This work addresses the use of Laponite® clay in various applications, focusing on its adsorption capacity and improvement of properties in water-based products. It describes the synthesis of Laponite® hybrids with the pigmented cyanobacterium phycocyanin (PC) to evaluate the adsorption capacity of the dye. This research addresses the importance of Laponite® clay in improving rheological properties of water-based products due to its ability to interact with components in aqueous solutions. Laponite® can be effectively dispersed in water, enhancing the dispersion of other elements in solution and preventing aggregation of solids. The structure and properties of synthetic Laponite® are described, highlighting its use in the textile industry as an additive for pigments and the formation of gels. Experimentally, Laponite® is used to adsorb phycocyanin, a cyanobacterial pigment, with the aim of achieving maximum adsorption. A design of experiments with different experimental conditions, including pH, addition of silane and surfactant, is used. A detailed analysis of the characterization of the resulting hybrids is presented, including colour, total solar reflectance (TSR), thermal analysis (TGA), EDS, FTIR and X-ray diffraction (XRD) measurements. The results show successful adsorption of the pigment in all experiments, with adsorption percentages above 97%. The colour of the hybrids is evaluated and the total solar reflectance is analyzed, highlighting their importance in applications such as coatings and printed textiles. Infrared spectroscopy and X-ray diffraction are used to study the structural properties of the hybrids. For future work, it is intended to be able to apply the hybrids obtained on an industrial scale in the colouring of textiles and plastic polymers.
Este trabajo aborda el uso de la arcilla Laponita® en diversas aplicaciones, centrándose en su capacidad de adsorción y mejora de propiedades en productos a base de agua. Así pues se describe la síntesis de híbridos de Laponita® con la cianobacteria pigmentada ficocianina (PC) para evaluar la capacidad de adsorción del colorante. Esta investigación aborda la importancia de la arcilla Laponita® en la mejora de propiedades reológicas de productos basados en agua debido a su capacidad para interactuar con componentes en soluciones acuosas. La Laponita® puede dispersarse eficazmente en agua, mejorando la dispersión de otros elementos en solución y previniendo la agregación de sólidos. La estructura y propiedades de la Laponita® sintética se describen, destacando su uso en la industria textil como aditivo para pigmentos y la formación de geles. Experimentalmente se utiliza Laponita® para adsorber ficocianina, un pigmento de cianobacteria, con el objetivo de lograr la máxima adsorción. Se utiliza un diseño de experimentos con diferentes condiciones experimentales, incluido el pH, la adición de silano y surfactante, Se presenta un análisis detallado de la caracterización de los híbridos resultantes, incluyendo mediciones de color, reflectancia solar total (TSR), análisis térmico (TGA), EDX, FTIR y difracción de rayos X (DRX). Los resultados muestran una adsorción exitosa del pigmento en todos los experimentos, con porcentajes de adsorción superiores al 97%. Se evalúa el color de los híbridos y se analiza la reflectancia solar total, destacando la importancia de estos en aplicaciones como revestimientos y textiles impresos. Se utiliza espectroscopia de infrarrojo y difracción de rayos X para estudiar las propiedades estructurales de los híbridos. Para futuros trabajos se pretende poder aplicar a escala industrial los híbridos obtenidos en coloración de textiles y polimeros plásticos.
In the realm of materials science, two unique elements have stood out for their innovative potential: Laponite®, a synthetic clay with interesting properties, and phycocyanin, a natural pigment extracted from certain algae and cyanobacteria. Each on its own is a treasure of nature, but when combined, they unleash a symphony of possibilities in the field of adsorption and beyond.
Laponite®, known for its nano-sized structure and ability to form gels and colloidal dispersions, has been the subject of intense research in materials science. Its layered structure allows the adsorption of organic and inorganic molecules on its surface, making it a promising material for a variety of applications, from pharmaceuticals to environmental remediation.
Laponite®, a hybrid-type synthetic clay, stands out for its exceptional adsorption capacity in various industrial and scientific applications. Its nanometric structure and modifiable surface charge make it highly efficient in the adsorption of organic and inorganic molecules in aqueous and non-aqueous systems. Laponite® acts as a stabilizing agent in emulsions and suspensions, improving the viscosity and texture of products such as paints, cosmetics and coatings. In addition, its ability to adsorb heavy metals and organic pollutants makes it a crucial material in environmental remediation and water purification. In scientific fields such as nanotechnology, laponite is used to create advanced nanocomposites, taking advantage of its exceptional adsorption capacity to improve the mechanical and structural properties of composite materials. Its versatility and adsorption efficiency make it an invaluable tool in numerous technological and environmental applications.
On the other hand, phycocyanin shows as an intense blue pigment and, beyond its visual appeal, it has antioxidant properties and applications in the food and health industry. Its ability to interact with other substances opens the door to a world of possibilities, especially when it meets Laponite®.
When phycocyanin interacts with Laponite®, something happens at the molecular level. The phycocyanin binds firmly to the surface of the Laponite® by adsorption forces, forming stable hybrids. This interaction is the starting point for a variety of applications. Laponite's ability to efficiently adsorb phycocyanin not only has implications in the field of catalysis and nanotechnology, but has also been explored in water purification and the creation of controlled drug release systems.
In order to make the most efficient use of nanoclays, there are elements that enhance their capabilities, such as silanes, which play a crucial role in improving the properties of nanoclays. These chemical compounds are used to modify the surface of clays at the nanometre level, optimizing their compatibility with various polymeric matrices. By applying silanes, a more uniform dispersion of nanoclays in composite materials is achieved, improving mechanical strength, thermal resistance and barrier properties. In addition, silanes strengthen the interactions between the polymer matrix and the nanoclays, resulting in stronger and lighter materials. This innovative process has promising applications in sectors such as automotive, aerospace and advanced materials.
On the other hand, the use of certain surfactants also improves the properties of certain nanoclays. The interaction between surfactants and nanoclays is essential in many industrial and scientific applications. Surfactants, by adsorbing on the surface of nanoclays, modify their properties and dispersion in polymeric matrices or liquids. This interaction improves colloidal stability, facilitates uniform dispersion and enhances the mechanical and thermal properties of the resulting composite material. This phenomenon is fundamental in nanotechnology, especially in the development of advanced materials and high-performance nanocomposites.
Phycocyanin is a very sensitive dye that must be stored in temperature and humidity conditions to avoid degradation [1,2]. By adsorbing it on a nanoclay, the aim is to improve its stability outside specific refrigeration conditions in order to avoid degradation. Laponite® tends to form gels relatively easily during the adsorption process and subsequent filtering [3,4], which is an ideal physical state for preserving this dye and makes it an appropriate clay for this purpose compared to others that do not form this type of physical state.
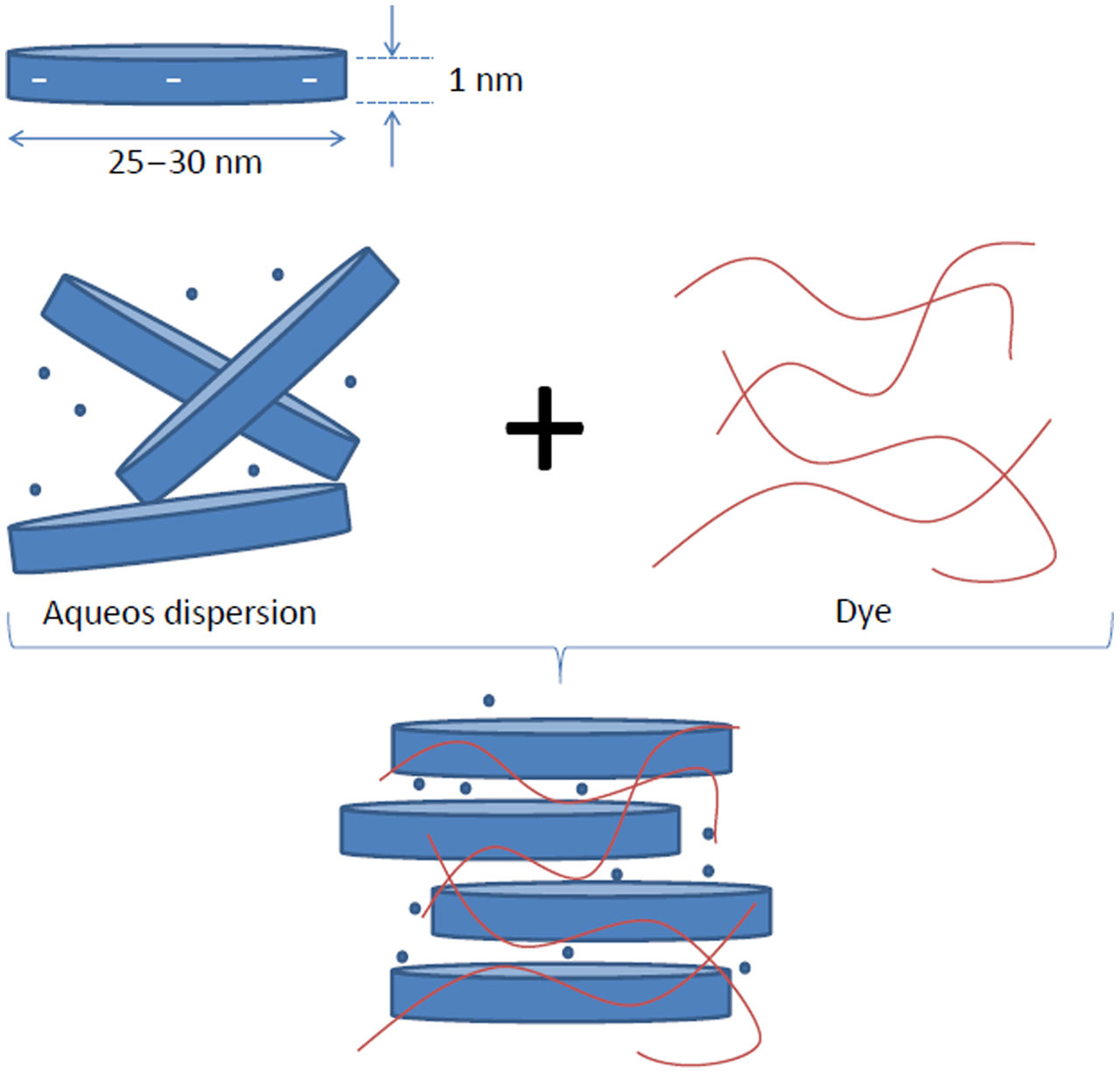
Materials and methodsMaterialsThe trade name Laponite® (Lap) clay, supplied by the merck distributor, is an inorganic layered silicate material commonly used to improve the rheological properties of various water-based products [5–7]. Its exceptional ability to interact with the components of aqueous formulations results in a substantial increase in viscosity after incorporation [8], reaching values of 87.8Pas. Studies [9–11] have shown that Laponite® can be effectively dispersed in water (as illustrated in Fig. 1) and can enhance the dispersion of other elements in solution. This is attributed to its unique ability to prevent aggregation of solids [3,12–15].
Synthetic Laponite® (Si8[Mg5.5Li0.4H4.0O24]0.7−Na0.7+) (Fig. 2) is a flat, disc-shaped silicate material with a thickness of approximately 1nm and a diameter of 25nm [16–18]. The untreated Laponite® was found to have a specific surface area (SSA) of 11.7m2g−1[19]. It carries permanent negative charges due to isomorphic substitution. Depending on factors such as temperature, concentration, and curing time, it can exist as a viscous gel that breaks in aqueous solution or transforms into a translucent fluid [20–23].
In the textile industry, Laponite® serves as an excellent additive for pigments, offering protection against environmental factors like temperature and oxygen, while enhancing the pigment's overall stability [24–27]. Studies have demonstrated the adsorption capacity of Laponite-based hydrogels [28], with a cation exchange capacity (CEC) of 74cmolkg−1[29]. The formation of Laponite-based hydrogels is attributed to the reticulation that occurs within the polymer. This gel can also be formed by introducing specific components, such as H2SO4 or KNO3, into the mix [20–23].
Additionally, researchers have utilized Laponite® membranes to achieve the removal of up to 100% of two organic dyes: Rhodamine B (a cationic dye) and Brilliant Blue (an anionic dye) [30]. This was accomplished through the synthesis of a superoleophobic membrane using polyacrylonitrile hydration.
