The decoration of ceramic tiles “ink-jet revolution” has brought significant advantages to tiles manufacturing, but the explosive diffusion, until 4–5 years ago, of this technique has not been accomplished by an adequate scientific research. Among all the features that have to be studied yet, one of the most important is the influence of the new generation inks on the total emission at ceramic chimneys. In this paper, we present a first characterization of emissions from a set of commercial inks and vehicles: they were collected by propter firing of samples in an electric kiln and analyzed by GC–MS. This study is part of a larger research project, which includes the chemical characterization and the evaluation of thermal behavior of inks and vehicles by TG-DTA and other techniques. The obtained results permit to divide common vehicles into 3 classes, depending on their thermal behavior and emissions pattern. Inks, most of which present an ester based formulation, sometimes mixed with glycols or paraffins, follow the behavior of their single components. The most common formulation leads to the presence in the emission pattern of evaporation products (2-ethylhexyl esters of lauric, myristic, decanoic and octanoic acids) and decomposition products (mainly aldehydes and 2-ethyl-1-hexanol).
La decoración de los azulejos cerámicos mediante tecnología Ink-jet ha supuesto numerosas ventajas en la producción de baldosas cerámicas, pero su rápida implantación no ha sido acompañada por una adecuada investigación científica, hasta hace 4–5 años. Entre todas las características que deberían ser estudiadas, una de las más importantes es la influencia de las nuevas tintas digitales en las emisiones totales de las chimeneas cerámicas. En este artículo presentamos una primera caracterización de las emisiones de un grupo de tintas y vehículos comerciales: las emisiones se recogieron durante la cocción en laboratorio y fueron analizadas por GC-MS. Este estudio es parte de una investigación más completa, que incluye la caracterización química y la evaluación del comportamiento térmico de tintas y vehículos por TG-DTA y otras técnicas. Los resultados permiten la clasificación de los vehículos más comunes en 3 clases, dependiendo de su comportamiento térmico y emisiones. La mayoría de las tintas que presentan una formulación a base de ésteres, algunas veces mezclados con glicoles o parafinas, siguen la conducta de cada uno de sus componentes. Las formulaciones más comunes presentan productos de evaporación (2-etilhexil ésteres de ácidos láurico, mirístico, decanoico y octanoico) y productos de descomposición (principalmente aldehídos y 2-etil-1-hexanol).
From 2001, the ink-jet revolution in decoration technologies for ceramic tiles has started and nowadays it has become the most widespread technique [1].
This incredible success is due to its well-known pros, in comparison with traditional methods such rotary serigraphy and Rotocolor® systems: almost unlimited possibilities of decoration, reduced hazards of mechanical stress on unfired tiles, non-flat and expanded surfaces are now decorable, easier storage of decoration models, etc.
Before its introduction in ceramic industry, ink-jet technology had been already applied to other industrial applications (microchips, home printers, LCD and plasma screens, etc.).
Among the different methods of jetting, decoration of tiles is based on drop-on-demand (DOD) technique, where a software controls the deposition of inks drop by drop [2,3].
Digital inks for ceramic decoration are composed, until now, mainly of two phases: an inorganic pigment and an organic liquid phase.
Pigments (20–45% of the total weight) are usually crystalline ground compounds (metal aluminates, silicates or oxides), characterized by thermal stability up to 1200–1250°C.
Pigment particles are kept in suspension by the liquid phase that must have also some specific rheological properties (high boiling point, low viscosity, etc.). This phase is divided in two main components: the vehicle (50–70% of the total weight) and the additives (5–10% of the total weight).
In spite of its success, ink-jet decoration has also some cons. For example, inks must have some restricted rheological properties to be jettable (diameter of pigment particles below 1μm, surface tension between 20 and 45mN/m, etc.) [4], at the same time, granulometry of pigments has to guarantee precise chromatic performances and avoid, as much as possible, sedimentation, which is generally more problematic to be overcome than in traditional inks.
As ink-jet decoration is a quite recent innovation for ceramic industry and according to the continuous and fast developments of this technique, until now scientific researches were focused just on few points, mainly related to rheological performances of inks [5,6].
For example, only during the last 2 years some studies on the chemical characterization of inks were proposed [7].
One of the most important aspect that still lacks a proper knowledge and comprehension, is how new digital ceramic inks contribute to emissions at chimneys, when they go through the firing processes of ceramic tiles. This problem has not to be underestimated because legislation regarding this matter is quite restrictive, at least in Italy, and understanding how new materials interact to modify gaseous emissions is fundamental to prevent and control the emission of potentially hazardous compounds.
Ceramic district of Sassuolo (Modena, Italy), for example, is under the control of ARPA Emilia-Romagna regulation that fixes specific limits for VOCs and aldehydes in emissions of ceramic industry [8].
Recently, Italian Confindustria Ceramica in collaboration with Centro Ceramico has published a report on the performances and environmental impact of producers of ceramic tiles between 2010 and 2013 [9]. This document underlines the increasing of VOCs in the period under consideration. Aldehydes, for example, have registered a significant increase in 2012 in complete manufacturing cycle, followed by a reduction in 2013, while in partial manufacturing cycles this value was still increasing in 2013. These are symptoms of changes in the ceramic industry, which may be linked to the introduction of new materials by inkjet technology.
Regardless of their growth, ARPA did not reported values higher than VOCs law limits, but some of these compounds (not fully identified yet) could be potentially irritating for throat, eyes or skin [10].
It is also necessary to take in consideration the particular conformation of ceramic kilns, which operate in counter-current [11]. This set-up implicates for tiles, with just printed inks on their surface, a thermal shock to even 500°C while entering in the pre-kiln. These temperatures are obtained thanks the hot air coming from the firing sectors, which passes above tiles surface and goes directly toward the chimney. Inks can, so, have a combustive reaction and their products can be removed from tile surface and interact with molecules transported by the air flow, or inks can simply evaporate to the chimney.
In this paper, we present first results of our study on the characterization of gaseous emissions from a set of commercial inks and vehicles, submitted, in Lab., to a slow or industrial-like fast firing. This study is part of a wider research that is collecting data on thermal behavior, chemical characterization and, of course, potentially polluting emissions of inks and common vehicles, to improve knowledge and comprehension of these materials.
Materials and methodsSamples and analytical procedureWe collected 55 inks (named I1, I2, …, I55) and 8 common vehicles (named as in Table 1) from inks suppliers in the ceramic district of Sassuolo (Modena, Italy). For the most of inks, no information was given about their composition. Vehicles are commercial products, with potentially low degree of purity.
Each sample was first characterized by infrared spectroscopy (FTIR) and thermogravimetric analysis coupled with differential thermal analysis (TG-DTA), in order to understand at least the class of the main organic compounds and their thermal behavior. The description of FTIR and TG-DTA procedures, as well as results of these two preliminary steps for vehicles and for the first 39 inks, have already been published [12].
Then, to study polluting products, samples have been fired in an electric tubular kiln and their gaseous emissions have been collected by Tenax TA tubes and analyzed in a GC–MS equipment.
Vehicles have been also characterized by direct injection in GC–MS to verify their purity, but results will not be discussed in detail in this paper.
Sampling of emissionsA known quantity of sample (10mg of ink, or 5mg of vehicle) was loaded in a quartz holder and inserted in an electric tubular kiln (Carbolite STF 15/50/450), equipped with a Quartz tube of internal diameter of 1cm. Then, samples were heated with the following temperature program: from ambient temperature to 800°C in 15min, dwell at 800°C for 10min.
During the whole heating program, air was pumped through the kiln to a flow splitter with 4 exits, at the end of the kiln tube. Each exit can be equipped with a sampling tube or used as flow regulator. Each flow of air through tubes can be adjusted separately, for a total air flow of 1l/min.
For the first qualitative screening of VOCs, Tenax TA (Supelco 60/80, glass tube, ¼in. OD×4mm ID×7in.) has been selected to collect air samples, because of its property and affinity to a wide range of VOCs [13–16]. Air through Tenax tube was set to 200ml/min. Tenax tubes were immediately analyzed with the next steps, or stored in closed boxes, avoiding light interaction, until their analysis.
Desorption and GC–MS analysisOne of the most useful characteristic of Tenax is its desorption procedure. After sampling, tubes were desorbed in a thermal desorption system (TDS), molecules were cryo-focused at −140°C with a cooled injection system (CIS) and, then, were sent to a gas chromatograph coupled with a mass spectrometer. Desorption and separation parameters have been selected on the basis of literature [16–18] and previous tests.
TDS starting temperature was set at 40°C, after 0.50min temperature was raised to 270°C at 60°C/min and maintained for 10min. Desorption was carried on with helium at 40ml/min, in splitless mode.
CIS was kept at −140°C with liquid nitrogen for all the desorption step, then temperature was rapidly raised to 280°C at 12°C/s and maintained for 2min.
Inlet was set to solvent vent mode, purged at 900ml/min after 0.40min and kept at 250°C.
