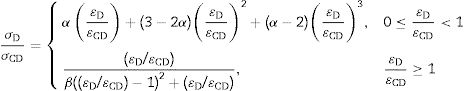This study investigates the characterization of two clays obtained from the Safi and Fez regions, focusing on their analysis for filtration membrane applications. Various analytical techniques were employed, including chemical composition analysis, elemental analysis, mineralogical characterization, carbonate content determination, color assessment, plasticity evaluation, thermal treatment analysis (DTA-TG), mineralogical transformation study, fusion tests, membrane tests, and scanning electron microscopy (SEM).
The results reveal significant differences between the two clays regarding their chemical composition. The red clay exhibits a mineralogical composition comprising quartz, calcite, dolomite, hematite, illite, and kaolinite, whereas the gray clay contains quartz, calcite, dolomite, illite, talc, and montmorillonite. Furthermore, upon thermal treatment, both clays exhibit changes in their physical properties.
Despite the decrease in porosity and water absorption, as well as the increase in compression strength for both clays, the permeability of the grey clay increases, unlike the red clay, which exhibits a constant permeability beyond 1000°C.
These findings highlight the diversity and industrial significance of clays from the Safi and Fez regions for filtration membrane applications. The contrasting properties of red and gray clays provide insights into their potential utilization in different industries. Exploring these clays’ behavior can lead to better filtration membranes and new industrial applications.
Este estudio investiga la caracterización de dos arcillas obtenidas de las regiones de Safi y Fez, centrándose en su análisis para aplicaciones de membranas de filtración. Se emplearon diversas técnicas analíticas, incluyendo análisis de composición química, análisis elemental, caracterización mineralógica, determinación del contenido de carbonato, evaluación del color, evaluación de plasticidad, análisis térmico (DTA-TG), estudio de transformación mineralógica, pruebas de fusión, pruebas de membrana y microscopía electrónica de barrido (SEM).
Los resultados revelan diferencias significativas entre las dos arcillas en cuanto a su composición química. La arcilla roja presenta una composición mineralógica que incluye cuarzo, calcita, dolomita, hematita, illita y caolinita, mientras que la arcilla gris contiene cuarzo, calcita, dolomita, illita, talco y montmorillonita. Además, tras el tratamiento térmico, ambas arcillas experimentan cambios en sus propiedades físicas.
A pesar de la disminución en la porosidad y absorción de agua, así como el aumento en la resistencia a la compresión para ambas arcillas, la permeabilidad de la arcilla gris aumenta, a diferencia de la arcilla roja, que muestra una permeabilidad constante más allá de los 1000°C.
Estos hallazgos resaltan la diversidad y la importancia industrial de las arcillas de las regiones de Safi y Fez para aplicaciones de membranas de filtración. Las características de las arcillas rojas y grises proporcionan información sobre su posible utilización en diferentes industrias. Explorar el comportamiento de estas arcillas puede conducir a mejores membranas de filtración y nuevas aplicaciones industriales.
Morocco has diverse clay deposits, each with special qualities appropriate for industrial and ceramic uses [1,2]. These clays, derived from dissimilar geological formations, have distinct properties, including flexibility, heat resistance, and chemical composition, making them valuable for different industrial uses [3–5]. Moroccan clays are in high demand because of their extraordinary flexibility in the pottery industry [6]. They are perfect for making tiles, pottery, and other ceramic products since they can be easily molded and molded into complicated shapes [7,8]. The chemical composition of Moroccan clays also contributes to their industrial value [9,10]. These clays contain minerals and elements, including silica, aluminum, iron oxide, and oligo-elements. These components enhance the distinctive qualities of clays, making them suitable for various industrial applications [11–14]. For instance, iron oxide gives some clays a distinctive reddish hue highly desired in the ceramics industry for its aesthetic appeal [15–17].
The coastal city of Safi, Morocco, has large deposits of excellent-quality red clay. Safi's clay deposits are distinguished by their purity, consistency, and bright red color, which results from iron oxide [18]. These characteristics make it a valuable resource of industrial importance. Safi red clay is widely used in the industry to produce ceramic tiles, bricks, and handicraft products. Safi red clay is wear-resistant, making it suitable for indoor and outdoor applications [19–21]. The availability of high-quality clay reserves in Safi has contributed to the growth of the local ceramics industry, facilitating economic development and establishing Morocco as a global competitor.
A city renowned for its cultural heritage, Fez is home to large reserves of gray clay [5,8,22]. The deposits of gray clay of Fez have unique properties that make them suitable for various applications [22]. The specific characteristics of gray clay, such as particle size and chemical composition, make it ideal for specific industrial applications. Its use in refractory materials contributes to the heat resistance and structural integrity required in the metallurgy and cement manufacturing industries [23].
In recent years, the use of clays for the preparation of filtration membranes has received considerable attention [24,25]. Clays, including the ones found in Morocco, have fine particles and a porous structure, which makes them suitable for creating membranes with high surface area and selective permeability [26–28]. Modifying the clay's properties by incorporating pore-forming agents and heat treatment makes it possible to adapt the membranes to specific filtration applications [29]. The technology of clay-based membranes provides several advantages for filtration applications: they are cost-effective, readily available, and environmentally friendly compared to traditional filtration materials [30].
Clay-based membranes’ scalability makes them a promising solution for large-scale filtration processes in water treatment, pharmaceuticals, and food processing industries. In addition, its unique properties, such as its high surface area and cation exchange capacity, help improve the performance of filtration membranes [31]. The fineness and porous structure of clays facilitate the retention and removal of contaminants, thus ensuring efficient filtration processes. Furthermore, the ability to modify the properties of clays allows for the optimization of membrane characteristics such as permeability, selectivity, and mechanical strength to meet specific filtration requirements.
Moroccan clays have various properties that contribute to their importance for industrial and ceramic applications. The red clay of Safi and the gray clay of Fez are particularly important because of their reserves and suitability for specific industries. Moreover, using these clays to prepare filtration membranes offers a promising solution for efficient and durable systems. The detailed characterization of these clays allows us to understand all the phenomena that will take place during the preparation of the membranes, and it will also provide valuable information on their filtration performances, allowing the development of advanced filtration technologies in Morocco and the world.
