pore size increases with the addition of waste. The microstructural analysis for the sample containing 30% of coffee waste (Fig. 10d) revealed a higher porosity due to the burning of organic matter. Calcite and dolomite rich particles presents in the raw clay also contributed to a less extent to the formation of the porous structure upon thermal treatment due to the loss of CO2. These CaO-rich particles in sample have a porous structure [33]. As it can be seen, largest pores about 1–2mm, co-exist with micro-pores less than 0.1mm. The pores are often isolated and have globular shape. The cracks are related to the increase of the interconnected pores between them.
ConclusionFrom the results presented above the following conclusions can be drawn. During firing up to 1150°C, the bodies undergo a complex series of physical and chemical reactions, involving: dehydroxylation of clay minerals, phase transformations and partial melting with formation of glassy viscous phase. These processes play an important role in the densification of the sintered specimens. As predicted, the coffee waste used as alternative raw material resources for the production of porous ceramics, strongly effects the microstructure, porosity and properties of fired products.
- -
The water absorption data (> 10%) showed that the elaborate ceramics belong to class BIII.
- -
The use of coffee waste increases the porosity, water absorption and decreases the bulk density, flexural strength and thermal conductivity.
- -
The rate of 30% seems to be optimal because it provides a highly porous material (P=42.81%, d=1.46g/cm3). The addition of more than 30% of coffee waste gives, after firing, ceramics characterized by a low flexural strength.
- -
Good dimensional stability obtained after firing at 1150°C.
- -
The ceramic materials contain mullite, quartz, diopside, spinel and anorthite.
This study confirms the fundamental role that research and development (R & D) can play in ceramics companies in improving quality and reducing the cost of production of their finished products.
The authors acknowledge support from National Center for Scientific and Technical Research (CNRST) and Hassan II Academy for Science and Technique (Project V2GV). Authors would like to thank “Analysis and Characterization Platform (PAC), faculty of science of Rabat” for supplying the DRX and TDA/TGA analysis.