Desorbed molecules were then directed to the GC–MS (Agilent 6890N), equipped with a Restek RXi-1MS column (60m×250μm×1μm). Oven initial temperature was kept at 35°C for 4min, then raised to 150°C at 3°C/min, and finally to 270°C at 8°C/min. The final temperature was maintained for 20min. Carrier gas was helium at 1ml/min.
MS operated in scan mode, in the range 15–450 m/z.
Results and discussionFor the extensive results of FTIR and TG-DTA, we refer to our previous paper [12]. On the basis of FTIR data, we divided our samples in 6 general groups, depending on the main classes of compounds identified and on the comparison to vehicles data. This classification has been revised and corrected after the emissions study and the new inks samples have been added (Table 2).
Classification of inks by FTIR and comparison with vehicles.
| Main vehicle class | Inks |
|---|---|
| Esters | I1, I3, I4, I5, I6, I8, I11, I12, I13, I14, I16, I18, I19, I20, I21, I22, I26, I27, I28, I29, I30, I31, I32, I33, I34, I35, I36, I37, I38, I39, I43, I44, I51, I53 |
| Glycols | I23, I41, I42, I52 |
| Paraffins | I2, I7, I10, I15 |
| Glycols+esters | I9, I50, I54, I55 |
| Paraffins+esters | I24, I25 |
| Water+glycols | I17, I40, I45, I46, I47, I48, I49 |
Referring to the emissions of vehicles, all the identified compounds are listed in Table 3.
Identified compounds in vehicles emissions.
| Compounds | 2-EHS | EHC | CCT | 2-EHL | EHP | TPM | TPnB | PRF1 | PRF2 |
|---|---|---|---|---|---|---|---|---|---|
| Aldehydes | |||||||||
| Butanal | X | X | X | ||||||
| Pentanal | X | X | X | X | X | ||||
| Hexanal | X | X | X | X | X | X | |||
| Heptanal | X | X | X | X | |||||
| Hexanal, 2-ethyl- | X | X | X | ||||||
| 2-Hexanal, 2-ethyl- | X | ||||||||
| Octanal | X | X | X | X | X | ||||
| Nonanal | X | X | X | X | |||||
| Decanal | X | X | X | ||||||
| Undecanal | X | ||||||||
| Dodecanal | X | ||||||||
| Tridecanal | X | X | |||||||
| 2-decenal, (E)- | X | ||||||||
| Ketones | |||||||||
| 2-Pentanone | X | X | |||||||
| 2-Hexanone | X | X | |||||||
| 3-Hexanone, 5-methyl- | X | ||||||||
| 2-Heptanone | X | ||||||||
| 3-Heptanone | X | ||||||||
| 2-Octanone | X | X | X | X | |||||
| 2-Nonanone | X | X | X | X | |||||
| 2-Decanone | X | X | X | ||||||
| 2-Undecanone | X | X | |||||||
| 2-Dodecanone | X | X | |||||||
| 2-Tridecanone | X | ||||||||
| Glycols and glycol ethers | |||||||||
| Tripropylene glycol monomethyl ether (isomers) | X | X | X | X | |||||
| 2-Propanol, 1-[1-methyl-2-(2-propenyloxy)ethoxy]- | X | ||||||||
| Ethanol, 2-(1,1-dimethylethoxy)- | X | ||||||||
| Hexaethylene glycol dimethyl ether | X | ||||||||
| Tri(1,2-propyleneglycol), monomethyl ether | X | ||||||||
| Propylene glycol monobutyl ether | X | ||||||||
| Dipropylene glycol (isomers) | X | ||||||||
| Tripropylene glycol n-butyl ether | X | X | X | X | |||||
| Organic acids | |||||||||
| Octanoic acid | X | ||||||||
| Nonanoic acid | X | X | X | ||||||
| n-Decanoic acid | X | X | |||||||
| Undecanoic acid | X | ||||||||
| Dodecanoic acid | X | X | |||||||
| n-Hexadecanoic acid | X | ||||||||
| Alcohols | |||||||||
| 1-Hexanol, 2-ethyl- | X | X | X | X | |||||
| Propanoic acid, 2,2-dimethyl- | X | ||||||||
| Dioxolanes | |||||||||
| 1,3-Dioxolane, 2-ethyl-4-methyl- | X | ||||||||
| Linear alkanes | |||||||||
| Hexane | X | X | X | X | |||||
| Heptane | X | X | X | X | |||||
| Octane | X | X | |||||||
| Nonane | X | X | |||||||
| Decane | X | X | |||||||
| Undecane | X | ||||||||
| Dodecane | X | X | X | ||||||
| Tridecane | X | ||||||||
| Tetradecane | X | X | X | X | |||||
| Pentadecane | X | X | X | X | |||||
| Hexadecane | X | X | |||||||
| Heptadecane | X | X | |||||||
| Octadecane | X | ||||||||
| Nonadecane | X | ||||||||
| Eicosane | X | ||||||||
| Branched alkanes | |||||||||
| Heptane, 3-methyl- | X | ||||||||
| Nonane, 2-methyl-5-propyl- | X | ||||||||
| Dodecane, 3-methyl- | X | ||||||||
| Dodecane, 2,6,10-trimethyl- | X | X | |||||||
| Dodecane, 2,7,10-trimethyl- | X | ||||||||
| Dodecane, 2,2,11,11-tetramethyl- | X | ||||||||
| Tetradecane, 2,6,10-trimethyl- | X | ||||||||
| Pentadecane, 2-methyl- | X | ||||||||
| Pentadecane, 2,6,10-trimethyl- | X | ||||||||
| Pentadecane, 2,6,10,14-tetramethyl- | X | ||||||||
| Hexadecane, 2-methyl- | X | ||||||||
| Hexadecane, 3-methyl- | X | ||||||||
| Hexadecane, 4-methyl- | X | ||||||||
| Hexadecane, 2,6,10-trimethyl- | X | ||||||||
| Hexadecane, 2,6,10,14-tetramethyl- | X | X | |||||||
| Heptadecane, 2-methyl- | X | ||||||||
| Heptadecane, 4-methyl- | X | ||||||||
| Heptadecane, 2,3-dimethyl- | X | ||||||||
| Octadecane, 3-methyl- | X | ||||||||
| Nonadecane, 9-methyl- | X | ||||||||
| Cyclic alkanes | |||||||||
| Cyclohexane | X | ||||||||
| Lactones | |||||||||
| Butyrolactone | X | ||||||||
| γ-Heptalactone | X | ||||||||
| γ-Octalactone | X | ||||||||
| δ-Octalactone | X | ||||||||
| γ-Decalactone | X | ||||||||
| δ-Decalactone | X | ||||||||
| γ-Undecalactone | X | ||||||||
| Alkenes | |||||||||
| Heptane, 3-methylene- | X | X | X | X | |||||
| 1-Nonadecene | X | ||||||||
| Fatty acids esters | |||||||||
| Acetic acid, 2-ethylhexyl ester | X | ||||||||
| n-Butyric acid 2-ethylhexyl ester | X | X | |||||||
| Pentanoic acid, 2-ethylhexyl ester | X | X | |||||||
| Hexanoic acid, 2-ethylhexyl ester | X | X | |||||||
| Octanoic acid, 2-ethylhexyl ester | X | X | X | X | |||||
| Decanoic acid, 2-ethylhexyl ester | X | X | |||||||
| Lauric acid, 2-ethylhexyl ester | X | X | X | X | X | ||||
| Myristic acid, 2-ethylhexyl ester | X | X | |||||||
| Palmitic acid, 2-ethylhexyl ester | X | ||||||||
| Hexanedioic acid, bis(2-ethylhexyl) ester | X | ||||||||
| Octanoic acid, methyl ester | X | ||||||||
| Decanoic acid, methyl ester | X | ||||||||
| Octanoic acid, 2-propenyl ester | X | ||||||||
| Butyl formate | X | ||||||||
| Butyl caprate | X | ||||||||
| Butyl caprylate | X | ||||||||
| Butyl palmitate | X | ||||||||
| n-Capric acid isopropyl ester | X | ||||||||
| Myristic acid, isopropyl (o 1-methylethyl) ester | X | ||||||||
| Palmitic acid, isopropyl (o 1-methylethyl) ester | X | ||||||||
| Stearic acid, isopropyl (o 1-methylethyl) ester | X | ||||||||
| Lauric acid, 1-methylethyl ester | X | X | X | X | X | ||||
| Elaidic acid, 1-methylethyl ester (o isopropyl) | X | ||||||||
| Heptanoic acid, 4-octyl ester | X | ||||||||
| Propanoic acid, 2-methyl-, 1-(1,1-dimethylethyl)-2-methyl-1,3-propanediyl ester | X | X | |||||||
| Glycerol tricaprylate | X | ||||||||
According to their thermal behavior, PRF1 and PRF2 are characterized mainly by evaporation products. Unfortunately, hydrocarbons peaks are often not well separable, so, especially in PRF1 the univocal identification of peaks has been difficult and, for most of the peaks, impossible. Anyway, the MS pattern of some branched alkanes can be recognized between principal compounds, among which 2,6,10,14-tetramethyl-pentadecane is identifiable, for example.