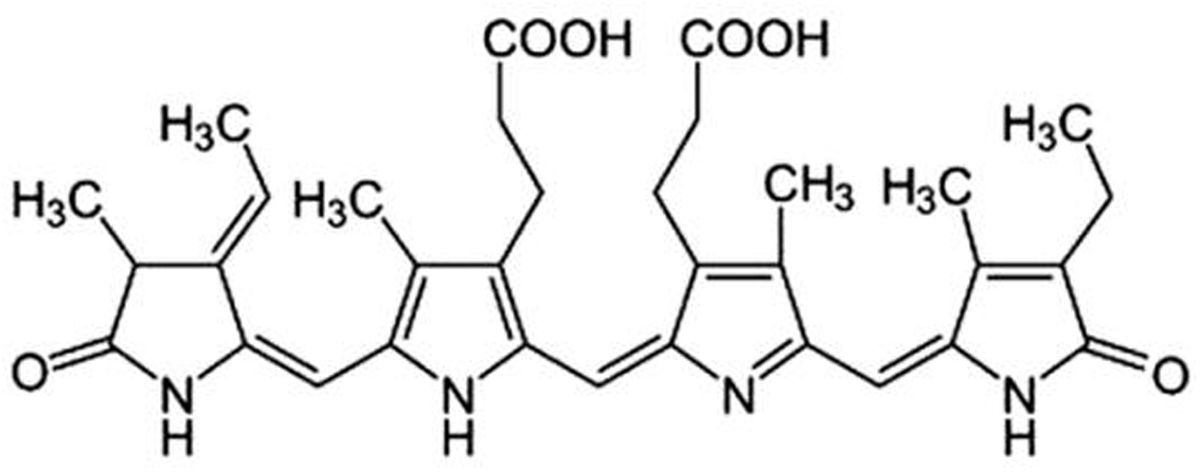
Phycocyanin (PC), known as C-phycocyanin (Fig. 3), has been utilized as a colouring material. It was extracted from freeze-dried biomass by dissolving it in a phosphate buffer (5mM, pH 6.5) at a ratio of 1:20 (w/v). The solution underwent stirring for 5min at 2000r.p.m. using a magnetic stirrer from Schwabach, Germany, leading to the release of intracellular material through cell osmotic shock. The resulting sample was then centrifuged at 12,000r.p.m. for 15min (Medtronic BL-S, P-Selecta, Barcelona, Spain) to retrieve the phycobiliproteins dissolved in the supernatant.
Following this step, the phycobiliproteins were precipitated by introducing a solution of ammonium sulfate (70% saturation) and left to stand overnight in darkness at 4°C. Subsequently, the purified biliprotein pellets were reconstituted in a small volume of phosphate buffer (5mM, pH 6.5) and dialyzed overnight at 4°C in the same buffer. To preserve the protein solution, 1% w/w sodium azide (NaN3) was added as a preservative. The samples were then stored at 4°C until further analysis.
The colour of these molecules is determined by the chromophore (prosthetic group), which is a linear tetrapyrrole group that binds to the apoprotein via a thioether bond to cysteine residues and whose absorption spectrum depends on the protein to which it is bound [31,32]. The prosthetic group bound to the apoprotein is a phycocyanobilin (phycocyanin).
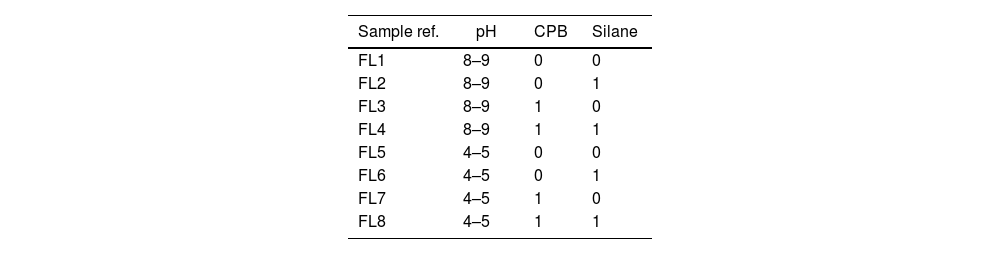
MethodsThe objective set for this experiment is to achieve the maximum possible adsorption of phycocyanin. In the experimental adsorption phase, 8 different solutions are prepared, all containing 7.5ml of dye dissolved in 400ml of distilled water. Immediately afterwards, 6g/L of laponite is added to each solution. Each of these solutions is subjected to different conditions of pH, addition of silane 3-aminopropyltriethoxysilane, H2N(CH2)3Si(OCH3)3, 179.29gmol−1, purity 99% or surfactant CPB (cetylpyridinium bromide hydrate) C21H38BrN·6H2O, 384.44g/mol, purity 98% both products were supplied by Sigma–Aldrich (Madrid, Spain). The specific conditions for each sample are given in Table 1. The pH was set at a slightly basic pH without any adjustment and an acidic pH between 4 and 5 by adding acetic acid to achieve this range.
The mixture is then stirred using a magnetic stirrer at 1600rpm for the first two hours, reducing the speed to 600rpm afterwards. This configuration allows for a first adsorption stage [33], favouring the penetration of the dye due to a higher centrifugal force. Afterwards, the speed is reduced to ensure that the dye remains adsorbed on the clay, which constitutes an “ageing” stage of the hybrid formed.
After the above time, the solution is filtered to separate and collect the Laponite® with the adsorbed dye from the aqueous solution, using filter paper. Once filtered, it is left in a resting phase for at least 48h to allow the water to dissipate by gravity completely and to achieve a complete separation of the clay. The clay on the filter paper is collected and freeze-dried to remove any water that may have been adsorbed [34,35] to remove any residual water present.
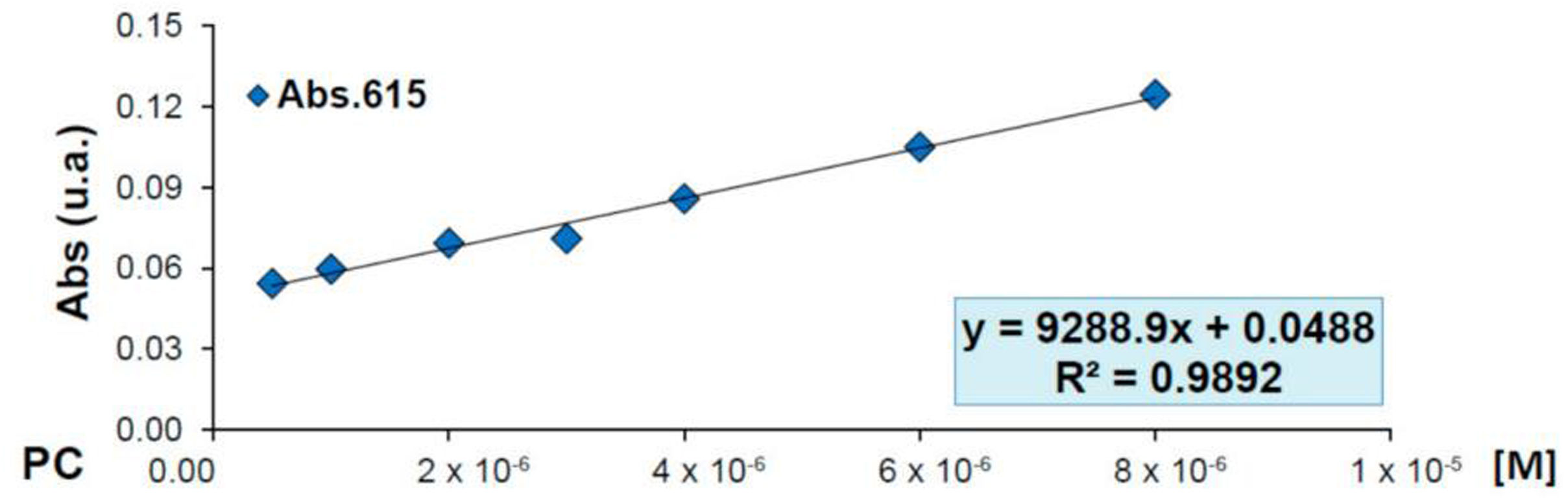
As described in Table 1, the amount of CPB added in the experiments listed as ‘1’ in the table is 0.3g/L while the amount of silane is 0.05% by volume. In order to know how to control the overdrawing after the described adsorption, the Lambert–Beer line [36] was obtained, which is shown in Fig. 4 with its corresponding equation, which will allow us to check the degree of absorption that each one of the experiments has had [37,38].
Looking at Fig. 4, we can see the equation obtained with a regression R of 0.9892, which indicates that it has a high accuracy. Using the equation obtained, the concentration of dye (x) in a solution can be calculated from an absorbance reading (y), making it possible to carry out a quantitative analysis in a simple and rapid manner.
CharacterizationThe colour and total solar reflectance (TSR) of clay-dye hybrids were measured using a Jasco V-670 dual UV–VIS/NIR spectrophotometer, operating in the range of 2500–280nm with a resolution of 0.5nm. This instrument features a double grating monochromator: the first grating, with a resolution of 1200grids-mm−1 and photomultiplier tube-based detectors, is used for UV–VIS range, while the second grating, with a resolution of 300lines/mm and a PbS detector, is employed for the infrared (IR) region. Both detectors have an automation system to manage changes and adjust them based on the required wavelength. Light sources included a halogen lamp (330–2700nm) and a deuterium lamp (190–350nm). Reflectance factors for hybrid pigments were applied using the CIE-1964 observer and D65 illumination for obtaining optical property values and making comparisons.
Topographical analysis of the samples’ surfaces was conducted using a scanning electron microscope (SEM) model PHENOM (FEI Company, Eindhoven, The Netherlands) at an electron acceleration of 5kV. Prior to analysis, the samples were coated with a gold-palladium alloy (5–7nm thickness) using an EMITECH sputter coater mod. SC7620 (Quorum Technologies Ltd., East Sussex, UK).
To evaluate thermal properties, thermogravimetric analysis (TGA) was performed using a TGA/SDTA 851 thermogravimetric analyzer (Mettler-Toledo Inc., Columbus, OH, USA). The analysis involved a temperature increase of 5°C per minute from 20 to 900°C, with an N2:O2 (4:1) atmosphere as the oxidation medium.
For characterization, Fourier transform infrared spectrophotometry (FTIR) was conducted using a horizontal attenuated total reflection (FTIR–ATR) setup with a ZnSe prism on a Jasco FTIR 4700 IRT 5200 spectrometer equipped with a DTGS detector. The spectrum was obtained through 64 scans with a resolution of 4cm−1.
Additionally, an X-ray diffraction (XRD) test was carried out to study changes in the shape and lamellar structure of the LDH before and after calcination and rehydration. A Bruker D8-Advance XRD instrument (Bruker, Billerica, MA, USA) with a Göebel mirror (power: 3000W, voltage: 20–60kV, and current: 5–80mA) was used. Readings were taken in an oxidizing atmosphere at an angular velocity of 1°/min, a step of 0.05°, and an angular range of 2.7–70°. This test was crucial for analyzing the basal space in the hydrotalcite structure resulting from the adsorption of the dyes.
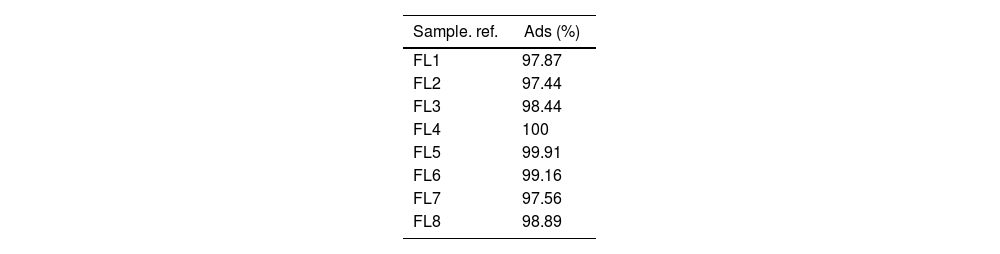
Results and discussionFinal concentration in solutionThe adsorption of the dye has been satisfactorily achieved in all experiments, with adsorption percentages above 97% (Table 2). This factor is taken as a reference for the performance of the adsorption process of the dye molecule, but the results are all excellent and so similar that they do not give statistically significant differences when working with the parameters established in the design of experiments. This demonstrates the adsorption capacity of LAP and its compatibility with the dye used. The amount of clay added has been estimated as sufficient to be able to adsorb the entire dye.