Material and methodsRaw materials and pretreatmentThe two clays investigated in the study have been sourced from two distinct regions in Morocco. The first clay was obtained from Safi (red clay), a coastal city in western Morocco, while the second clay was procured from Fez (gray clay), located in the country's northern central part. The clay samples were collected and then prepared for analysis. This entailed drying the samples and grinding them into a fine powder, which was then passed through a 100μm sieve to remove impurities and coarse particles. The resulting powder was subsequently utilized for all subsequent analyses.
Powder characterization techniquesWe analyzed the chemical composition of each clay using X-ray fluorescence spectroscopy (XRF). This technique allowed us to determine the elemental composition of each sample, including significant elements. A Panalytical ZETIUM X-ray fluorescence instrument (Malvern Panalytical, Malvern, U.K.) was used in this study.
X-ray powder diffraction was also used to examine the mineralogical makeup of the raw materials and ceramics heated to 900, 1000, and 1100°C (XRD). The materials were crushed into a fine powder, and Rigaku SmartLab's CuK radiation diffractometer was used to gather data across a range of 5–60 2Theta by degrees with a 0.04-degree step size (Tokyo, Japan). The PDF 2004 database's diffractogram was compared to diffraction peaks to identify minerals.
Heavy metals were determined using inductively coupled plasma optical emission spectroscopy (ICP-AES, Ultima Expert, Horiba Inc., Toronto, Ontario, Canada). The samples were made as follows: in Teflon digesting vessels, 20mg of the clay sample were precisely weighed, and 1mL of a concentrated HNO3 solution and 4mL of an HF solution were added (Sigma-Aldrich, St. Louis, Missouri, USA). For 75min, the samples were heated in a microwave. The colorless solutions were quantitatively transferred to 100mL volumetric flasks after cooling, and the volume was then filled to the desired level with deionized water.
The thermal analyzer is used to assess the thermal behavior of both clays (DTA-TG) (STA PT 1600, Linseis, Selb, Germany). At a heating rate of 10°C/min, the results were achieved in the air between 25 and 1050°C. Using this method, we could ascertain the temperatures at which the clays underwent mineralogical transition and weight loss.
Using a Mastersizer 2000 laser particle size analyzer, the size distribution of the clay particles was determined (Malvern Panalytical, Malvern, U.K.). Then, the oversized agglomerates were broken up by sonicating the solution for 1min after adding 40mg of the powder to 40mL of water. The plasticity limit is evaluated according to the Moroccan standard Nm iso 17892-12.
The Bernard technique is a good choice for quickly determining the carbonate content % [11]. Five grams of clay powder were combined with 10mL of concentrated hydrochloric acid (Sigma-Aldrich, Missouri, United States) in a graduated cylinder after it was first filled with water (1N). The interaction between the acid and clay particles forced water out of the graduated cylinder, which created CO2. The sample's carbonate quantity was then determined by directly weighing the gas emitted.
Preparation of clay ceramic membraneIn this study, we aimed to investigate precisely the physicochemical, mechanical properties, and workability of the two different types of clays as filtration membranes. We prepared three different sizes of pellets red and gray clay, to achieve this. The powders were carefully prepared by weighing the desired amount of red and gray clay with a precision balance. Next, the pellets made from clay were used in a dry method. Finally, the powder was axially compressed by 2.4 tons to produce pellet (a) and by one ton for pellets (b) and (c), which were then heated to different temperatures.
- •
(a): 20mm in diameter and 2mm in thickness.
- •
(b): 13mm in diameter and 17mm in thickness.
- •
(c): 13mm in diameter and 1.5mm in thickness.
The heat treatment was performed in an electric furnace (LH 15/12 System, Nabertherm Lilienthal, Germany). The thermal cycle is explained below:
The target temperatures of 900, 1000, and 1100°C were attained by applying a temperature ramp of 5°C/min. Following 2h at the predetermined temperature, the samples were allowed to cool naturally while the oven was turned off. Fig. 3 shows the effect of temperature and composition on sintered pellets.
Characterization of clay ceramic membraneThe Archimedes technique was used to calculate the samples’ bulk density, open porosity, and water absorption values as part of the investigation into the physical characteristics of the samples. First, the ceramic pieces were submerged in water for 24h after being dried until their weight (W1) remained constant. Then, the samples’ mass suspended in water was calculated (W2). Then, the pieces were taken out of the water. Finally, before weighing them, the water on the surface was quickly blotted with a paper towel (W3). The following three equations were then used to determine the samples’ water absorption, apparent porosity, and bulk density values [29].
- ■
Water adsorption (%)=((W3−W1)/W1)×100
- ■
Apparent porosity (%)=((W3−W1)/(W3−W2))×100
- ■
Bulk density (%)=((W1)/(W1−W2))×100
Compressive strength was measured using an Instron 3369 apparatus with a load and loading speed of 50kN and 0.1mm/min, respectively, and the pellet size was 13mm×17mm. The morphology and microstructure of the membranes were analyzed using the Hitachi SC 2500 scanning electron microscope (Hitachi High-Technologies Corporation, Japan). An acceleration voltage of 5kV was used for this examination.
Filtration (permeability of membrane)The laboratory-scale frontal filtration pilot comprises three components: a 300mL supply tank, an air compressor, and a pressure gauge. The pressure gauge regulates the pressure of the fluid on the membrane. Before usage, the membrane was immersed in distilled water for 24h and then inserted into the membrane housing, which has an effective filtration surface area of 2cm2. All filtration experiments were conducted at room temperature.
A membrane's permeability (P) is an intrinsic characteristic that depends on its structure. In practical terms, permeability can be defined as the ratio of the permeation flux (JP) to the applied pressure (ΔPm) [32].