PRF2 emissions are characterized by the same mixture of linear and branched alkanes (with chains from C12 to C20) found by direct injection.
In TPM emissions, we can recognize evaporation products as well (tripropylene glycol monomethyl ether), with some impurities, due to the not high purity of the commercial product and to residues in the GC column.
TPnB emissions seems to confirm the thermal behavior recognized by TG-DTA, with a weak exothermic peak after the main endothermic phenomenon. Its main emission is tripropylene glycol n-butyl ether, indeed, but some decomposition compounds as dipropylene glycol, tripropylene glycol monomethyl ether or unsaturated glycols are present [19–21].
Same considerations can be done for 2-EHL. The analysis of the liquid vehicle revealed that the main compound was not 2-ethylhexyl laurate, as it has been claimed, but 1-methylethyl laurate (1-MEL). The same ester is the main emission product, due to evaporation. Secondary components, as lauric acid, comes from the decomposition of 1-MEL, while 1-methylethyl esters of heavier fatty acids (C14–C18) could be impurities.
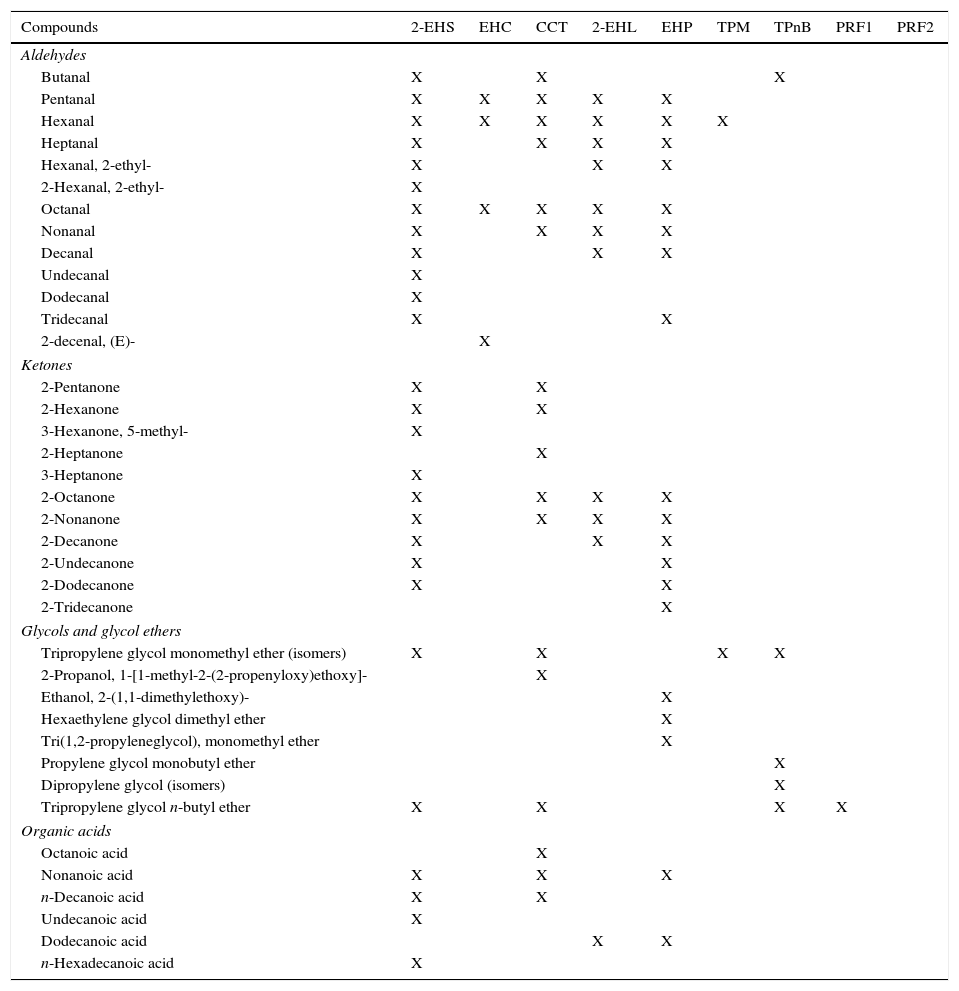
EHC is a mixture of 2-ethylhexyl esters of C8, C10, C12, C14, C16 fatty acids, that are present as evaporation products in EHC emissions. The fast firing of EHC causes also the decomposition of some molecules into aldehydes (pentanal, hexanal, octanal, nonanal), 2-ethyl-1-hexanol and other light compounds.
Sample 2-EHS has been revealed to be not pure 2-ethylhexyl stearate, but a mix of this ester with 2-ethylhexyl palmitate. The chromatogram of 2-EHS emissions highlights the predominance of decomposition phenomena (identified as exothermic events by TG-DTA) that 2-EHS meets with. The most abundant compound is 2-ethyl-1-hexanol, from the cracking of 2-ethylhexyl esters. Various aldehydes, organic acids, ketones and hydrocarbons complete the emission chart, underlining the uncomplete combustion of 2-EHS due to the removal of intermediate products from the reaction site by the air flow [22].
CCT is composed mainly of glycerol tricaprylate. We found it also in CCT emissions, but just as minor component. In fact, the chromatogram of CCT emissions presents numerous substances, coming from the exothermic decomposition of glycerol tricaprylate. Among these, aldehydes, ketones, fatty acids esters (especially, different esters of octanoic acid, not always perfectly identifiable), some glycol ethers, and lactones. Some studies, for example, have demonstrated that lactones form spontaneously from triglycerides, from the hydroxy-fatty acids liberated by heating [23]. Selke et al. identified the same classes of compounds as products of the thermal oxidation of tristearin [24] and triolein [25].
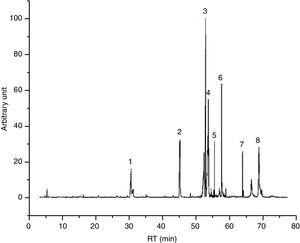
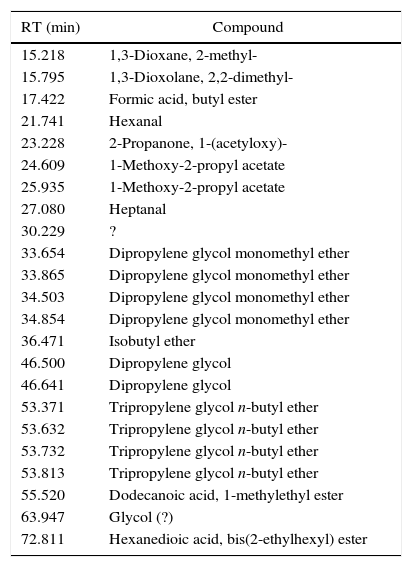
Emissions of inksGlycol based inks (4/55), like I23 (Fig. 1), are characterized mostly by evaporation products, tripropylene glycol monomethyl ether and tripropylene glycol n-butyl ether above all. We found also few aldehydes and dioxolanes, as well as some lighter glycol ethers, deriving from the decomposition of the main components. I23 DTA shows two events, indeed. The first one, endothermic, could be the evaporation of glycols; the second one, exothermic, could confirm the decomposition of evaporation products. In Table 4 all the identified compound in I23 emission are reported.
Identified compounds in I23 emissions.