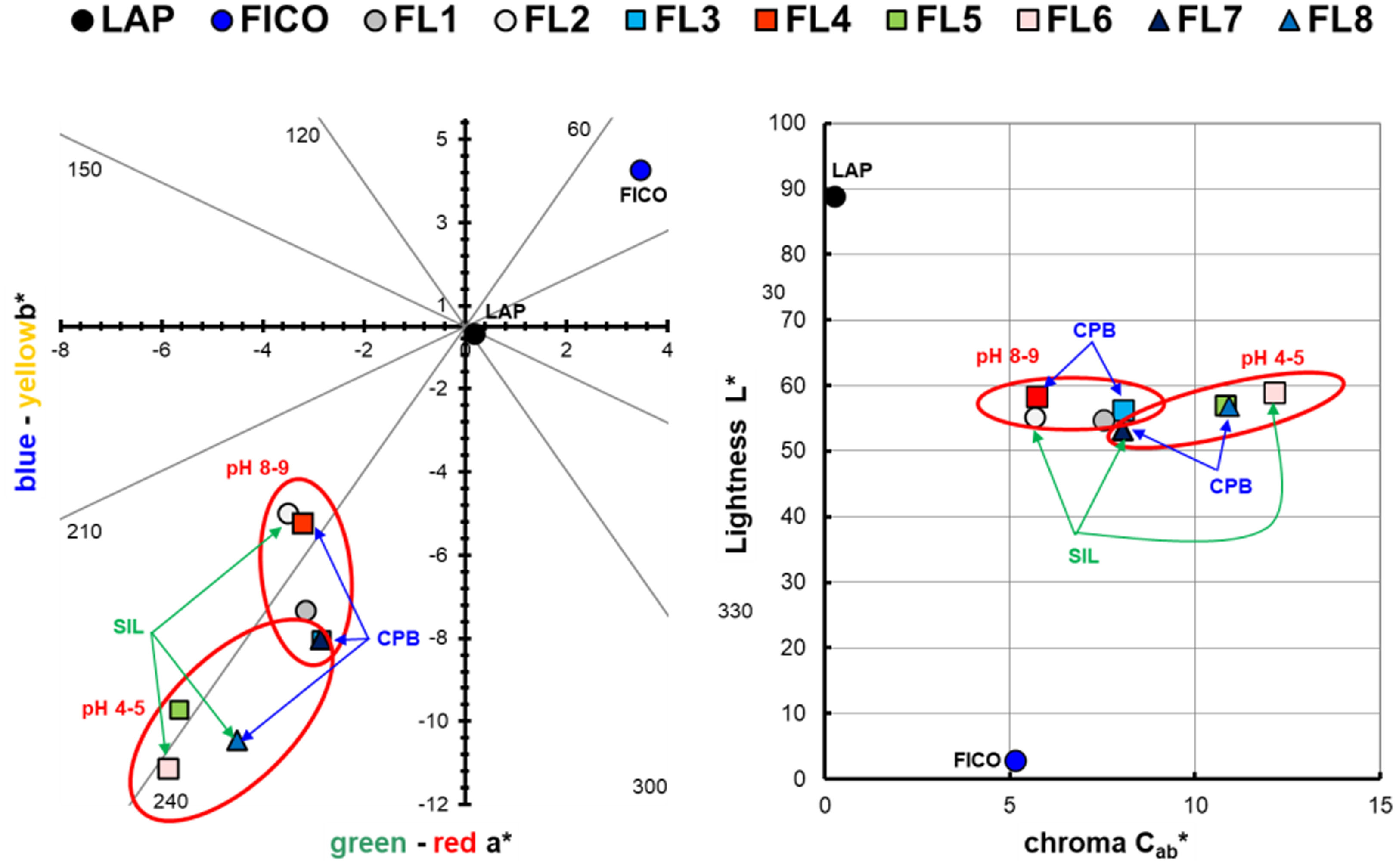
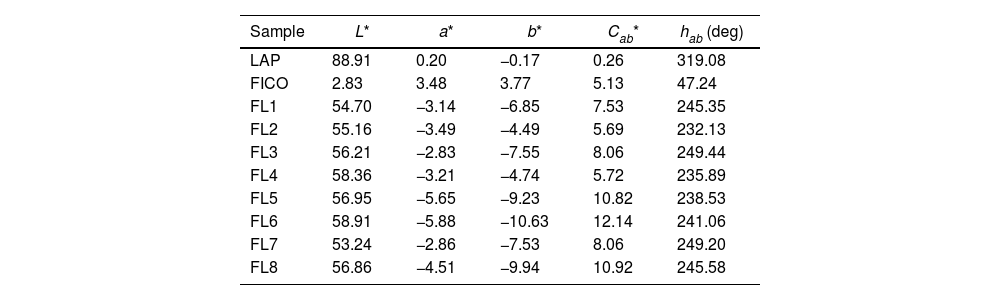
Hybrid colour measurementsTable 3 and Fig. 5 depict the measurements conducted to ascertain the colour of the eight hybrids generated, as illustrated in a chromatic diagram. The determination of the primary colour calculations for each hybrid pigment involved utilizing the reflectance values (λ) of these dye-LDH hybrids, in adherence to the guidelines outlined by the International Commission on Illumination CIE 15:2004 [39]. These guidelines facilitate an unbiased comparison of absolute and relative colour values through the application of CIELAB colorimetric parameters, as per the CIE 1931 XYZ standard and the D65 standard illumination.
L*, a*, b*, Cab* values of each dye and each hybrid.
| Sample | L* | a* | b* | Cab* | hab (deg) |
|---|---|---|---|---|---|
| LAP | 88.91 | 0.20 | −0.17 | 0.26 | 319.08 |
| FICO | 2.83 | 3.48 | 3.77 | 5.13 | 47.24 |
| FL1 | 54.70 | −3.14 | −6.85 | 7.53 | 245.35 |
| FL2 | 55.16 | −3.49 | −4.49 | 5.69 | 232.13 |
| FL3 | 56.21 | −2.83 | −7.55 | 8.06 | 249.44 |
| FL4 | 58.36 | −3.21 | −4.74 | 5.72 | 235.89 |
| FL5 | 56.95 | −5.65 | −9.23 | 10.82 | 238.53 |
| FL6 | 58.91 | −5.88 | −10.63 | 12.14 | 241.06 |
| FL7 | 53.24 | −2.86 | −7.53 | 8.06 | 249.20 |
| FL8 | 56.86 | −4.51 | −9.94 | 10.92 | 245.58 |
The CIEa*b* and CIE-Cab*L* diagrams presented in Fig. 5 validate that the dye extracted from the solutions has been effectively sequestered within the nanoadsorbent, manifesting the colours indicative of the resulting pigments.
Depending on the synthesis parameters, differences in the perceived colour attributes of the synthesized samples can be observed. The factor that appears to have the greatest influence on the colour of the samples is pH. Samples at high pH can be seen to have more yellowish and reddish tones in general, while samples at low pH are more bluish and greenish. In all cases the shade varies with respect to the original FICO dye, which shows a much more yellowish and reddish hue than the samples adsorbed on the clay.
The shade also varies as a function of the addition of surfactant and silane, but the variability here makes it difficult to interpret on the graph, and this will need to be looked at with an ANOVA to determine whether or not the factor significantly influences the shade obtained.
In terms of brightness and chroma, the eight samples synthesized are very similar. In all of them the effect of the clay is observed, which causes the colours obtained to be lighter than that of the initial colouring material, and of course, darker than the clay itself. It is interesting that the saturation increases in all the cases with respect to both raw materials, that is to say that the increase of clarity entails an increase of the saturation in the samples increasing the range of colour obtained in all the conditions of synthesis.
On the other hand, trying to differentiate the effects of the synthesis factors on the clarity and tone obtained, again only some conclusions can be drawn regarding pH. To determine if there are significant differences in these responses with respect to surfactant or silane, analysis of variance (ANOVA) will be performed. What seems clear is that at higher pH levels the chroma decreases and vice versa, it is the more acidic pHs that achieve the highest saturation levels. As for the clarity no differences can be seen in this representation, we will also wait to see the results of the ANOVA, which will confirm this conclusion.
Total solar reflectance (TSR)When applying coatings like textile printing paste, assessing their behaviour and resistance to external factors is vital. The total solar reflectance factor (TSR) becomes crucial in this evaluation. TSR measures a material's ability to reflect incident solar radiation as a percentage of the total radiation incident on its surface. High TSR values indicate effective solar radiation reflection, reducing heat absorption and surface temperatures. Calculated through sunlight reflectance measurements across various wavelengths, TSR considers the full solar spectrum. Solar weighting factors adjust for spectral distribution. TSR is especially significant in applications such as roof coatings and solar shading fabrics, where minimizing heat absorption is essential for energy efficiency. Higher TSR contributes to lower surface temperatures and decreased cooling loads. These calculations adhere to ASTM G173-03 [40] guidelines, providing a standardized approach to evaluating materials in diverse applications.
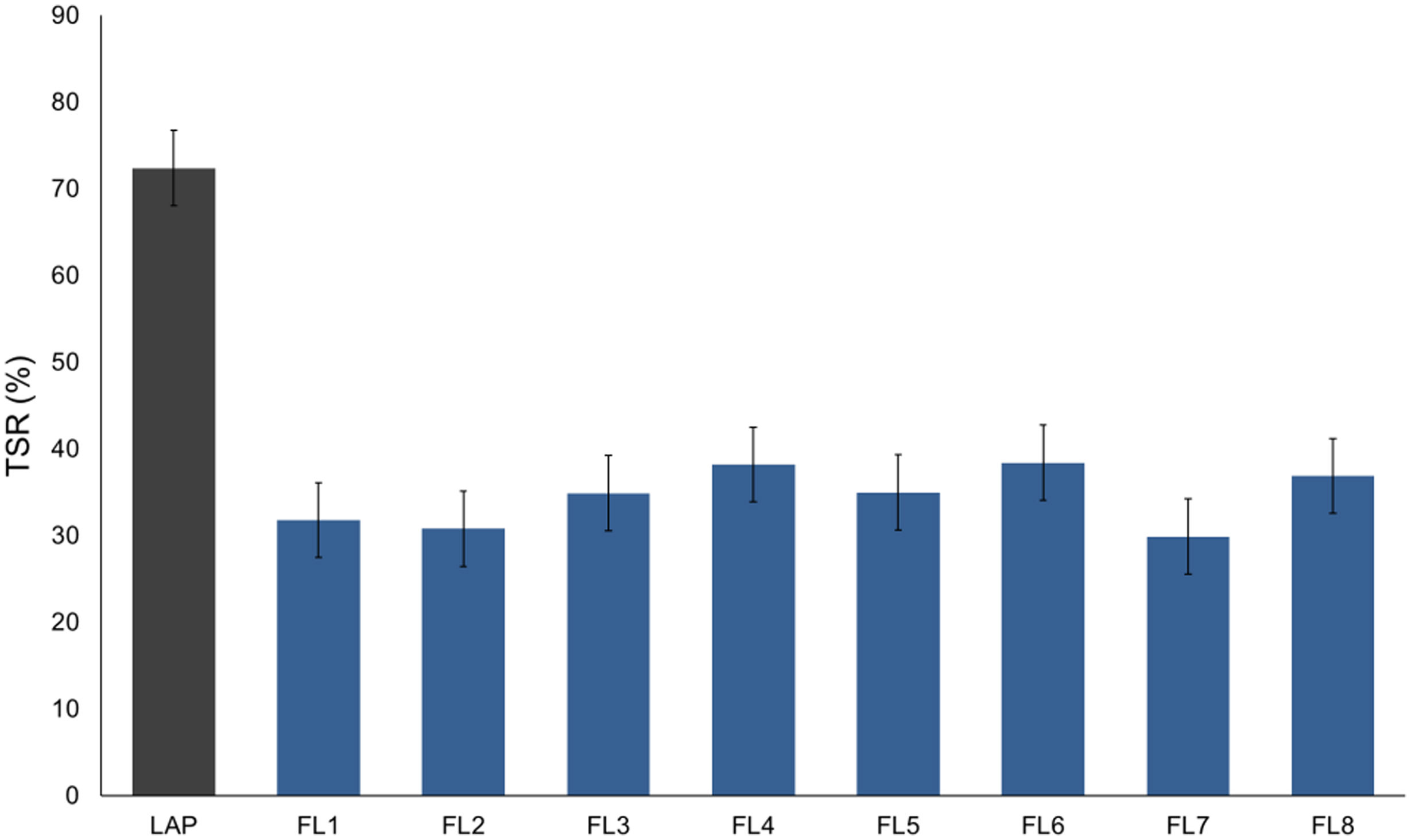
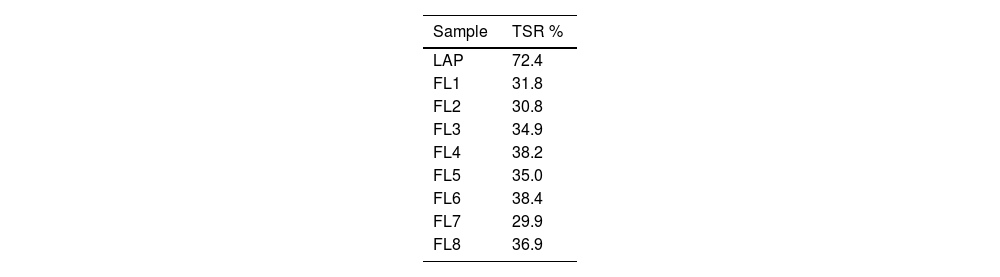
Table 4 below shows the TSR results obtained for each of the synthesized hybrids and the pure form LAP before dye adsorption. Initially, the nanoclay has a much higher L* luminosity than the resulting hybrids, as can be seen in Table 3 and Fig. 5. As a result, elements with a lower TSR and therefore warmer than that initially offered by the LAP alone are obtained.
By examining and comparing both the L* and TSR results for each hybrid, it can be seen that all samples have a very similar lightness ranging from 53 to 59, with sample FL6 showing the highest lightness value and FL7 the lowest value. The TSR values are also very similar, ranging from 29.9 to 38.4%. Fig. 6 shows a graphical comparison of the TSR values obtained for each sample, from which it can be concluded that the hybrid with the highest TSR is FL6 and the lowest is FL7, which corresponds to the values offered by the brightness.
However, to better refine and see if there are significant differences in terms of the synthesis parameters, we will have to wait to see the results of the analysis of variance (ANOVA).
This data enables us to assess and contrast the capabilities of various hybrids concerning their overall solar reflectance. This aspect proves significant in situations where the objective is to optimize the reflection of solar radiation and minimize heat absorption.