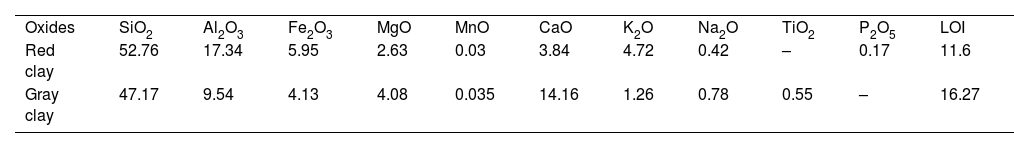
where P is the permeability (in L/hm2bar), JP is the permeation flux (in L/hm2) and ΔPm is the applied pressure (in the bar).Result and discussionChemical compositionsThe chemical compositions of red and gray clay are listed in Table 1. The result of red clay shows that the percentage of SiO2 is very high compared to the other oxides, representing approximately 52.76% of the total weight. Al2O3 is also present in a significant percentage, 17.34%. Fe2O3 and CaO are in smaller quantities than SiO2 and Al2O3, each representing approximately 5.95% and 3.84%, respectively. K2O and Na2O are also in moderate amounts, representing about 4.72% and 0.42%, respectively. MgO, TiO2, and P2O5 are in smaller quantities, representing less than 3% of the total weight.
Gray clay displays a different chemical composition with a relatively high proportion of SiO2, 47%. CaO and Al2O3 are also in significant quantity, representing approximately 14.16% and 9.5% of the total weight, respectively. The material also contains substantial amounts of Fe2O3 and MgO, each comprising around 4% of the total weight. Other oxides, MnO, K2O, Na2O, and TiO2, are in smaller amounts, each comprising less than 1%.
An in-depth reading of the chemical composition shows notable differences between the clay samples “Red” and “Gray” in their oxide percentages. The red clay has a higher ratio of SiO2, Al2O3, Fe2O3, K2O, Na2O, and a lower percentage of CaO and LOI than gray clay. In contrast, gray clay has higher MgO, TiO2, and MnO rates. These variations in oxide percentages may be attributed to differences in the two clays’ origin, mineralogy, and geological history. In general, by comparing the chemical composition of the two clays, it is possible to identify their distinct characteristics and the potential consequences of these disparities on their properties and uses. For example, red clay's SiO2/Al2O3 ratio is 3.02, while gray clay's is 4.94.
Both percentages are significantly higher than the theoretical value of 1.18 for pure kaolinite. They suggest that both samples contain a significant amount of free quartz, aluminosilicate, and other minerals [9,11]. Finally, the loss on ignition (LOI) percentage represents the weight loss due to removing water, organic matter, and decomposition of carbonates during heating. Red clay has a lower LOI percentage, indicating a lower carbonate and organic matter content than gray clay.