| RT (min) | Compound |
|---|---|
| 15.218 | 1,3-Dioxane, 2-methyl- |
| 15.795 | 1,3-Dioxolane, 2,2-dimethyl- |
| 17.422 | Formic acid, butyl ester |
| 21.741 | Hexanal |
| 23.228 | 2-Propanone, 1-(acetyloxy)- |
| 24.609 | 1-Methoxy-2-propyl acetate |
| 25.935 | 1-Methoxy-2-propyl acetate |
| 27.080 | Heptanal |
| 30.229 | ? |
| 33.654 | Dipropylene glycol monomethyl ether |
| 33.865 | Dipropylene glycol monomethyl ether |
| 34.503 | Dipropylene glycol monomethyl ether |
| 34.854 | Dipropylene glycol monomethyl ether |
| 36.471 | Isobutyl ether |
| 46.500 | Dipropylene glycol |
| 46.641 | Dipropylene glycol |
| 53.371 | Tripropylene glycol n-butyl ether |
| 53.632 | Tripropylene glycol n-butyl ether |
| 53.732 | Tripropylene glycol n-butyl ether |
| 53.813 | Tripropylene glycol n-butyl ether |
| 55.520 | Dodecanoic acid, 1-methylethyl ester |
| 63.947 | Glycol (?) |
| 72.811 | Hexanedioic acid, bis(2-ethylhexyl) ester |
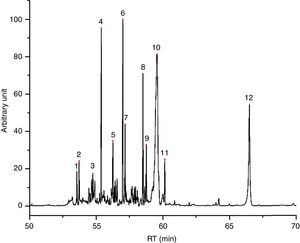
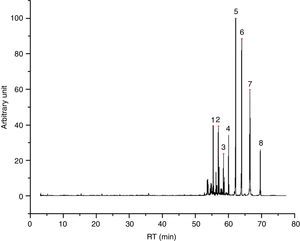
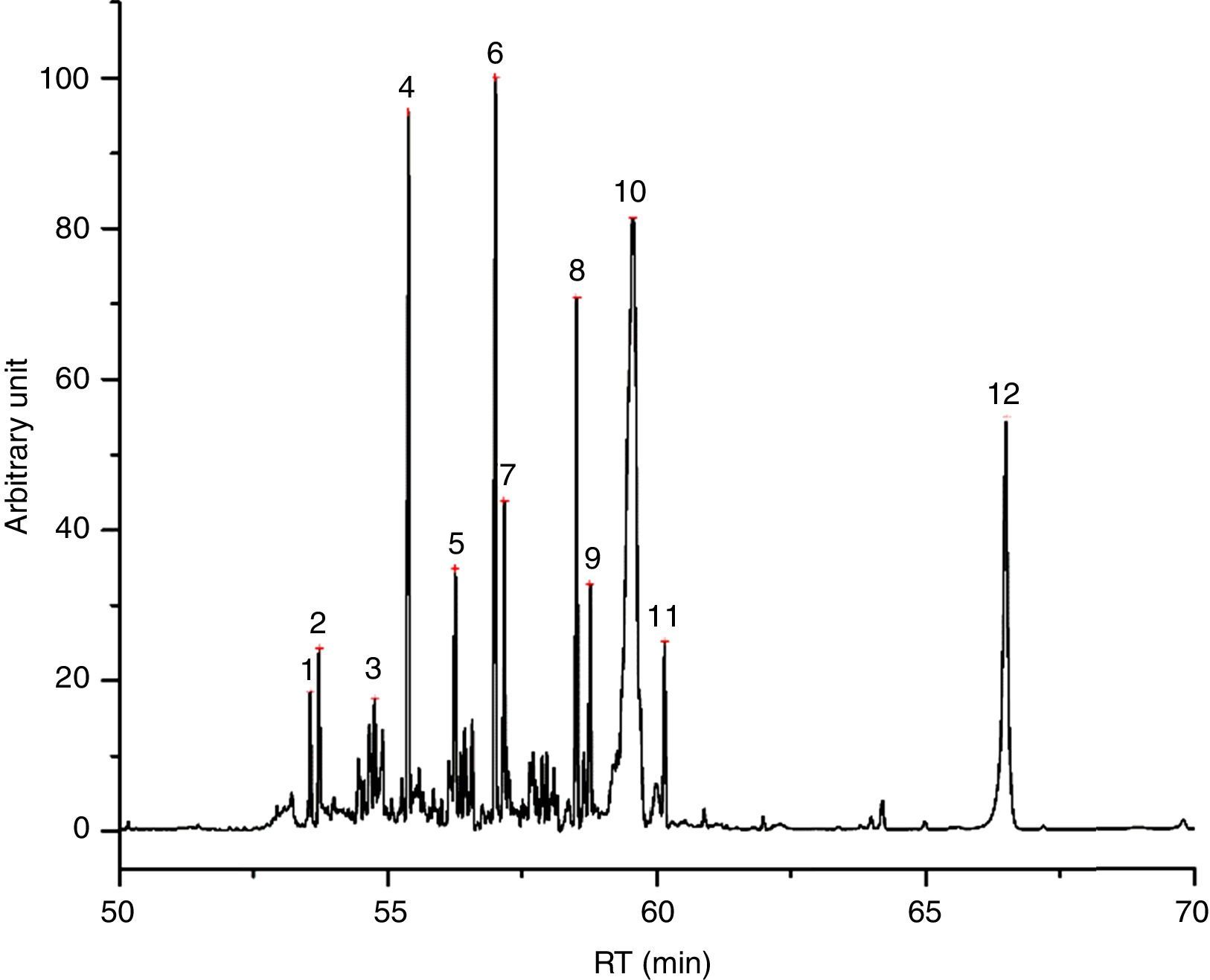
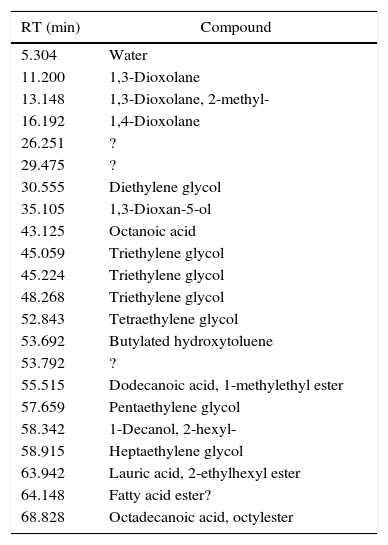
Water+glycol based inks (7/55), of which I49 is an example (Fig. 2), release less quantity of substances, being equal the weight in comparison with another type of ink. Their chromatograms display the evaporation products from the glycol part, which of course depend on the chosen glycol. I49, for instance, seems to be a mix of triethylene, tetraethylene and pentaethylene glycols, to which a fraction of esters is added. In the first part of the chromatogram, there are some weak peaks recognized as dioxolanes, therefore the same considerations already done for I23 are valid for I49 too. Table 5 shows the characterization of I49 emissions.
Identified compounds in I49 emissions.
| RT (min) | Compound |
|---|---|
| 5.304 | Water |
| 11.200 | 1,3-Dioxolane |
| 13.148 | 1,3-Dioxolane, 2-methyl- |
| 16.192 | 1,4-Dioxolane |
| 26.251 | ? |
| 29.475 | ? |
| 30.555 | Diethylene glycol |
| 35.105 | 1,3-Dioxan-5-ol |
| 43.125 | Octanoic acid |
| 45.059 | Triethylene glycol |
| 45.224 | Triethylene glycol |
| 48.268 | Triethylene glycol |
| 52.843 | Tetraethylene glycol |
| 53.692 | Butylated hydroxytoluene |
| 53.792 | ? |
| 55.515 | Dodecanoic acid, 1-methylethyl ester |
| 57.659 | Pentaethylene glycol |
| 58.342 | 1-Decanol, 2-hexyl- |
| 58.915 | Heptaethylene glycol |
| 63.942 | Lauric acid, 2-ethylhexyl ester |
| 64.148 | Fatty acid ester? |
| 68.828 | Octadecanoic acid, octylester |
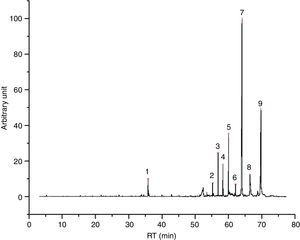