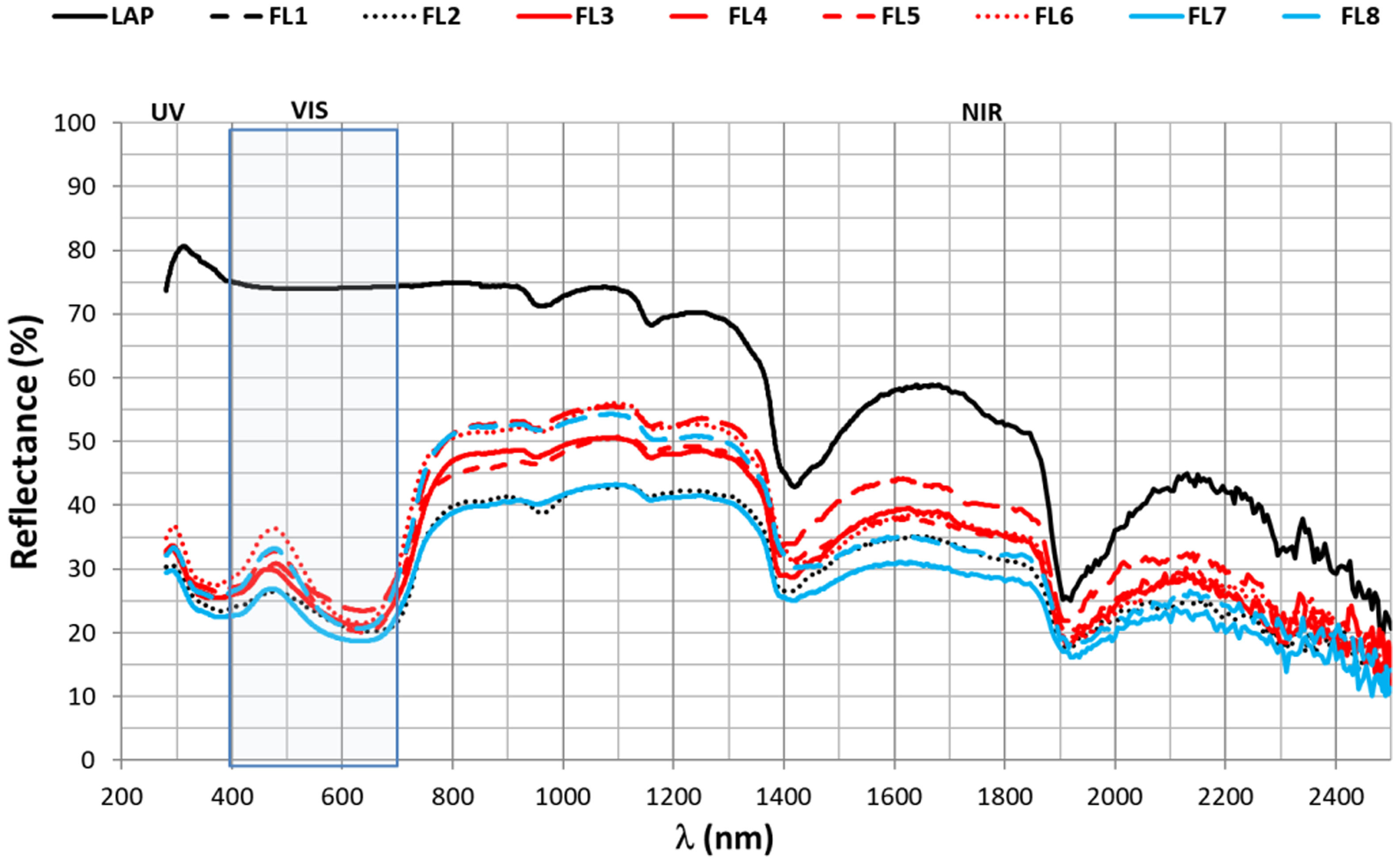
Fig. 7 shows the reflectivity spectra of the 8 hybrids and laponite. The analysis of this spectrum can be performed in 4 zones, the ultraviolet (200–400nm), the visible (400–700nm), the near infrared (700–1200nm) and the infrared (1200–2400nm). The 8 hybrids have a very similar reflectivity across the whole spectrum, while laponite alone has a much higher reflectivity in the UV, visible and near-infrared regions, i.e. between 200 and 1200nm. However, from wavelengths above 1200nm, the intensity of the LAP reflectance begins to attenuate, reaching values very similar to those of the hybrids.
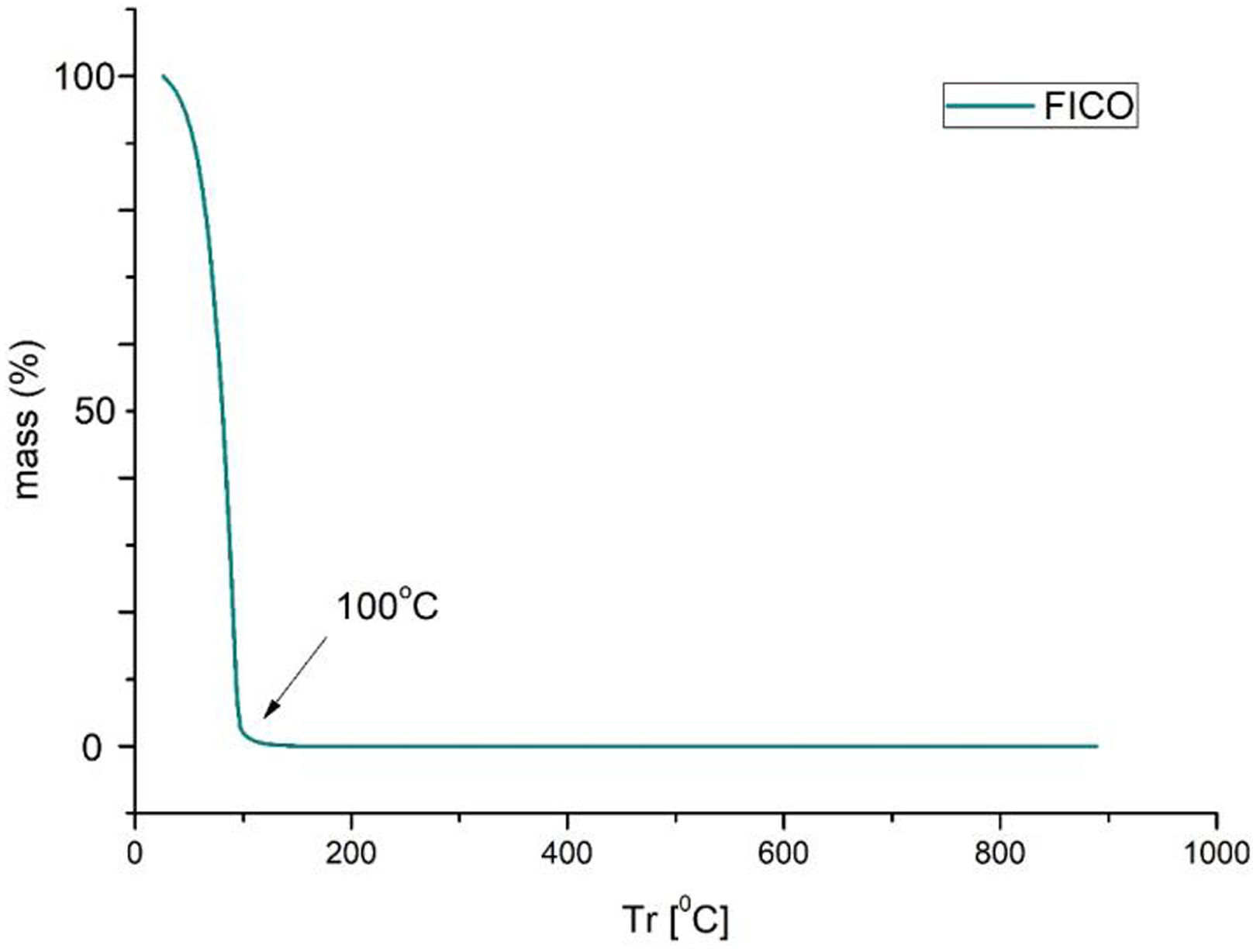
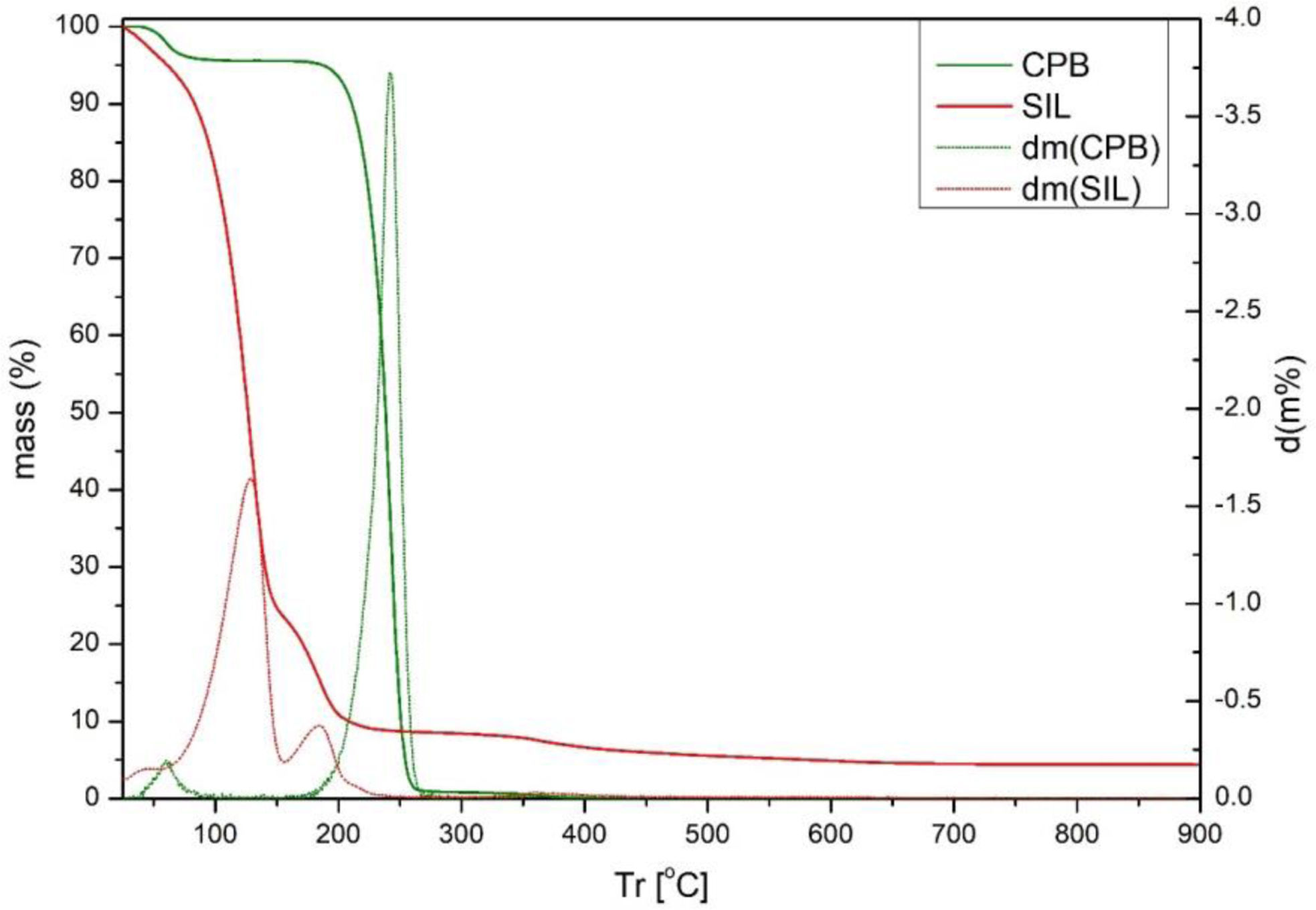
Thermogravimetry (TGA-DTGA)Thermal analyses were conducted by evaluating the mass loss across specific temperature ranges and examining the derivative curves to compare the peaks of maximum degradation. Thermal degradation tests on phycocyanin show in Fig. 8 that total degradation occurs rapidly at 100°C. This shows the high sensitivity of this dye to temperature and its high volatility. This is why it is very interesting to combine it with nanoclays to increase its resistance to temperatures.