Elemental analysisTable 2 shows the concentration of three heavy metals, As, Cd, and Pb, measured in the two clays. Red clay has a concentration of 129ppb for arsenic, 53ppb for cadmium, and 15ppb for lead. On the other hand, gray clay has a higher concentration for all three elements, with 172ppb for arsenic, 41ppb for cadmium, and 10ppb for lead. Comparing the two clays, it is clear that gray clay has a much stronger concentration of these. Specifically, gray clay has around 33% higher concentration of arsenic, 22% lower concentration of cadmium, and 31% lower concentration of Lead.
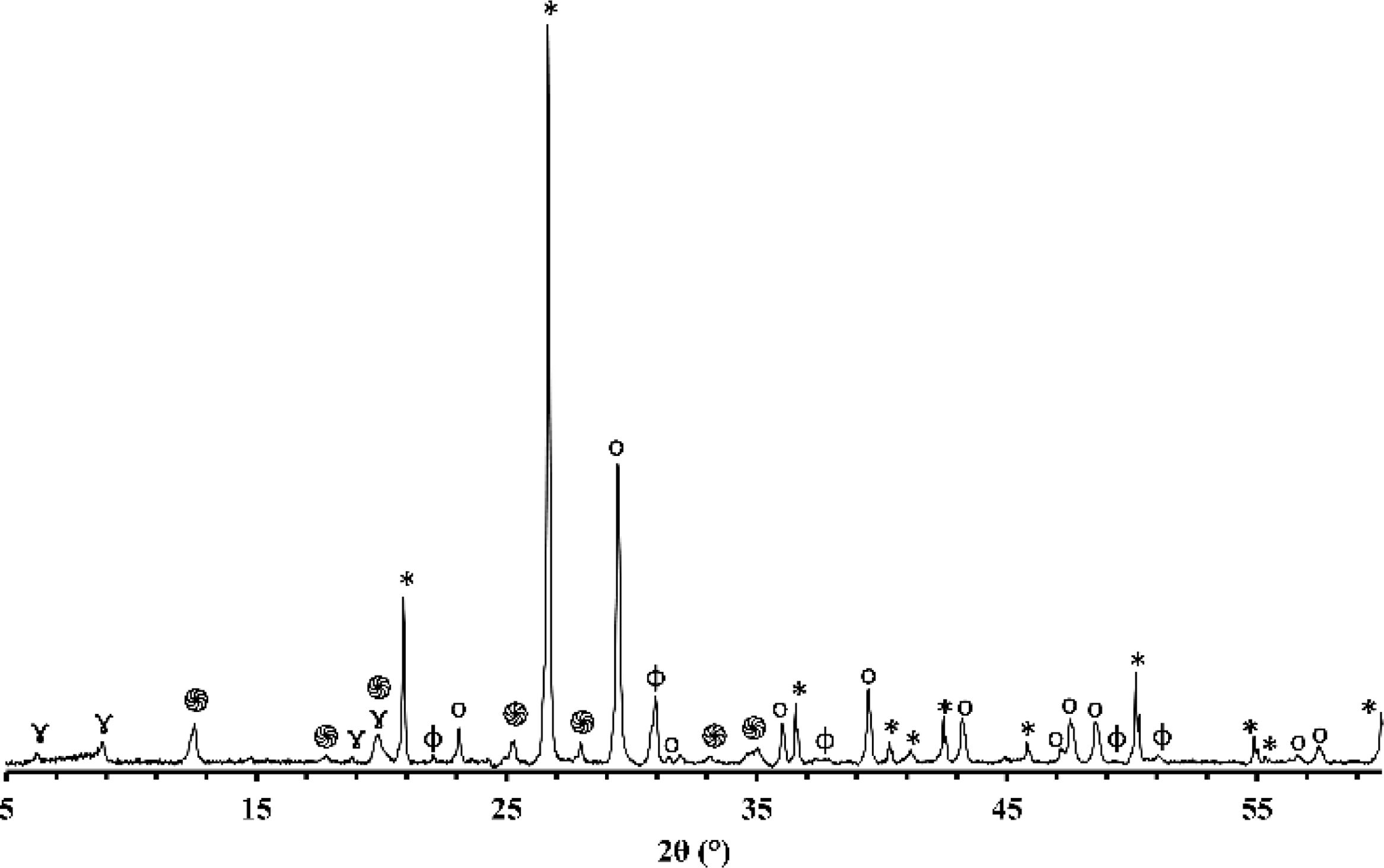
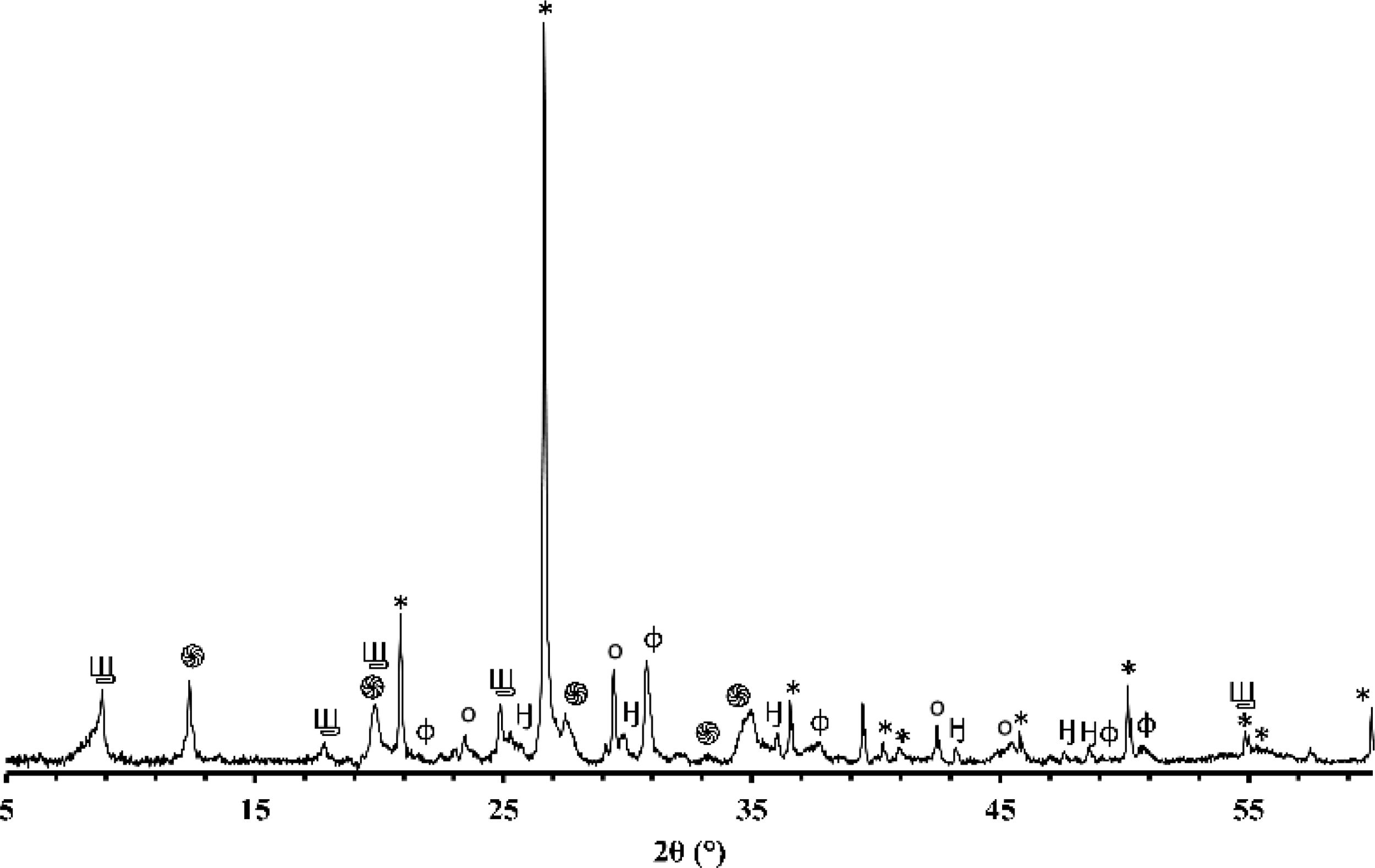
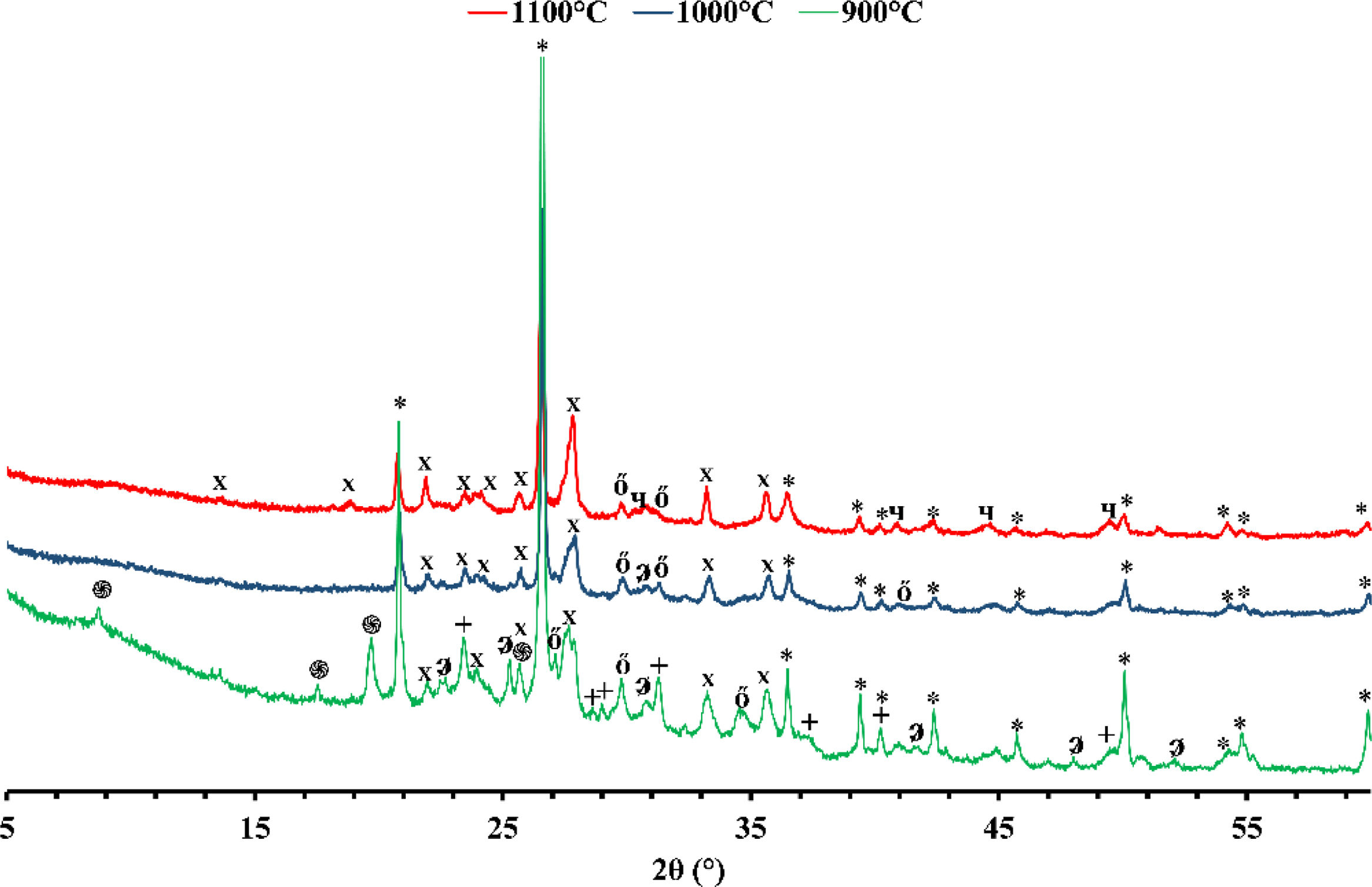
X-ray diffraction (XRD)Figs. 1 and 2 depict the XRD patterns of red and gray clay powder samples, respectively. Red clay contains minerals, including quartz, calcite, dolomite, hematite, illite, and kaolinite. In contrast, gray clay contains slightly different minerals: quartz, calcite, dolomite, illite, talc, and montmorillonite. These results provide valuable information on the mineralogical characteristics of these two raw clay samples, which may have consequences on their potential use in various industrial and commercial applications. For example, the presence of clay minerals may affect the plasticity and workability of the clay, as well as its firing temperature. Upon meticulous examination of the two diffractograms, the intensity of calcite peaks is clearly greater than that of red clay, which is expected due to the significant proportion of calcium oxide present in the chemical composition of the gray clay. However, despite the red clay containing approximately twice the amount of magnesium oxide, the intensity of the dolomite is considerably weak in contrast to red clay. This phenomenon can be explained by forming another primary phase, talc, by this oxide.
The differences in carbonate content between the two clays can be explained by the variance in the clays’ calcium oxide (CaO) and magnesium oxide (MgO) content. Calcium carbonate (CaCO3) is a principal component of various clays and is often used as an indicator of the clays’ overall fertility and productivity. The red clay has a lower carbonate content (4%) than the second clay (16%), implying that the latter possesses a higher concentration of calcium carbonate and dolomite. This deduction is supported by the observation that