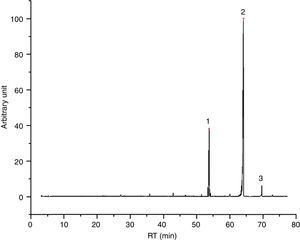
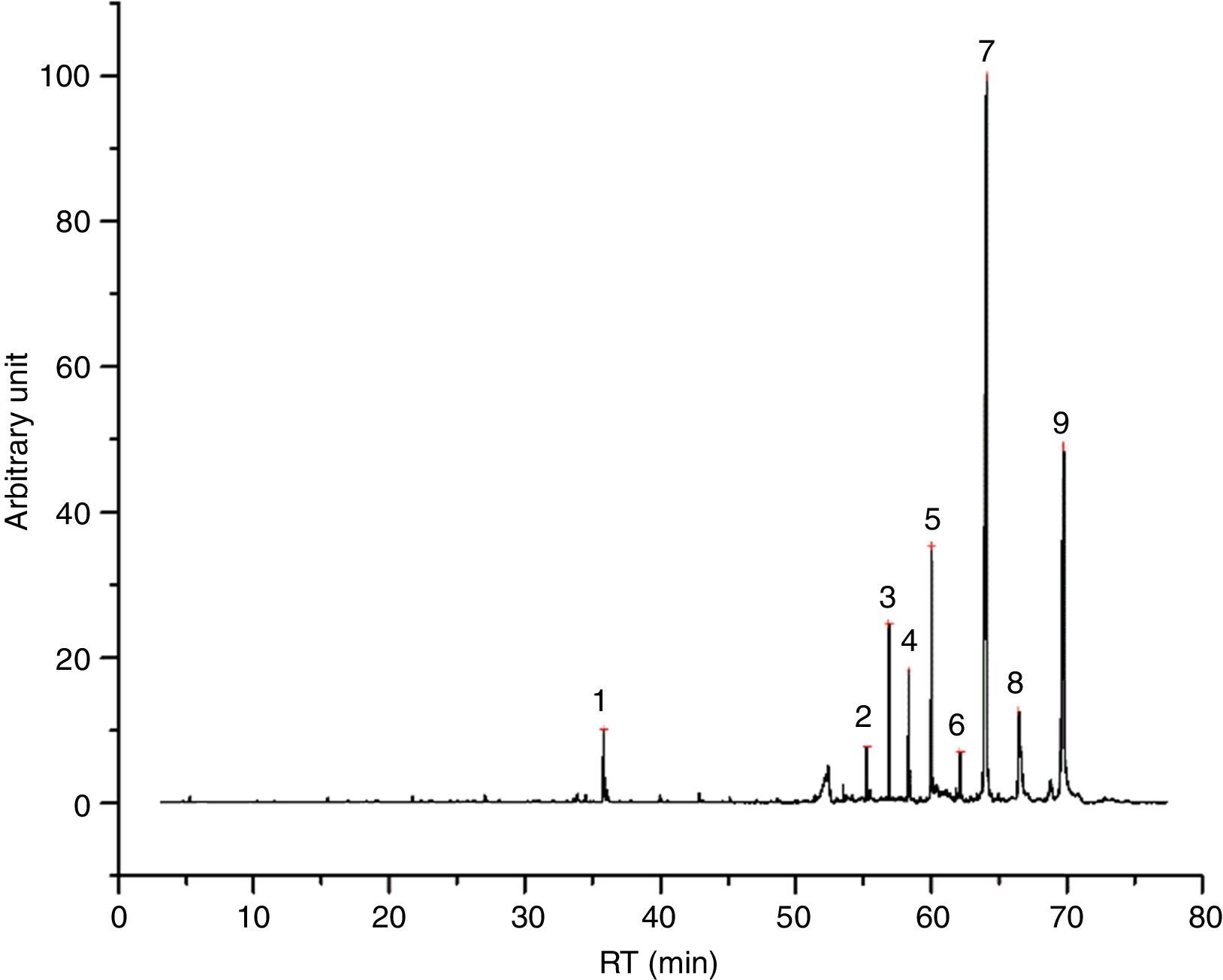
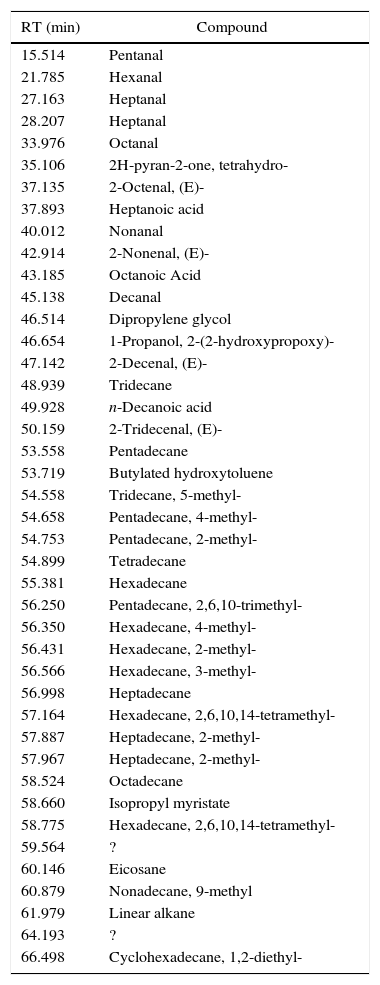
Also paraffin based inks (4/55), as I2 (Fig. 3) show mainly compounds coming from evaporation, confirming the behavior registered for PRF1 and PRF2. Their emissions are characterized by C14–C20 alkanes, linear or branched. All the inks of this group are also characterized by the presence of some light aldehydes and carboxylic acids (octanoic acid, above all), which may derive from a hybrid formulation between paraffins and one or more esters, as minor components (see Table 6 for details). I2 thermal behavior show one principal weight loss connected to an endothermic event, according to paraffins behavior.
I2 chromatogram – (1) pentadecane, (2) butylated hydroxytoluene, (3) 2-methyl-pentadecane, (4) hexadecane, (5) 2,6,10-trimethyl-pentadecane, (6) ?, (7) 2,6,10,14-tetramethyl-hexadecane, (8) octadecane, (9) 2,6,10,14-tetramethyl-hexadecane, (10) ?, (11) eicosane and (12) 1,2-diethyl-cyclohexadecane.
Identified compounds in I2 emissions.
| RT (min) | Compound |
|---|---|
| 15.514 | Pentanal |
| 21.785 | Hexanal |
| 27.163 | Heptanal |
| 28.207 | Heptanal |
| 33.976 | Octanal |
| 35.106 | 2H-pyran-2-one, tetrahydro- |
| 37.135 | 2-Octenal, (E)- |
| 37.893 | Heptanoic acid |
| 40.012 | Nonanal |
| 42.914 | 2-Nonenal, (E)- |
| 43.185 | Octanoic Acid |
| 45.138 | Decanal |
| 46.514 | Dipropylene glycol |
| 46.654 | 1-Propanol, 2-(2-hydroxypropoxy)- |
| 47.142 | 2-Decenal, (E)- |
| 48.939 | Tridecane |
| 49.928 | n-Decanoic acid |
| 50.159 | 2-Tridecenal, (E)- |
| 53.558 | Pentadecane |
| 53.719 | Butylated hydroxytoluene |
| 54.558 | Tridecane, 5-methyl- |
| 54.658 | Pentadecane, 4-methyl- |
| 54.753 | Pentadecane, 2-methyl- |
| 54.899 | Tetradecane |
| 55.381 | Hexadecane |
| 56.250 | Pentadecane, 2,6,10-trimethyl- |
| 56.350 | Hexadecane, 4-methyl- |
| 56.431 | Hexadecane, 2-methyl- |
| 56.566 | Hexadecane, 3-methyl- |
| 56.998 | Heptadecane |
| 57.164 | Hexadecane, 2,6,10,14-tetramethyl- |
| 57.887 | Heptadecane, 2-methyl- |
| 57.967 | Heptadecane, 2-methyl- |
| 58.524 | Octadecane |
| 58.660 | Isopropyl myristate |
| 58.775 | Hexadecane, 2,6,10,14-tetramethyl- |
| 59.564 | ? |
| 60.146 | Eicosane |
| 60.879 | Nonadecane, 9-methyl |
| 61.979 | Linear alkane |
| 64.193 | ? |
| 66.498 | Cyclohexadecane, 1,2-diethyl- |
Emissions of ester based inks (34/55), as well as their thermal behavior, depend on the nature of the ester or of the ester mixture.