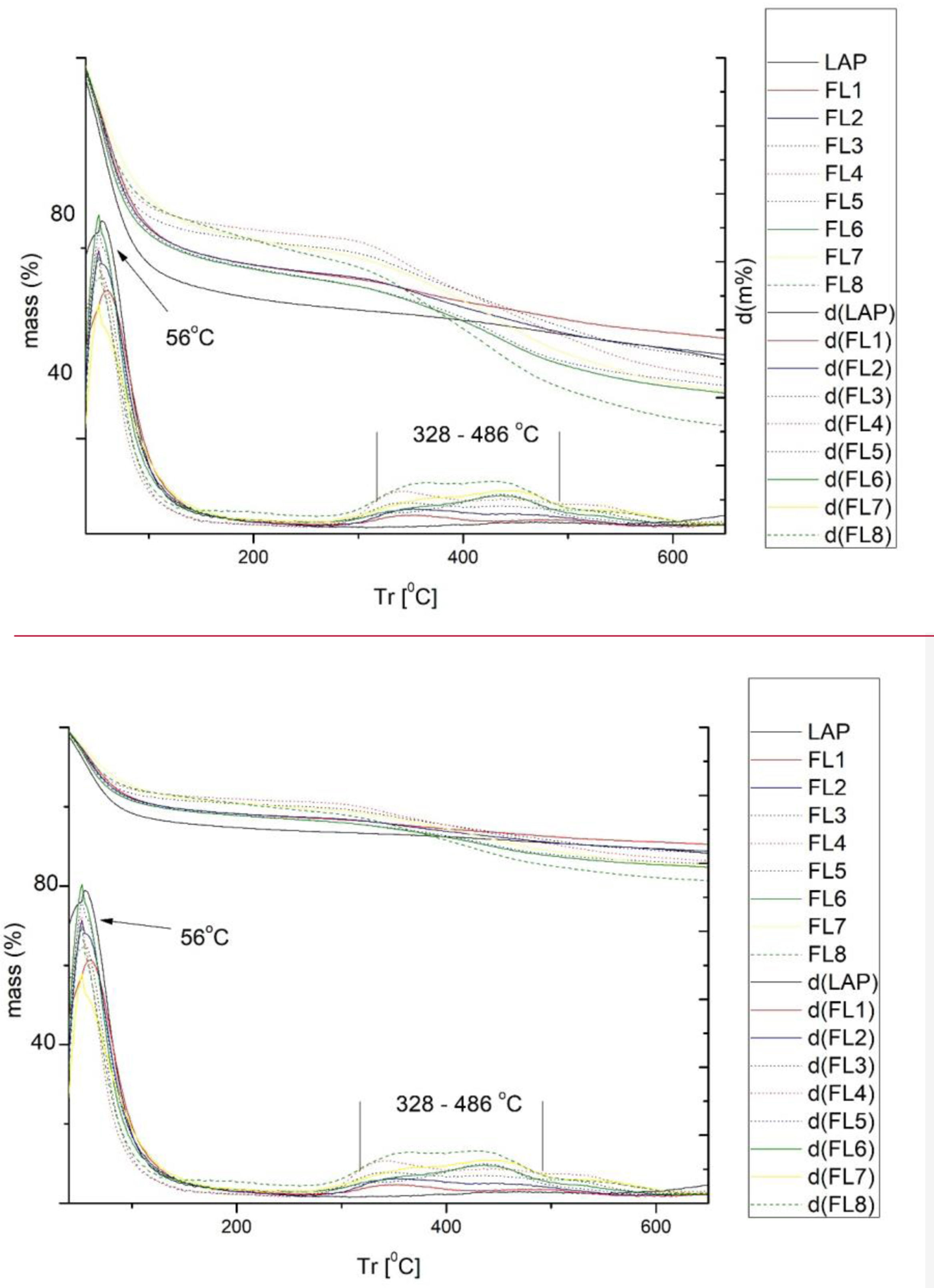
Fig. 9 illustrates the primary peaks originating from the original raw materials, specifically nanoclays. The initial mass loss is evident around 56°C, corresponding to the removal of surface-absorbed water (free water) and interlayer water [41]. There is another zone of mass loss fluctuations located between 328 and 486°C. Looking at Figs. 8 and 9, it is obvious what a substantial change is taking place. From the high volatility of phycocyanin, the hybrids have become almost as stable as LAP alone, protecting the phycocyanin. Research conducted by various scholars has demonstrated a notable enhancement in the thermal stability of dye-clay hybrids, attributed to the impact of laponite [42,43]. This improvement is twofold: firstly, the lamellar structure of the nanoclay exerts a protective influence, diminishing the volatility of dye components; secondly, under elevated temperatures, there is an energy transfer from the dye to the clay. The nanoclay absorbs this transferred energy, thereby mitigating the overall energy impact on the dye [44,45].
In addition, there is a loss around 200°C which can be attributed to the degradation suffered by the dye, although it degrades at lower temperatures, its combination with the clay causes this loss of mass to occur at higher temperatures. The degradation curves of silane and CPB have also been studied, which have significant peaks at 125°C and 250°C respectively as can be seen in Fig. 10. These peaks are not reflected in Fig. 9 of the hybrids, partly because the concentrations of these elements are very low in relation to the clay.
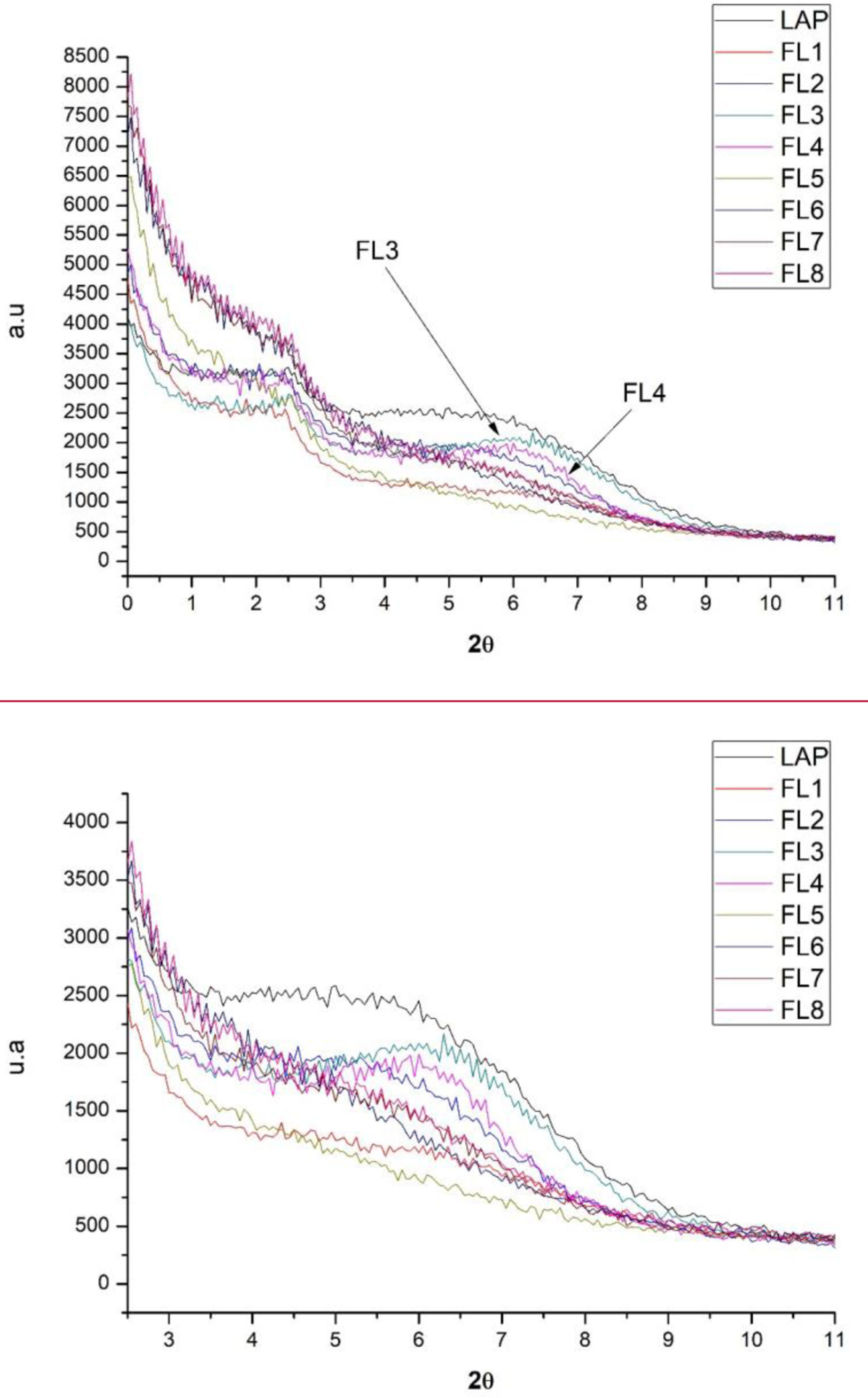
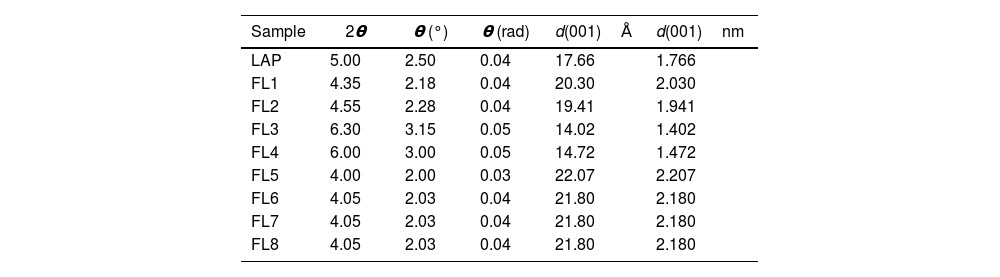
X-ray diffraction (XRD)X-ray diffraction (XRD) patterns illustrate alterations in the crystal structures of nanoclays attributed to the adsorption of phycocyanin. Within the LAP nanoclay category, distinct variations were observed among samples. In some instances, a noteworthy increase in interlayer space was evident, while in other samples, the basal space remained comparable to that of the original nanoclay, or was even reduced (Fig. 11 and Table 5). For these particular samples, it was challenging to ascertain that the interactions with phycocyanin occurred within the basal space. Our deduction led us to infer that the interactions between the dye and the clay transpired on the surface of the nanoclay, resulting in a comparatively lower level of protection compared to the remaining samples.
Calculated interlaminar spacing d(001)nm for laponite and synthesized hybrids.
| Sample | 2θ | θ (°) | θ (rad) | d(001)Å | d(001)nm |
|---|---|---|---|---|---|
| LAP | 5.00 | 2.50 | 0.04 | 17.66 | 1.766 |
| FL1 | 4.35 | 2.18 | 0.04 | 20.30 | 2.030 |
| FL2 | 4.55 | 2.28 | 0.04 | 19.41 | 1.941 |
| FL3 | 6.30 | 3.15 | 0.05 | 14.02 | 1.402 |
| FL4 | 6.00 | 3.00 | 0.05 | 14.72 | 1.472 |
| FL5 | 4.00 | 2.00 | 0.03 | 22.07 | 2.207 |
| FL6 | 4.05 | 2.03 | 0.04 | 21.80 | 2.180 |
| FL7 | 4.05 | 2.03 | 0.04 | 21.80 | 2.180 |
| FL8 | 4.05 | 2.03 | 0.04 | 21.80 | 2.180 |
Considering the synthesis conditions of the hybrids, it can be seen that samples FL3 and FL4, which were worked with at a basic pH and using CPB surfactant, show a smaller interlaminar space than the one initially offered by the laponite. For the rest of the samples with the different variables in terms of pH, surfactant and use of silane, there is an increase in the interlaminar space.
Moreover, upon a more in-depth examination of Fig. 11, it is crucial to bear in mind the differentiation between the amorphous state of the dye and the crystalline state of the nanoadsorbent [46–48]. As the structures progress towards increased crystallinity, the intensity of the X-ray diffraction (XRD) peak exhibits a corresponding augmentation. Thus, in scenarios where the LAP harbours a greater dye concentration, the compound tends to shift towards an amorphous state, resulting in a notable reduction in the intensity of the corresponding band.
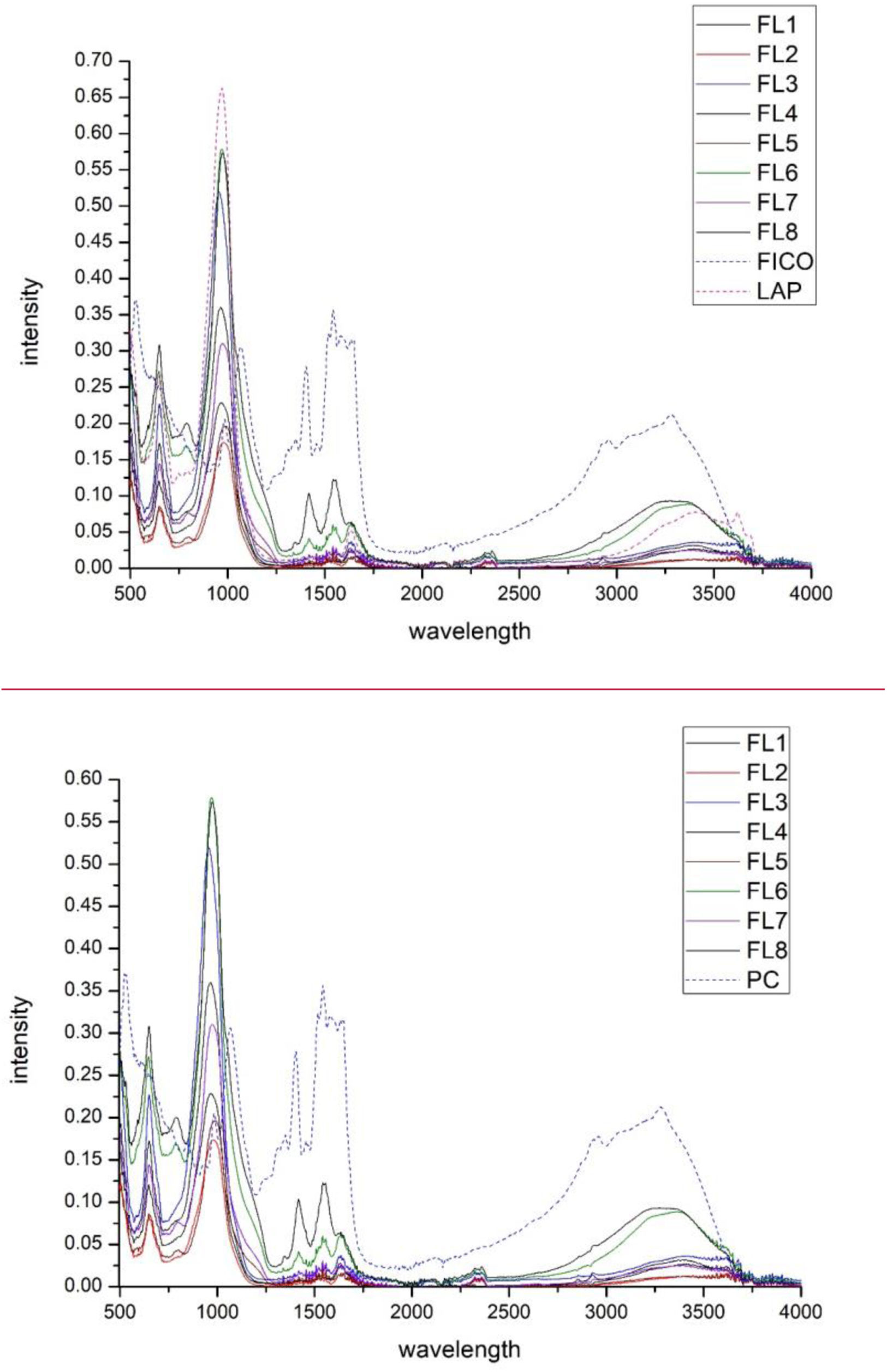
Fourier transform infrared spectroscopy FTIR–ATR analysisWhen analysing Fig. 12 showing the different FTIR spectra, a zone between 3000 and 3600cm−1 is observed which is attributed to the presence of the NH bonds and in the spectral range of 1600–1800cm−1, the presence of the CO bond is evident, with the peak of phycocyanin spectra occurring at a wavelength between 1550 and 1600cm−1[49–51]. Besides the main characteristics of PC have absorption at 3390cm−1 (NH), 1633cm−1 (amide I), 1557cm−1 (amide II) (stretching CO bond), and 1421cm−1 CN) signals [50]. The vibrations produced by the COH bond produce a peak in length at around 1000cm−1[51]. After adsorption of the phycocyanin by the nanoadsorbent, the bands between 1250 and 1750cm−1 have considerably reduced in intensity, as have those in the 2500–3500cm−1 range. A disappearance of the peak at 1100cm−1 is observed in the dye curve, which is covered by the curves offered by the laponite, and therefore does not show up in the component hybrids.