the gray clay exhibits a considerably higher percentage of CaO (14.16%) than the red clay (3.94%). Magnesium is also noteworthy as it is a significant component of dolomite. The gray clay contains a higher percentage of MgO (4%) than the red clay (2.53%). While the presence of organic matter can influence the carbonate content of clay, it was not considered in this study. This was because the DTA curves of the two clays did not exhibit any exothermic peaks, which would indicate significant thermal decomposition of the organic matter. As a result, the impact of organic matter on the carbonate content was considered negligible for this investigation.
Color of claysThe difference in color between the two clays, red and gray, can be attributed to their mineral compositions. Red clay contains hematite and kaolinite minerals. Hematite is a reddish-brown mineral that gives red clay its reddish hue [33]. Conversely, kaolinite is a white or gray mineral typically not colored, but it can sometimes appear slightly yellow or red [34–37]. Gray clay, on the other hand, contains smectite, montmorillonite, and talc minerals. These minerals do not contain significant amounts of iron oxide like hematite, responsible for the reddish color. Instead, these minerals are gray, which is likely why the clay appears gray [38–40].
PlasticityThe plasticity of clays is primarily determined by mineralogical composition, particle size, particle shape, and water content. However, the chemical composition of the clay also plays a significant role in deciding its plasticity [41]. First, the red clay with a low carbonate content and high Al2O3 content has higher plasticity (25%) than the gray clay (15%) with a higher carbonate content and low montmorillonite content. This is because the Al2O3 content in the Safi clay can contribute to forming clay minerals, resulting in an increased surface charge and a strong affinity for water molecules, leading to high plasticity. Additionally, the Safi clay's illite and kaolinite clay phases are known to have high plasticity [42]. On the other hand, according to the bibliography, the characteristics of the talc include low specific surface areas. Furthermore, it is generally accepted that the basal surfaces of talc and pyrophyllite are hydrophobic, and the edges are hydrophilic. From these results, it can be inferred that the plasticity of the gray clay is adversely affected by the presence of talc.