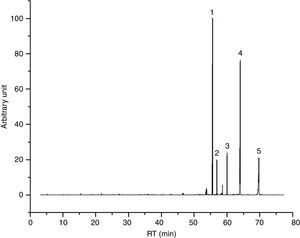
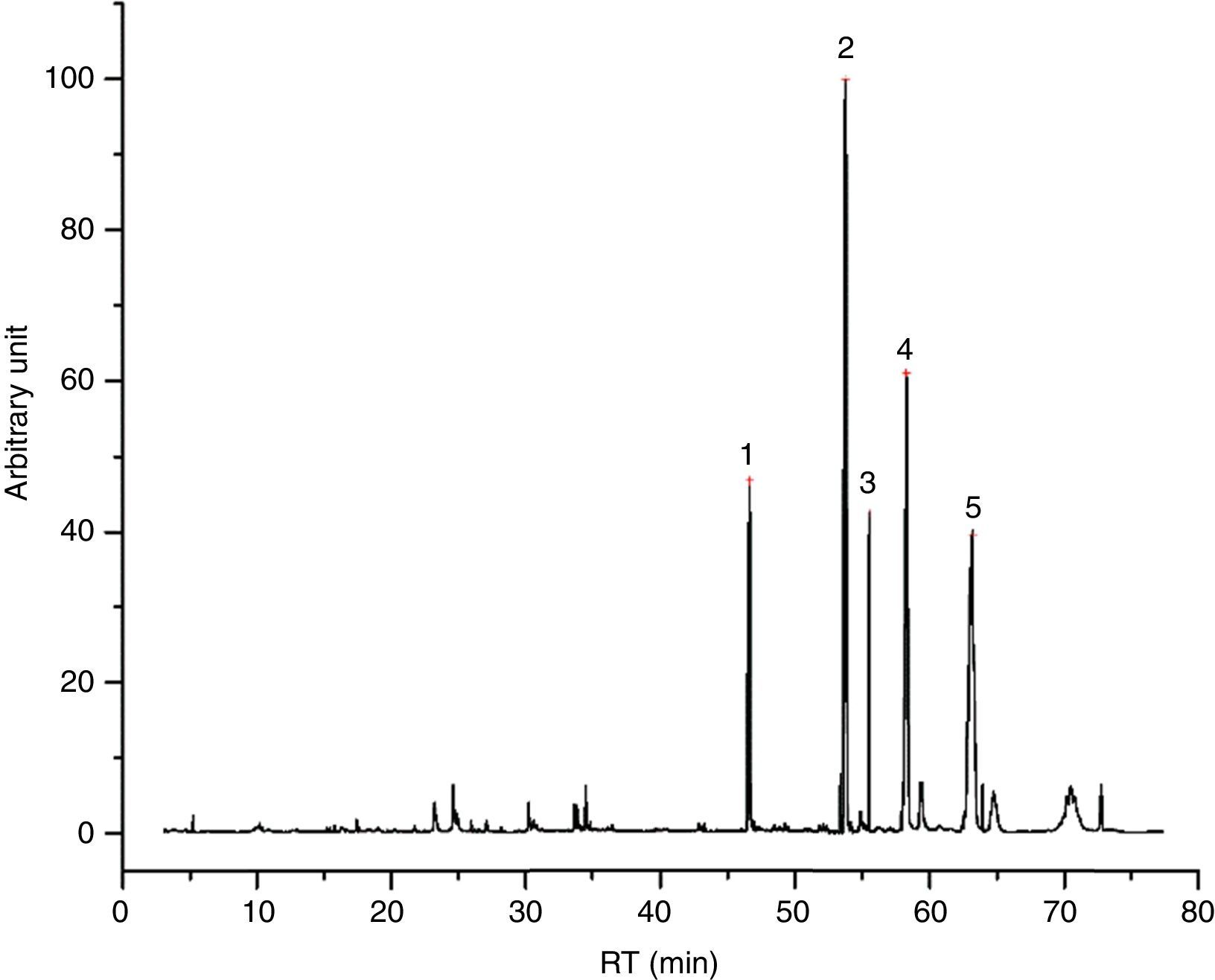
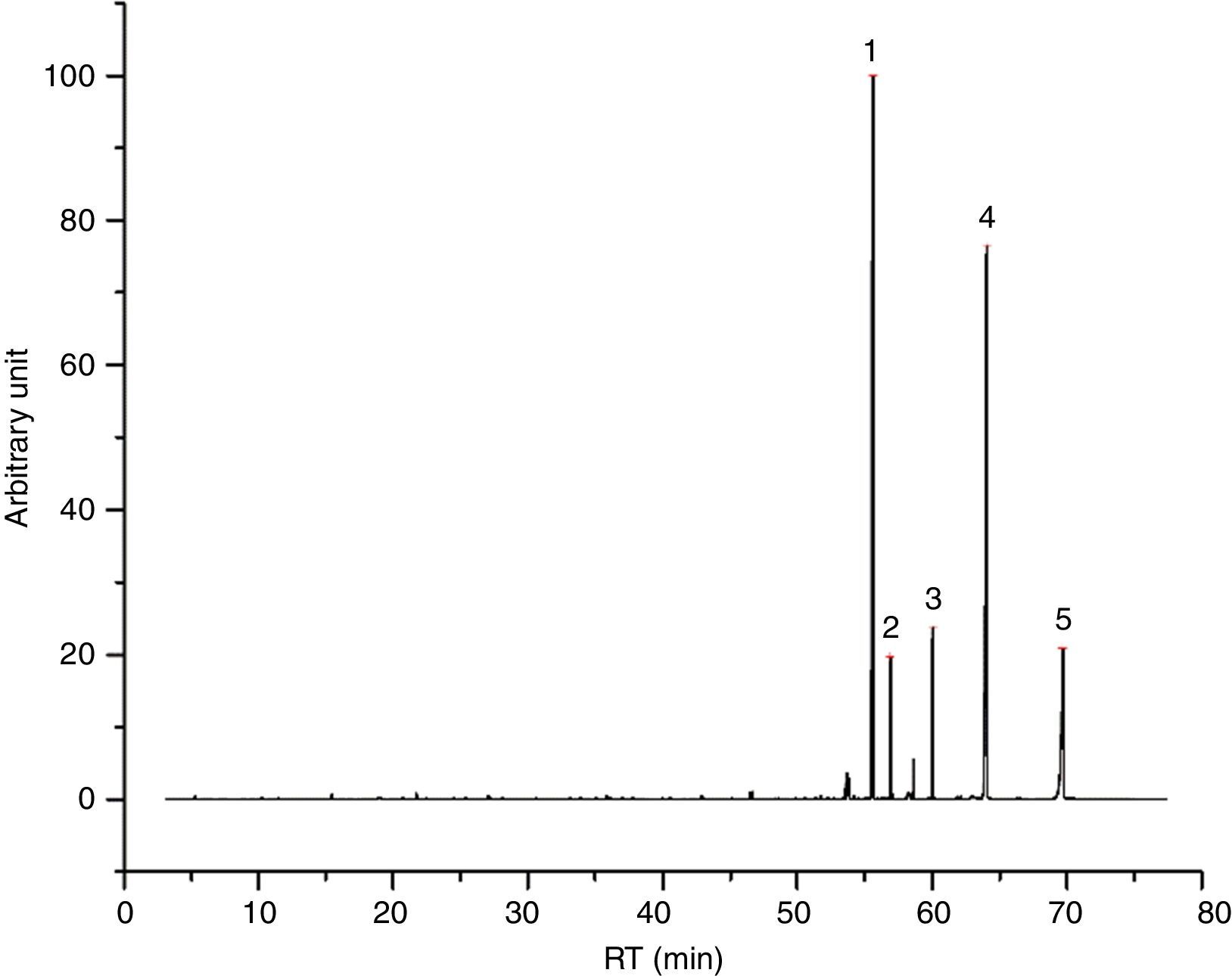
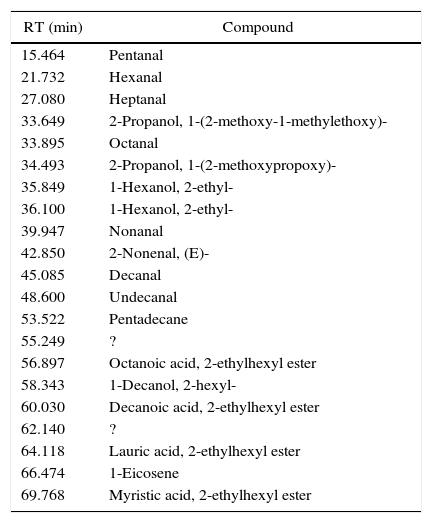
I33 (Fig. 4) is a good example for 2-ethylhexyl esters mixtures, as main vehicle fraction. In its chromatogram, 2-ethylhexyl laurate, myristate, decanoate and octanoate represent the main peaks, underlining evaporation phenomena. Together, we found few aldehydes and alcohols (especially 2-ethyl-1-hexanol) from the decomposition of these esters, potentially correlated to the exothermic event seen in DTA graph.
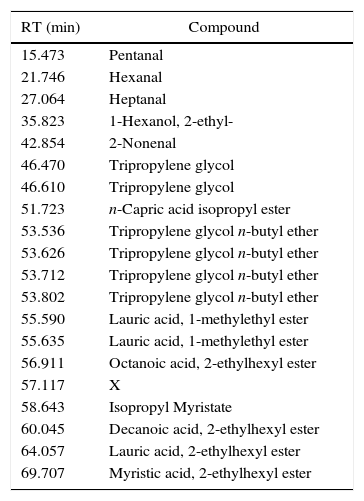
I53 emissions are characterized mainly by 1-methylethyl laurate (Fig. 5), mixed with a 2-ethylhexyl esters mix, as made clear by the presence of 2-ethylhexyl laurate, decanoate, myristate and octanoate. Compared to I33, the principal weight loss is linked more to the first endothermic event, rather than to the second exothermic one, according to the behavior of 1-methylethyl laurate. Some decomposition products are observed as well (aldehydes, 2-ethyl-1-hexanol, etc.), together with a quite small portion of glycols. Tables 7 and 8 report the entire results of I33 and I53 analyses.