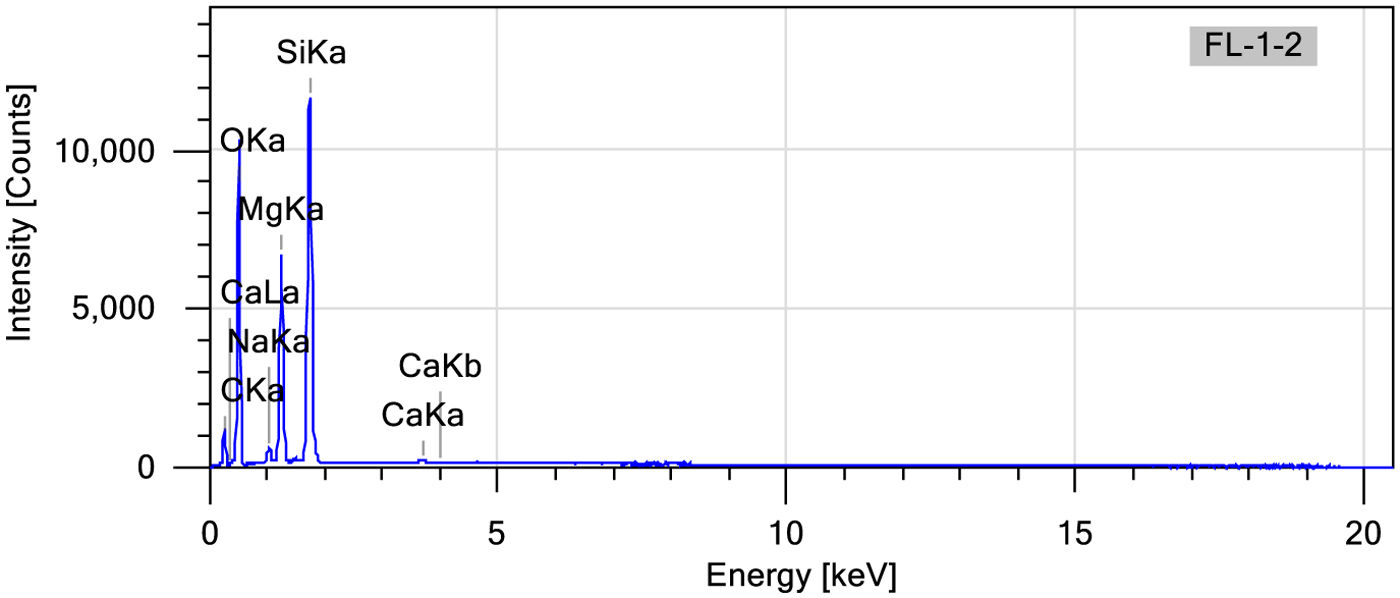
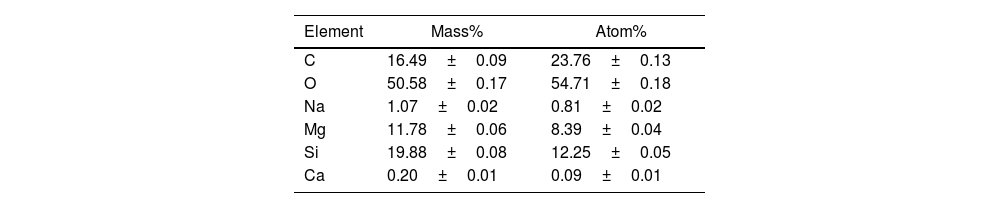
Scanning electron microscopy energy+dispersive X-ray spectroscopy (SEM–EDX)Energy dispersive analysis (EDX) was employed to verify the existence of the component elements within the hybrids, the LDH, and the dye. The spectra derived from this analysis are depicted in Fig. 13. From the already known composition of both laponite and phycocyanin, peaks at 0.2, 0.5, 1.1, 1.2, 1.7 and 3.7keV corresponding to the elements C, O, Na, Mg, Si, Ca respectively can be observed. There is one element of the phycocyanin which does not appear in this analysis, N. This again gives us signs that the dye has been placed in the interlaminar space of the clay and as EDX is a surface analysis it is not able to detect the presence of nitrogen, which reinforces the results obtained with the XDR which already suggested that the dye had been placed in this basal space. Table 6 shows the mass and atom % values for each element detected.
Analysis of variance (ANOVA)The analysis of variance (ANOVA) is performed to determine whether the results analyzed in the previous sections are significantly influenced by any of the parameters selected in the experimental design. In this section we will show only those responses in which significant differences are found, with p-values lower than the significance level a=0.05, or those cases in which the trends suggest that with a greater number of experiments significant differences could be achieved.
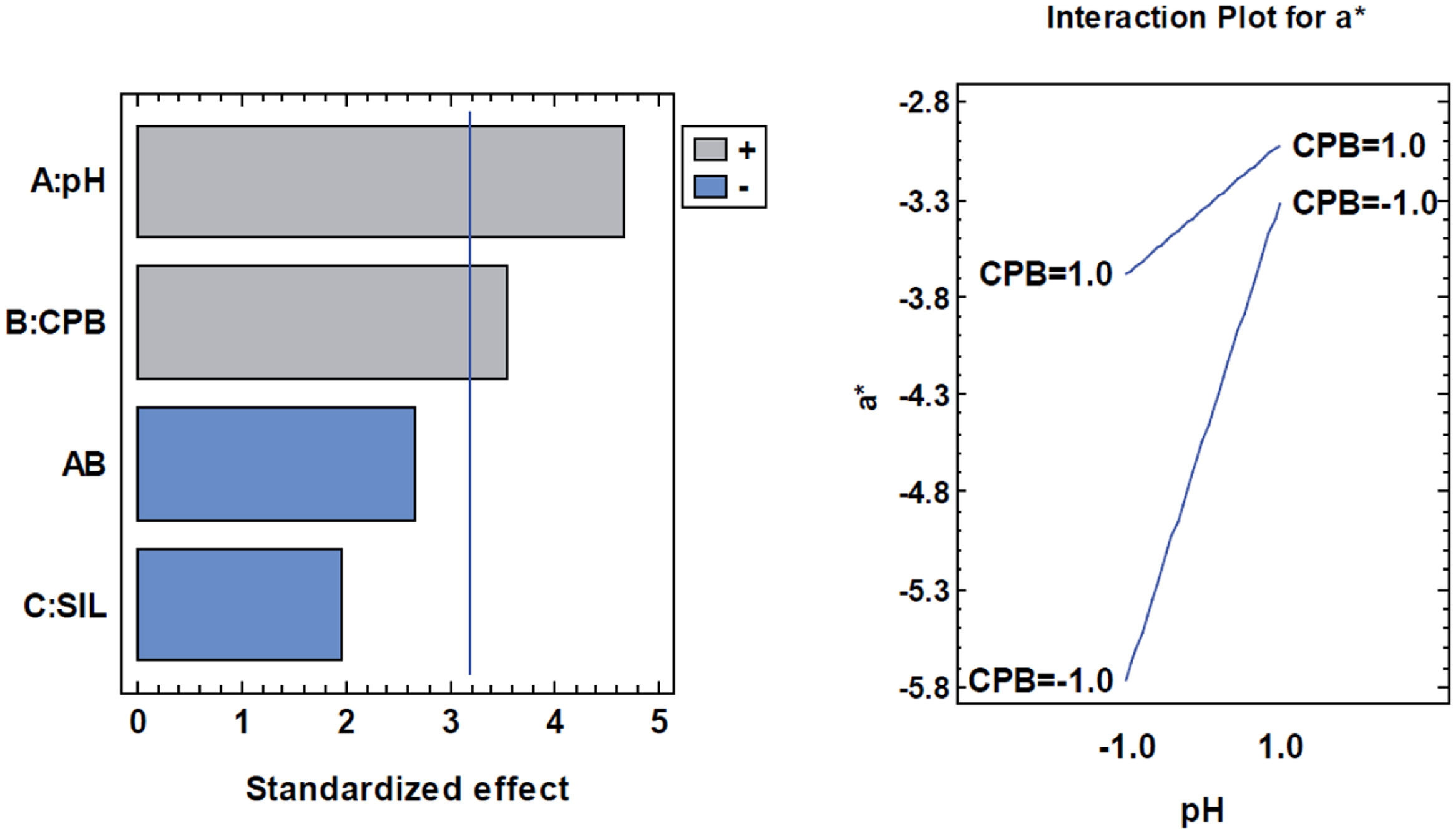
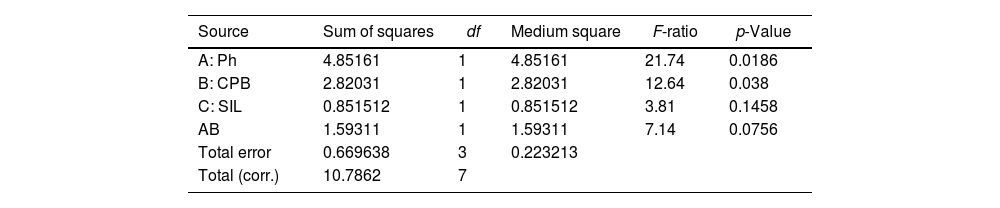
a* parameterThe parameter a* is influenced by the synthesis factors. As can be seen in the ANOVA table (Table 7), both pH and surfactant and their interaction are significant in this parameter that determines how the red-green shades turn.
Analysis of variance for a*.
| Source | Sum of squares | df | Medium square | F-ratio | p-Value |
|---|---|---|---|---|---|
| A: Ph | 4.85161 | 1 | 4.85161 | 21.74 | 0.0186 |
| B: CPB | 2.82031 | 1 | 2.82031 | 12.64 | 0.038 |
| C: SIL | 0.851512 | 1 | 0.851512 | 3.81 | 0.1458 |
| AB | 1.59311 | 1 | 1.59311 | 7.14 | 0.0756 |
| Total error | 0.669638 | 3 | 0.223213 | ||
| Total (corr.) | 10.7862 | 7 |
R-squared=93.7917%.
The interaction shows that the parameter a* is significantly affected by the surfactant when working at acidic pH levels. In this case the samples become significantly redder with surfactant (CPB) (Fig. 14).