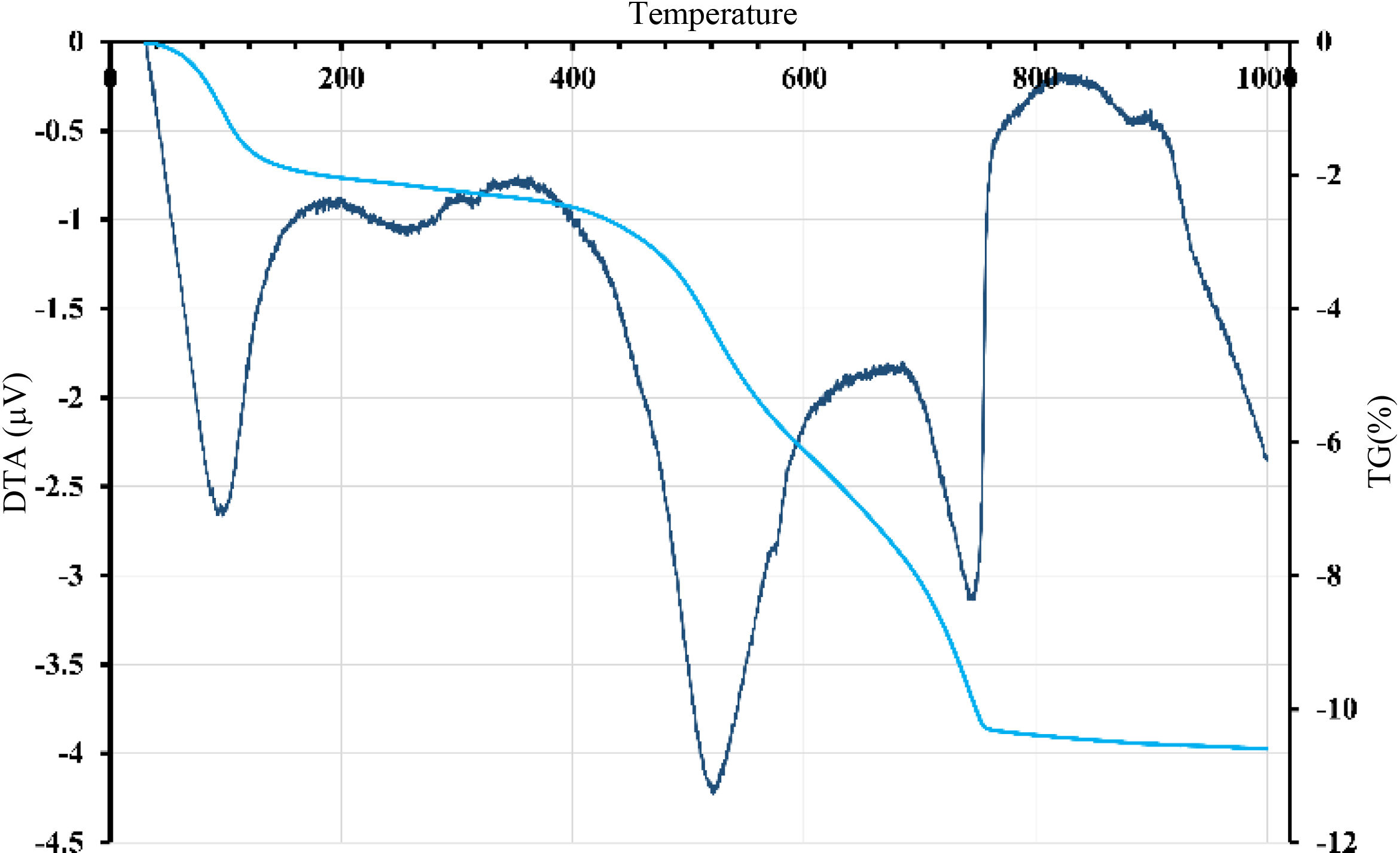
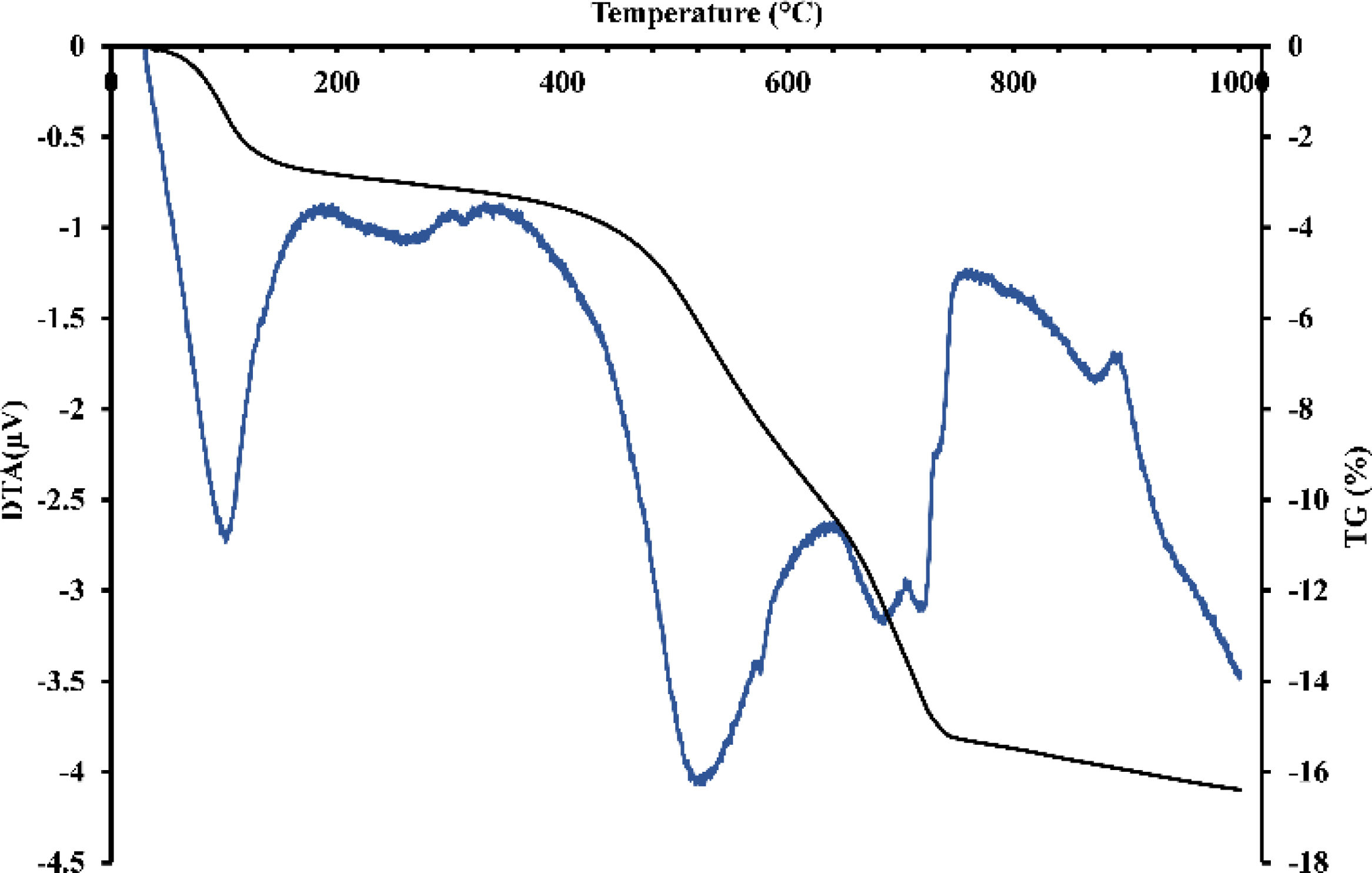
Thermal treatment analysisDTA-TGFig. 3 revealed endothermic peaks at different temperatures with corresponding mass loss. The initial peak at 95°C resulted in a mass loss of about 0.65%, attributed to removing hygroscopic water. A more prominent endothermic peak with a center temperature of 575°C caused a significant mass loss likely caused by the dehydroxylation of the kaolinite. A small peak observed at 595°C could be related to the allotropic transformation of quartz. Around 750°C, the last peak resulted in a significant mass loss and could indicate the calcite's and dolomite's decarbonization. These results suggest that the material undergoes multiple transformations when heated and that each transformation could develop distinct mineralogical phases. The thermal behavior of gray clay (Fig. 4) was similar to that of red clay. Several endothermic peaks were observed. The initial peak detected at 95°C resulted in a mass loss of around 3%, indicating the removal of hygroscopic water. The significant loss in mass is due to smectite, a type of clay mineral with a high surface area that can swell in the presence of water, which can cause a decrease in the bulk density of the material in which it is present.
A larger endothermic peak was observed at 575°C, resulting in a consequent loss of mass, which could signify the dehydroxylation of smectite, talc, and montmorillonite. Another minor peak was observed at 595°C, which could be linked to the allotropic transformation of quartz. Finally, the last two peaks, centered around 700 and 750°C, resulted in significant mass loss, suggesting the decomposition of calcite and dolomite.