Identified compounds in I33 emissions.
| RT (min) | Compound |
|---|---|
| 15.464 | Pentanal |
| 21.732 | Hexanal |
| 27.080 | Heptanal |
| 33.649 | 2-Propanol, 1-(2-methoxy-1-methylethoxy)- |
| 33.895 | Octanal |
| 34.493 | 2-Propanol, 1-(2-methoxypropoxy)- |
| 35.849 | 1-Hexanol, 2-ethyl- |
| 36.100 | 1-Hexanol, 2-ethyl- |
| 39.947 | Nonanal |
| 42.850 | 2-Nonenal, (E)- |
| 45.085 | Decanal |
| 48.600 | Undecanal |
| 53.522 | Pentadecane |
| 55.249 | ? |
| 56.897 | Octanoic acid, 2-ethylhexyl ester |
| 58.343 | 1-Decanol, 2-hexyl- |
| 60.030 | Decanoic acid, 2-ethylhexyl ester |
| 62.140 | ? |
| 64.118 | Lauric acid, 2-ethylhexyl ester |
| 66.474 | 1-Eicosene |
| 69.768 | Myristic acid, 2-ethylhexyl ester |
Identified compounds in I53 emissions.
| RT (min) | Compound |
|---|---|
| 15.473 | Pentanal |
| 21.746 | Hexanal |
| 27.064 | Heptanal |
| 35.823 | 1-Hexanol, 2-ethyl- |
| 42.854 | 2-Nonenal |
| 46.470 | Tripropylene glycol |
| 46.610 | Tripropylene glycol |
| 51.723 | n-Capric acid isopropyl ester |
| 53.536 | Tripropylene glycol n-butyl ether |
| 53.626 | Tripropylene glycol n-butyl ether |
| 53.712 | Tripropylene glycol n-butyl ether |
| 53.802 | Tripropylene glycol n-butyl ether |
| 55.590 | Lauric acid, 1-methylethyl ester |
| 55.635 | Lauric acid, 1-methylethyl ester |
| 56.911 | Octanoic acid, 2-ethylhexyl ester |
| 57.117 | X |
| 58.643 | Isopropyl Myristate |
| 60.045 | Decanoic acid, 2-ethylhexyl ester |
| 64.057 | Lauric acid, 2-ethylhexyl ester |
| 69.707 | Myristic acid, 2-ethylhexyl ester |
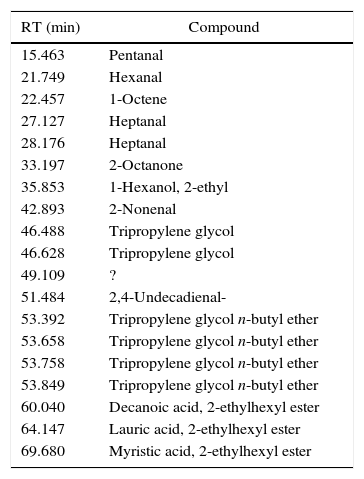
Emissions of glycol+ester based inks (4/55) show the characteristic emissions of both the fractions. Glycols produce evaporation products (as described for I23). Emissions from the ester fraction depend on the nature of the ester itself. In I50 (Fig. 6), for example, we found 2-ethylhexyl laurate as major component, with aldehydes and 2-ethyl-1-hexanol, coming from the decomposition of a portion of the ester part. The thermal behavior of this ink in DTA is described by an endothermic event, at temperatures comparable to TPnB evaporation, followed by an exothermic phenomenon, probably linked to the decomposition of the ester. Two weight losses are clearly visible and linkable to these two events, one for each. Table 9 displays all the recognized molecules.
Identified compounds in I50 emissions.
| RT (min) | Compound |
|---|---|
| 15.463 | Pentanal |
| 21.749 | Hexanal |
| 22.457 | 1-Octene |
| 27.127 | Heptanal |
| 28.176 | Heptanal |
| 33.197 | 2-Octanone |
| 35.853 | 1-Hexanol, 2-ethyl |
| 42.893 | 2-Nonenal |
| 46.488 | Tripropylene glycol |
| 46.628 | Tripropylene glycol |
| 49.109 | ? |
| 51.484 | 2,4-Undecadienal- |
| 53.392 | Tripropylene glycol n-butyl ether |
| 53.658 | Tripropylene glycol n-butyl ether |
| 53.758 | Tripropylene glycol n-butyl ether |
| 53.849 | Tripropylene glycol n-butyl ether |
| 60.040 | Decanoic acid, 2-ethylhexyl ester |
| 64.147 | Lauric acid, 2-ethylhexyl ester |
| 69.680 | Myristic acid, 2-ethylhexyl ester |
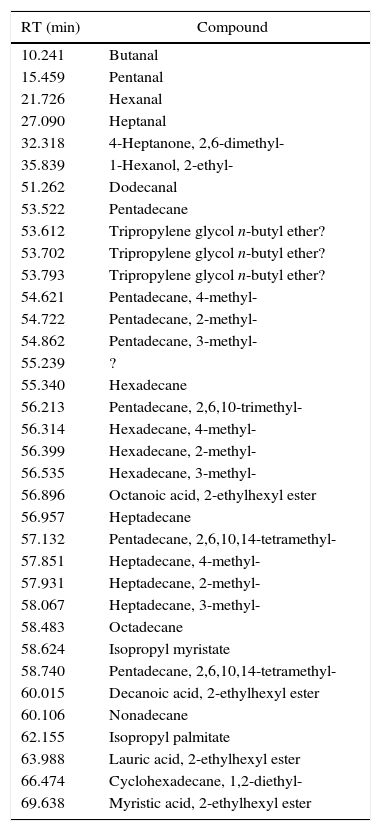
The pattern of emissions of paraffin+ester inks (2/55) is well represented by I24 (Fig. 7). Its emissions (Table 10) are characterized by evaporation products from the paraffin (linear and branched alkanes), aldehydes and 2-ethylhexanol from the decomposition of part of the ester fraction, 2-ethylhexyl decanoate, laurate and myristate, and isopropyl palmitate from the evaporation of part of the ester mix. As for I50, DTA proposes two events, the first endothermic, the second exothermic, but it seems that just one major weight loss is present and principally connected to the endothermic reaction. This could be the sum of paraffin and ester evaporation, the products of which then go through a combustion process.
Identified compounds in I24 emission.
| RT (min) | Compound |
|---|---|
| 10.241 | Butanal |
| 15.459 | Pentanal |
| 21.726 | Hexanal |
| 27.090 | Heptanal |
| 32.318 | 4-Heptanone, 2,6-dimethyl- |
| 35.839 | 1-Hexanol, 2-ethyl- |
| 51.262 | Dodecanal |
| 53.522 | Pentadecane |
| 53.612 | Tripropylene glycol n-butyl ether? |
| 53.702 | Tripropylene glycol n-butyl ether? |
| 53.793 | Tripropylene glycol n-butyl ether? |
| 54.621 | Pentadecane, 4-methyl- |
| 54.722 | Pentadecane, 2-methyl- |
| 54.862 | Pentadecane, 3-methyl- |
| 55.239 | ? |
| 55.340 | Hexadecane |
| 56.213 | Pentadecane, 2,6,10-trimethyl- |
| 56.314 | Hexadecane, 4-methyl- |
| 56.399 | Hexadecane, 2-methyl- |
| 56.535 | Hexadecane, 3-methyl- |
| 56.896 | Octanoic acid, 2-ethylhexyl ester |
| 56.957 | Heptadecane |
| 57.132 | Pentadecane, 2,6,10,14-tetramethyl- |
| 57.851 | Heptadecane, 4-methyl- |
| 57.931 | Heptadecane, 2-methyl- |
| 58.067 | Heptadecane, 3-methyl- |
| 58.483 | Octadecane |
| 58.624 | Isopropyl myristate |
| 58.740 | Pentadecane, 2,6,10,14-tetramethyl- |
| 60.015 | Decanoic acid, 2-ethylhexyl ester |
| 60.106 | Nonadecane |
| 62.155 | Isopropyl palmitate |
| 63.988 | Lauric acid, 2-ethylhexyl ester |
| 66.474 | Cyclohexadecane, 1,2-diethyl- |
| 69.638 | Myristic acid, 2-ethylhexyl ester |
To resume the obtained results, the most utilized vehicles for the production of ceramic inks can be divided in 3 groups, considering their thermal behaviors and, consequently, their emissions:
- -
Vehicles with evaporating behavior: paraffins and TPM.
- -
Vehicles with hybrid behavior: TPnB, 2-EHL (or, more precisely, 1-MEL), EHC.
- -
Vehicles with decomposing behavior: 2-EHS and CCT.
Among the decomposition products, esters release aldehydes (from butanal to tridecanal), ketones (from 2-pentanone to 2-tridecanone), carboxylic acids (from octanoic to hexadecanoic acid), alcohols (most of all 2-ethyl-1-hexanol), alkanes, lactones (found only in CCT), fatty acids esters.
TPnB can decompose into some aldehydes and, above all, minor ethers and 2-ethyl-4-methyl-1,3-dioxolane.
Inks, whether they are based on mixtures or nearly pure vehicles, follow the thermal behavior and the emissions pattern of their single components. The set of commercial inks analyzed underline how fatty acids esters are quite common, used as single vehicle or in mixtures with glycols or paraffins. Considering that every analyzed sample of ester-based vehicle seems to meet with a total exothermic reaction or, at least, with a partial one, decomposition products of esters characterize the majority of inks samples.
Moreover, it seems that EHC is the most common ester used for ester based inks. Its hybrid thermal behavior leads to the presence of both evaporation (2-ethylhexyl laurate, myristate, decanoate and octanoate) and decomposition products (aldehydes – pentanal, hexanal, octanal, nonanal – 2-ethyl-1-hexanol).
These results should clearly be implemented by experimentations at real chimneys or by firing in combustion ovens, because the different nature of the industrial ovens could change somehow the actual behavior of inks.