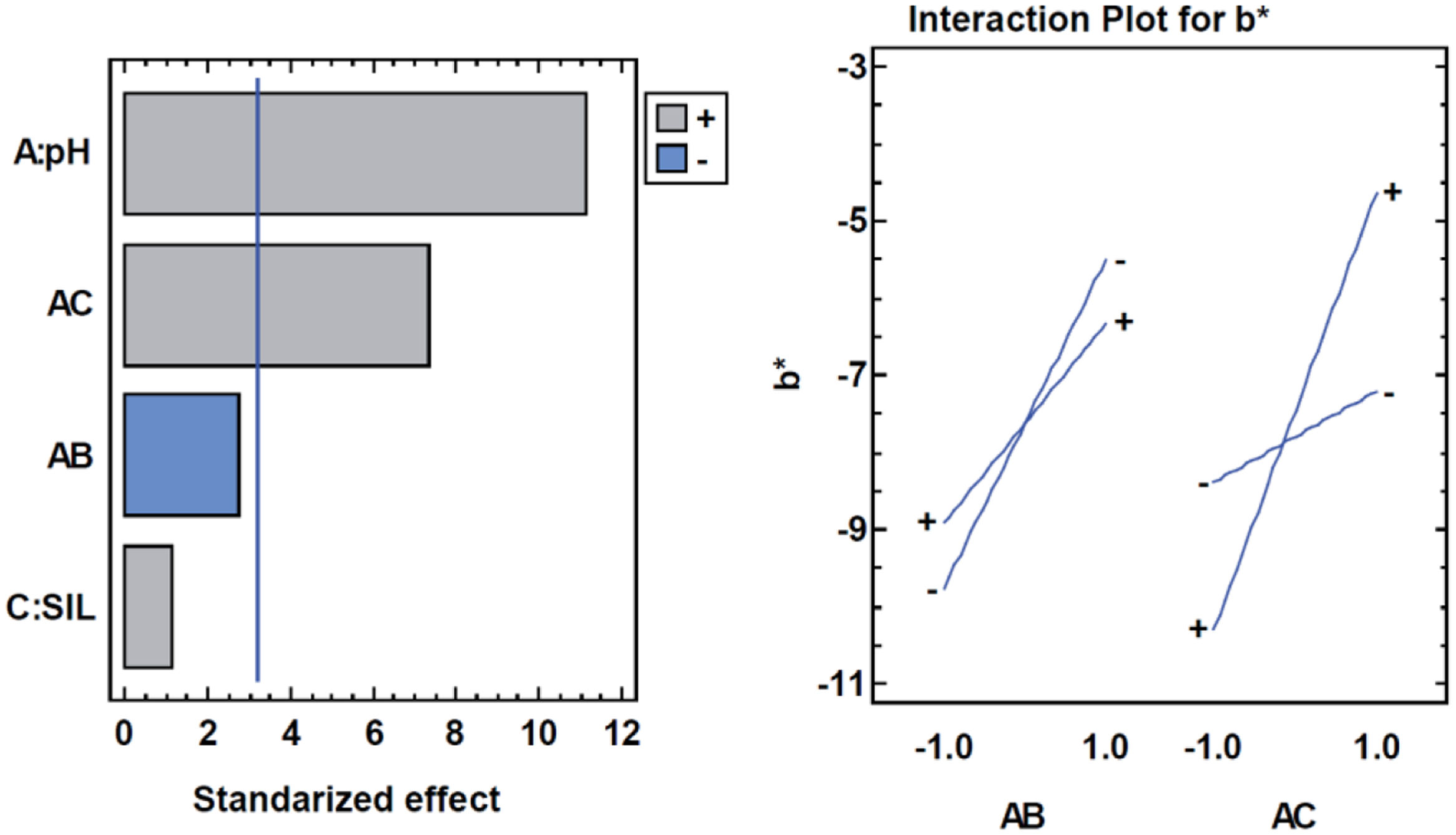
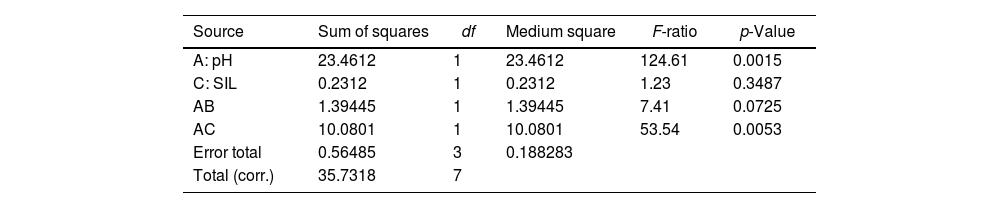
b* parameterAnother parameter that shows significant differences depending on the synthesis conditions is the b* coordinate. In this case, the pH level and Silane are significant, and the pH level is again almost significant with CPB (Table 8).
Analysis of variance for b*.
| Source | Sum of squares | df | Medium square | F-ratio | p-Value |
|---|---|---|---|---|---|
| A: pH | 23.4612 | 1 | 23.4612 | 124.61 | 0.0015 |
| C: SIL | 0.2312 | 1 | 0.2312 | 1.23 | 0.3487 |
| AB | 1.39445 | 1 | 1.39445 | 7.41 | 0.0725 |
| AC | 10.0801 | 1 | 10.0801 | 53.54 | 0.0053 |
| Error total | 0.56485 | 3 | 0.188283 | ||
| Total (corr.) | 35.7318 | 7 |
R-squared=98.4192%.
The effect of the surfactant depends on the pH. At acidic pH with CPB the samples yellow, while at high pH (8–9) the samples yellow more without the surfactant. The opposite is true for silane. At low pH they yellow more without silane, and at higher pH they yellow more with the presence of silane (Fig. 15).
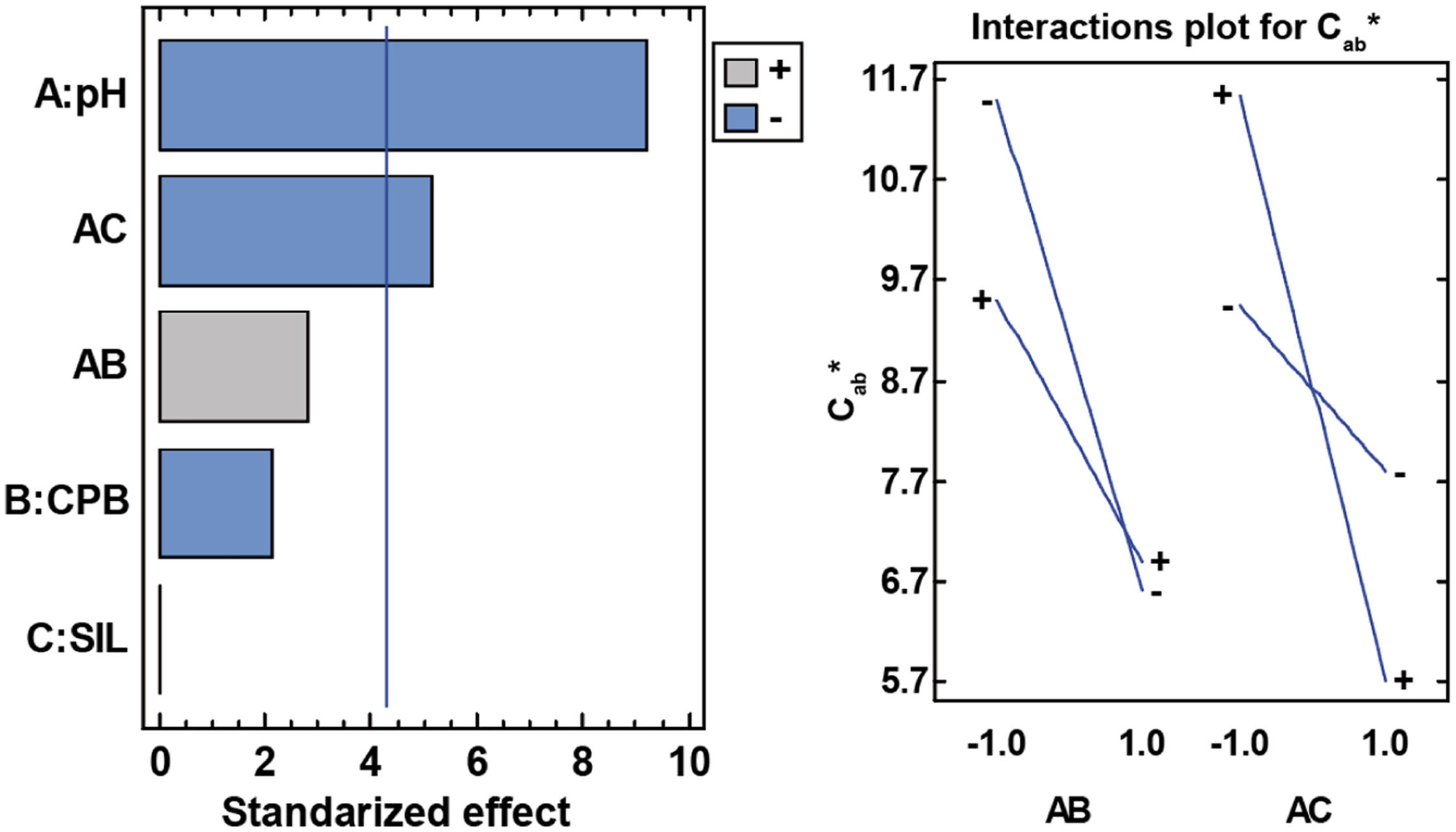
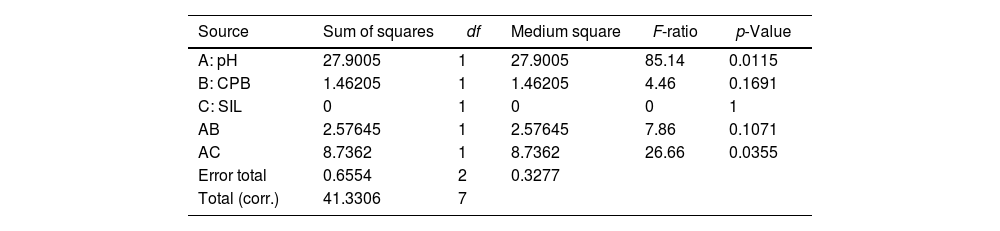
Cab* parameterSince the a* and b* parameters are influenced by the factors selected in the synthesis, it is to be expected, as is the case, that chroma is also significantly influenced. The model fit is very high, as in the previous cases, and in this case the pH interaction with silane is again significant, while the silane factor is not significant at all. The pH is also significant, and its interaction with CPB is close to being significant as in the previous case (Table 9).
Analysis of variance for Cab*.
| Source | Sum of squares | df | Medium square | F-ratio | p-Value |
|---|---|---|---|---|---|
| A: pH | 27.9005 | 1 | 27.9005 | 85.14 | 0.0115 |
| B: CPB | 1.46205 | 1 | 1.46205 | 4.46 | 0.1691 |
| C: SIL | 0 | 1 | 0 | 0 | 1 |
| AB | 2.57645 | 1 | 2.57645 | 7.86 | 0.1071 |
| AC | 8.7362 | 1 | 8.7362 | 26.66 | 0.0355 |
| Error total | 0.6554 | 2 | 0.3277 | ||
| Total (corr.) | 41.3306 | 7 |
R-squared=98.4142%.
At high pHs, CPB does not influence the chroma at all, whereas at low pHs without CPB, higher saturation values are reached. Silane influences both at low and high pH, but inversely. At low pH it is preferable to include silane to achieve the highest saturation values, while at high pH it is better not to include silane (Fig. 16).
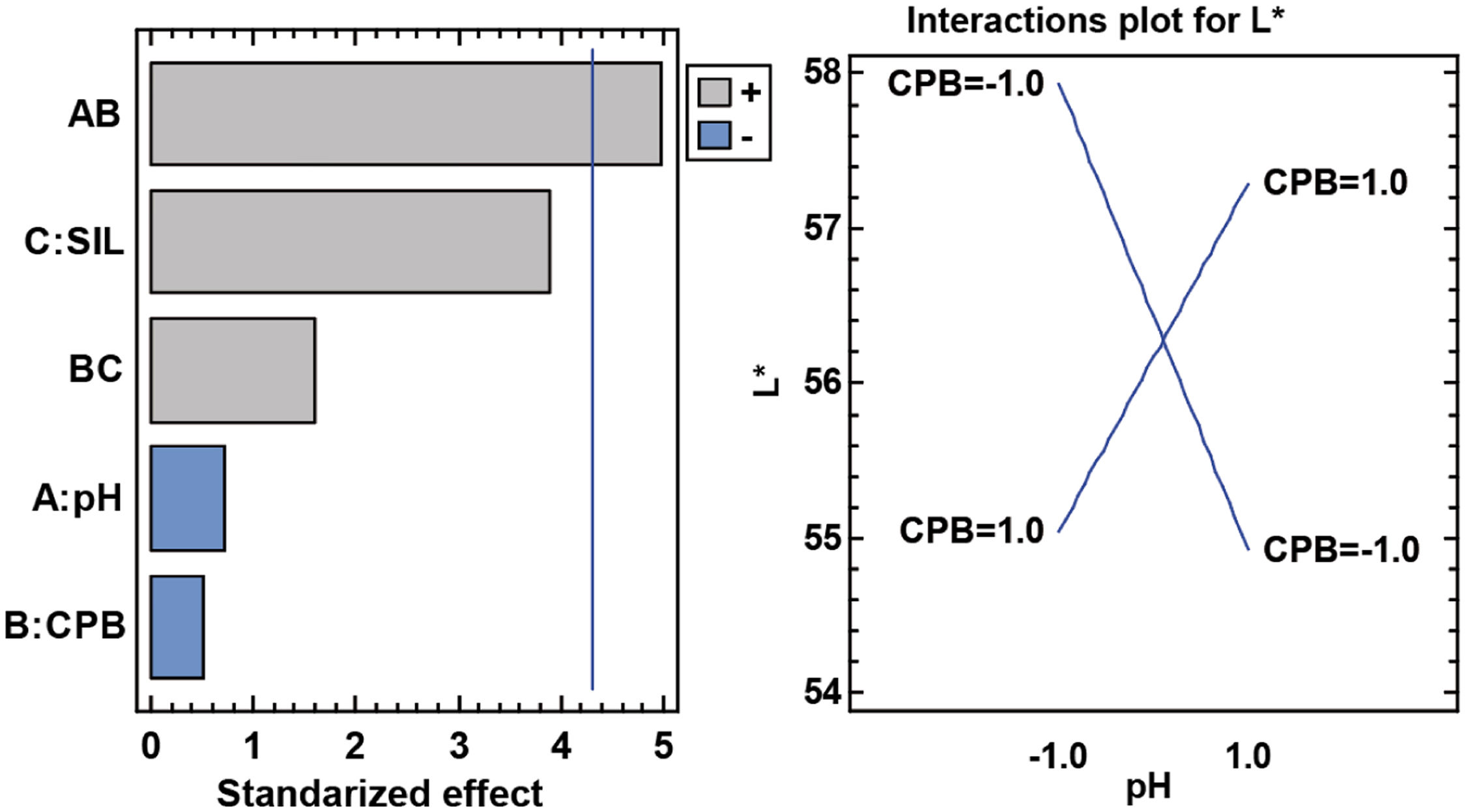
L* parameterAnother parameter that did not appear to be influential in the first graphical analysis was the clarity L*, and in the analysis of variance differences appear in one of the interactions. In this case, significant differences are only seen in the AB interaction, which corresponds to pH and CPB surfactant. In this case, the darkest samples are obtained either with the surfactant at acidic pH or at basic pH without the surfactant (Fig. 17).