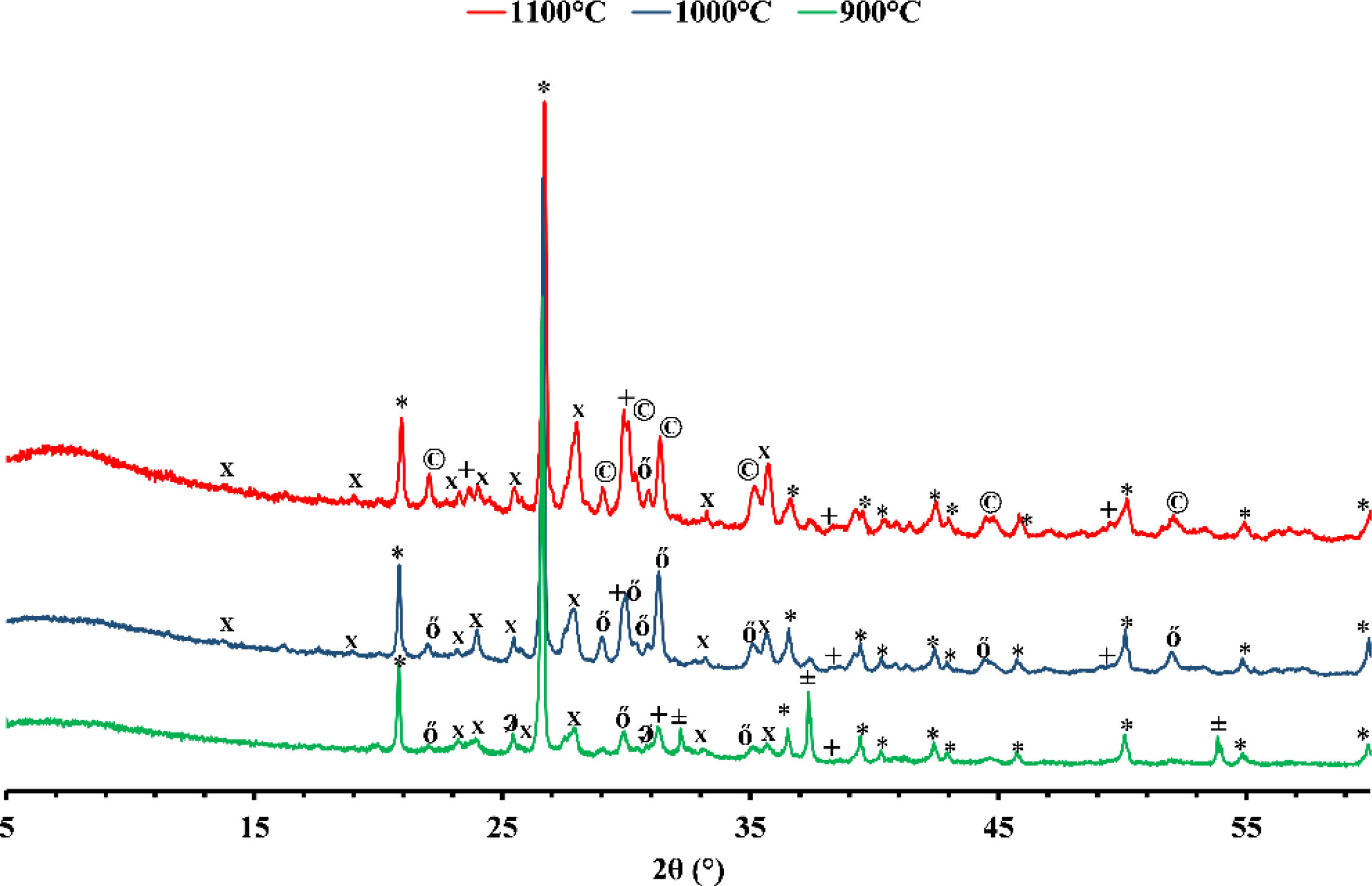
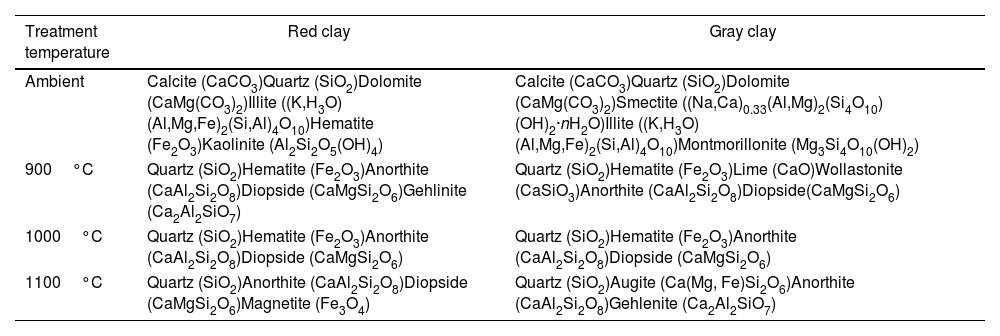
Mineralogical transformationFigs. 5 and 6 show the phases formed during the treatment at different temperatures. These minerals already contain the phases that exist at room temperature, which are calcite (CaCO3), quartz (SiO2), dolomite (CaMg(CO3)2), illite ((K,H3O)(Al,Mg,Fe)2(Si,Al)4O10), hematite (Fe2O3), and kaolinite (Al2Si2O5(OH)4). As the temperature increases, various phase transformations occur. At 900°C, the minerals react to form anorthite (CaAl2Si2O8), diopside (CaMgSi2O6), and gehlinite (Ca2Al2SiO7). At 1000°C, the minerals present at 900°C continue to form, except for gehlinite (Ca2Al2SiO7), which disappears. The evolution of the mineral phases present can be deduced by examining the variation of their intensity on the diffractogram. Gehlinite (Ca2Al2SiO7) is a mineral phase that forms at around 900°C, as shown in Table 3. However, it is not present at 1000°C. This is because, at 1000°C, the Ca2Al2SiO7 phase undergoes a phase transformation into the CaAl2Si2O8 phase when there is a substantial amount of free quartz [11,18].
Minerals transformation after thermal treatment.
| Treatment temperature | Red clay | Gray clay |
|---|---|---|
| Ambient | Calcite (CaCO3)Quartz (SiO2)Dolomite (CaMg(CO3)2)Illite ((K,H3O)(Al,Mg,Fe)2(Si,Al)4O10)Hematite (Fe2O3)Kaolinite (Al2Si2O5(OH)4) | Calcite (CaCO3)Quartz (SiO2)Dolomite (CaMg(CO3)2)Smectite ((Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2·nH2O)Illite ((K,H3O)(Al,Mg,Fe)2(Si,Al)4O10)Montmorillonite (Mg3Si4O10(OH)2) |