Anyway, assuming the configuration of industrial ceramic kilns, the huge air flow causes the removal of decomposition and evaporation products, conducting them to chimneys, where they can exit into the atmosphere, if they are not able to react completely or to be retained by specific systems.
Among the emission products, the specialized legislation on ceramic industry in Italy puts attention to quantification of aldehydes, so future steps of this study could imply their quantification.
This first characterization is just the initial step to investigate the entire problem. On the basis of these data, we are carrying on comparative studies between emissions of inks alone, collected in laboratory, and emissions of the same inks applied to ceramic tiles, gathered both in laboratory and at a ceramic chimney, to understand the real influence of inks on total emissions.





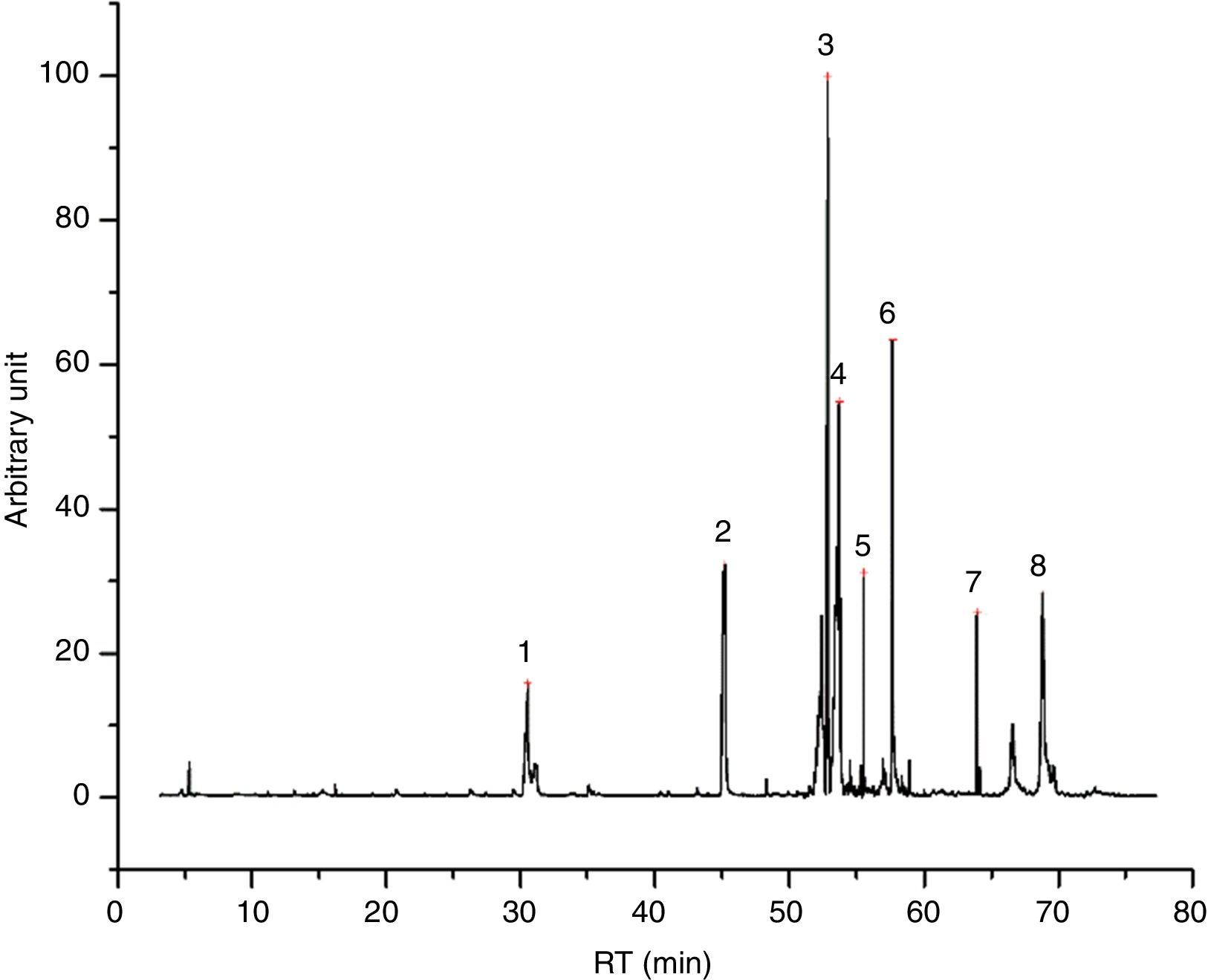
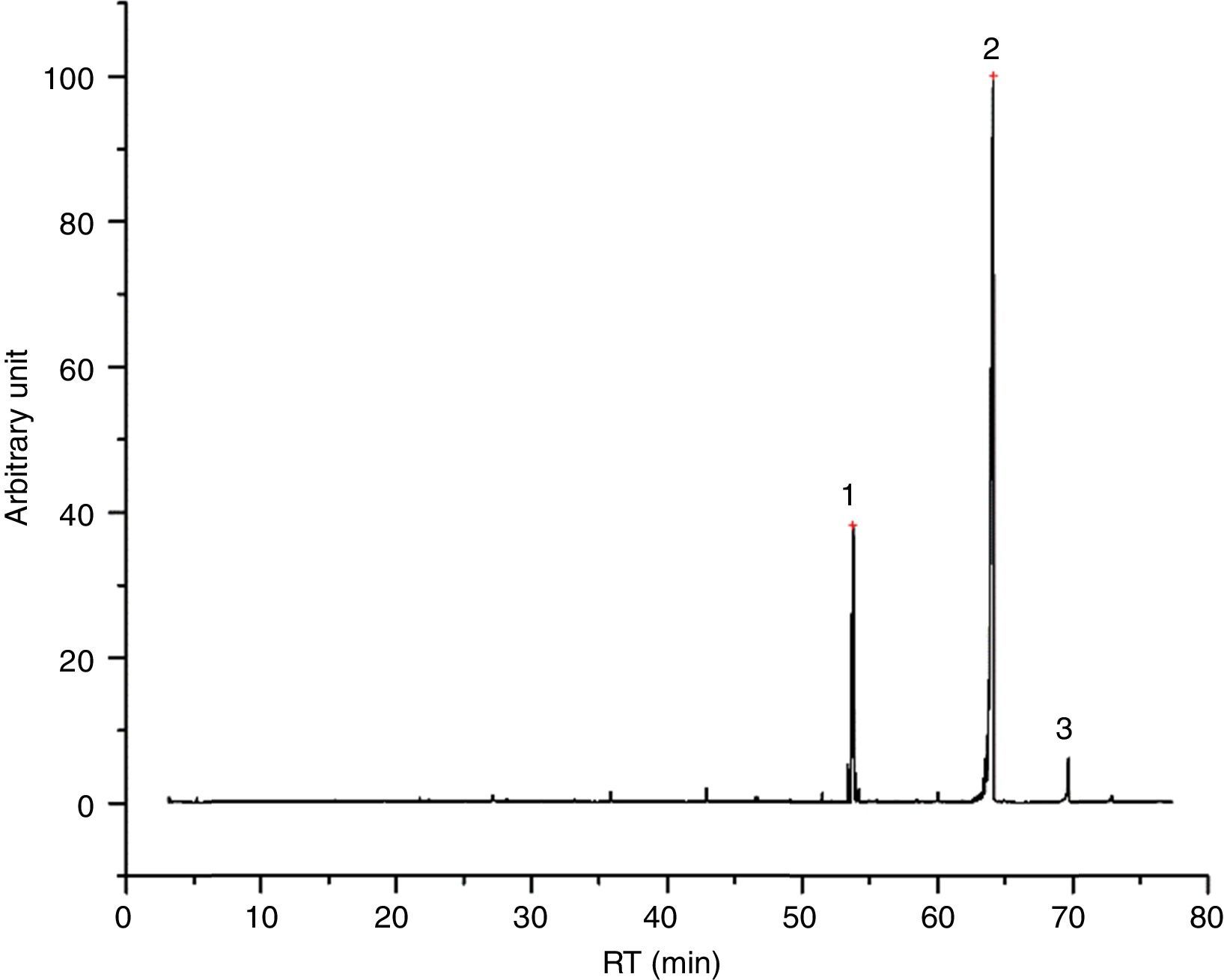
![I23 chromatogram – (1) 1,1′-[(1-methyl-1,2-ethanediyl)bis(oxy)]bis-2-propanol, (2) tripropylene glycol n-butyl ether, (3) 1-methylethyl laurate, (4) and (5) ? = unidentified compound. I23 chromatogram – (1) 1,1′-[(1-methyl-1,2-ethanediyl)bis(oxy)]bis-2-propanol, (2) tripropylene glycol n-butyl ether, (3) 1-methylethyl laurate, (4) and (5) ? = unidentified compound.](https://static.elsevier.es/multimedia/03663175/0000005600000005/v1_201710120026/S0366317517300420/v1_201710120026/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)