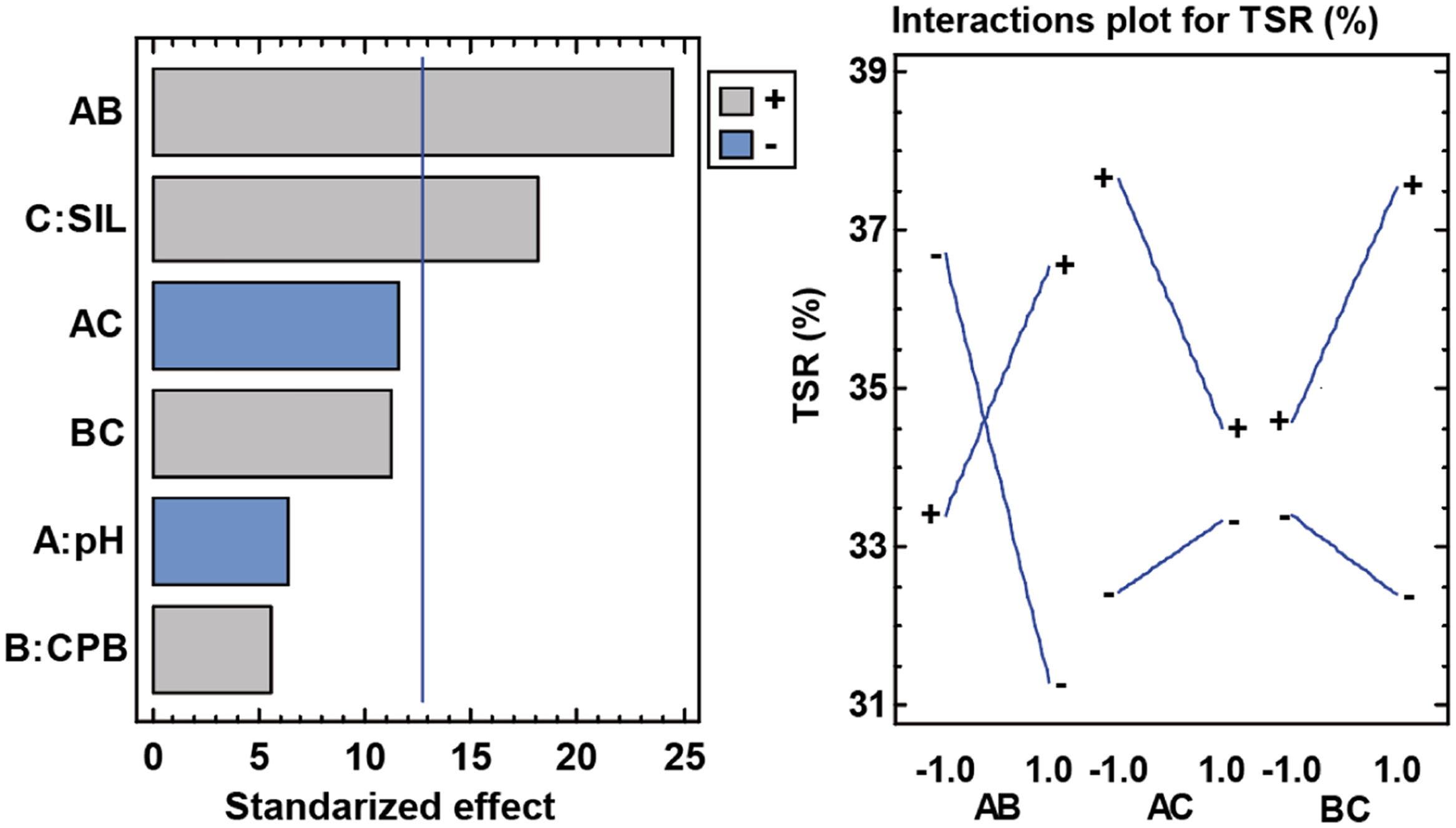
TSR (%) responseThe TSR values (%) are affected by the synthesis parameters. Most influential in this case is the pH and CPB interaction. It is followed by the simple effect of silane, and very close to being significant the interactions pH with silane and CPB with silane. A priori it seems that silane by itself increases the TSR value (%). When looking at the interactions it can be seen that this is accentuated at acidic pH values and when in the presence of CPB (Fig. 18).
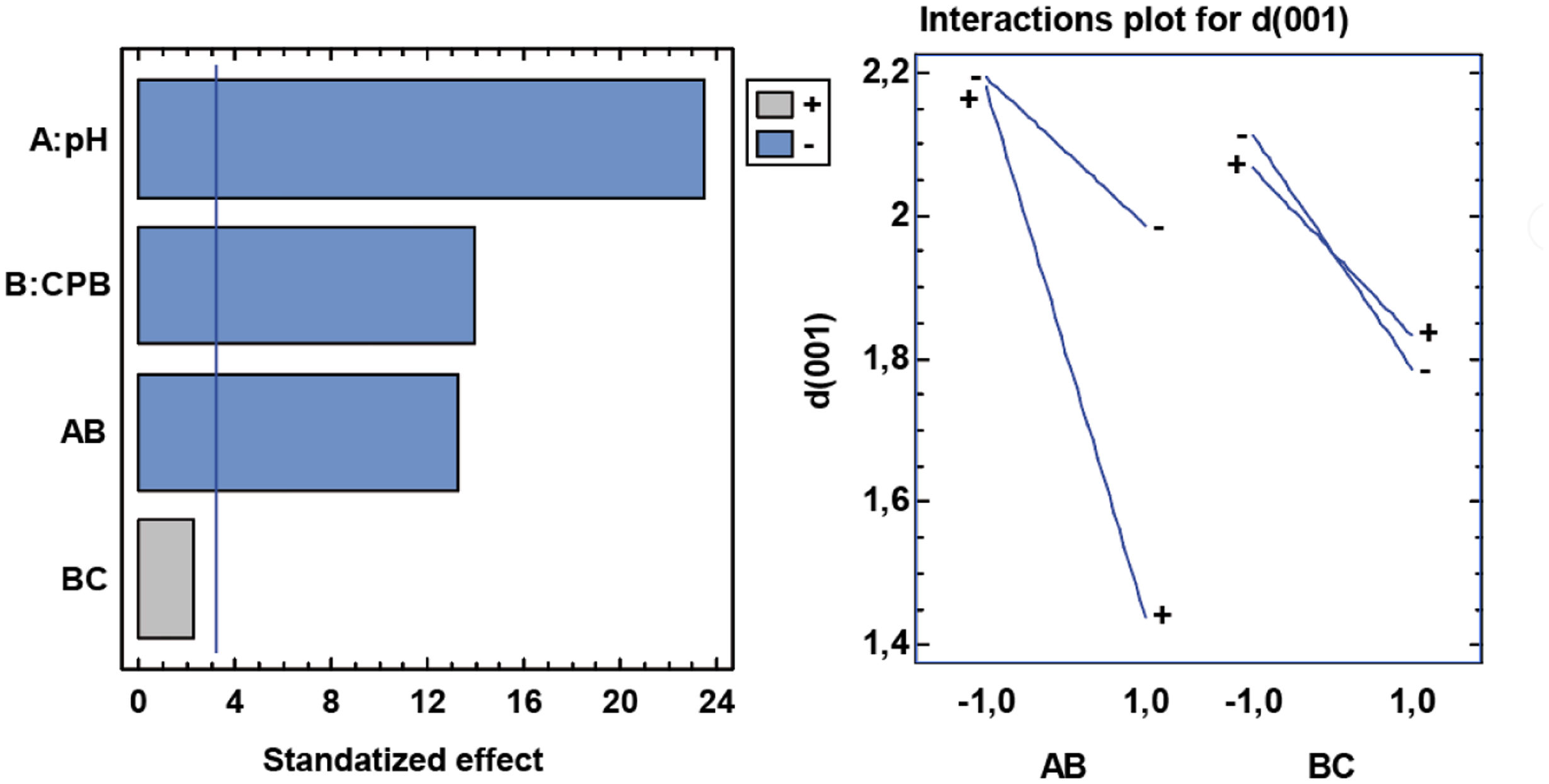
d(001) responseThe values for the nanoclay basal spacing d(001)nm are affected by the synthesis parameters. The analysis of the design of experiments shows that at low or acidic pH, the distance in the d(001) plane increases significantly. The surfactant also affects in such a way that in its presence this gap decreases. In this case there is one interaction that is clearly significant and one that is close to being significant. The significant one is the pH with the CPB, so that at acidic pH levels the presence or not of the surfactant does not affect, while at basic levels the presence of the CPB does have an influence, considerably decreasing the basal space of the clay. As for the combination of the modifiers, silane has almost no effect, but the highest basal space is achieved without either of the two modifiers, or at most with silane and without CPB (Fig. 19).
ConclusionsThe versatility and diverse applications of Laponite clay are remarkable, with a specific focus on its ability to enhance the properties of water-based products. Laponite, an inorganic layered silicate clay, is noted for its exceptional ability to interact with components in aqueous solutions, resulting in a significant increase in viscosity after incorporation. Its unique ability to prevent aggregation of solids makes it a valuable industrial additive as it improves the stability of pigments against environmental factors such as temperature and oxygen.
The application of Laponite has been tested in the adsorption of phycocyanin, a cyanobacterial pigment. The results show successful adsorption, with percentages above 97%, indicating the effectiveness of Laponite in retaining the pigment. Using different experimental conditions, including different pH values, addition of silane and surfactant, which provides valuable information on how to manipulate these variables to optimize adsorption. Although the adsorption values in all cases have been of high value, certain differences have been observed, such as the interlaminar space analyzed in the X-ray diffraction.
Results on the thermal stability of the hybrids, assessed by thermogravimetry, highlight the ability of Laponite to enhance the strength of phycocyanin at elevated temperatures. X-ray diffraction provides in-depth insight into the changes in the crystalline structure of Laponite after phycocyanin adsorption, highlighting the importance of the interaction between clay and pigment. Infrared spectroscopy and scanning electron microscopy (SEM–EDX) analysis reinforces the evidence for the successful placement of the pigment on the clay.
The hybrid obtained can then be put to different uses, such as incorporating it into a printing paste and colouring a textile substrate, incorporating it into a polymer for plastic injection, etc. Although all these applications open up the possibility of continuing to advance in this work, it is necessary to take into account the solidities that they can present to factors such as light, water, rubbing, etc. The results must be in accordance with minimum requirements and if they are not achieved, the applications must be reformulated in order to improve them.
In conclusion, the feasibility of the applicability of Laponite in various fields, from the improvement of rheological properties to the efficient adsorption of pigments, with a specific focus on phycocyanin, has been demonstrated. The experimental results and detailed characterisations provide a valuable basis for the future understanding and application of these hybrids in industrial and scientific applications. Laponite's ability to improve specific properties, such as thermal stability and colour behaviour, positions it as a promising material in the synthesis of new advanced materials.
From the DoE analysis part, it is concluded that, to obtain more turquoise, greenish and bluish shades, it is better to work with acid pH, without CPB and with Silane. As for the colour range, the chroma increases with acid pH as well as without CPB and with silane, and the L* decreases with acid again but in the presence of CPB the sample still becomes darker, which is consistent with the chroma, since if the clarity is greatly reduced it is more difficult to obtain pure and saturated tones. In general, the most interesting colour parameters are obtained at acid pH, without CPB and with silane.
As for the separation between the planes that make up the basal space, they are obtained at acid pH with CPB or basic without CPB, and silane has no influence. The last response in which significant differences are obtained is the TSR%, which increases at acid pH, without CPB and with silane, or at basic pH with CPB and with silane as well.
The project ‘Microbiomat: R+D new dyes for application in vegetable textiles and alternative biomaterials to leather to obtain products for the fashion sector’ is financed by the Valencian Agency for Innovation through the Strategic Projects programme with file number INNEST/2022/214 and is made up of the following consortium José Gisbert S.L. Perchados Textiles S.A., Guerola S.A., Mapelor S.L., Acabados Pegasa S.L. and UPV.