| 900°C | Quartz (SiO2)Hematite (Fe2O3)Anorthite (CaAl2Si2O8)Diopside (CaMgSi2O6)Gehlinite (Ca2Al2SiO7) | Quartz (SiO2)Hematite (Fe2O3)Lime (CaO)Wollastonite (CaSiO3)Anorthite (CaAl2Si2O8)Diopside(CaMgSi2O6) |
| 1000°C | Quartz (SiO2)Hematite (Fe2O3)Anorthite (CaAl2Si2O8)Diopside (CaMgSi2O6) | Quartz (SiO2)Hematite (Fe2O3)Anorthite (CaAl2Si2O8)Diopside (CaMgSi2O6) |
| 1100°C | Quartz (SiO2)Anorthite (CaAl2Si2O8)Diopside (CaMgSi2O6)Magnetite (Fe3O4) | Quartz (SiO2)Augite (Ca(Mg, Fe)Si2O6)Anorthite (CaAl2Si2O8)Gehlenite (Ca2Al2SiO7) |
Finally, when red clay is subjected to a temperature of 1100°C, some minerals undergo significant changes. However, few transformations occur, except for the appearance of a new phase, magnetite (Fe3O4), and the disappearance of hematite. The intensity of anorthite increases while that of quartz decreases.
Gray clay is a complicated mixture of several materials at room temperature, including calcite (CaCO3), quartz (SiO2), dolomite [CaMg(CO3)2], smectite [(Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2·nH2O], illite [(K,H3O)(Al,Mg,Fe)2(Si,Al)4O10], and montmorillonite [Mg3Si4O10(OH)2]. At 900°C, the gray clay is primarily composed of quartz (SiO2), hematite (Fe2O3), and a range of high-temperature minerals that form through the decomposition of illite and calcite. The decomposition of illite can result in feldspars, such as CaAl2Si2O8 and KAlSi3O8, while calcite decomposition can lead to wollastonite formation (CaSiO3) and lime (CaO). By heating clay minerals to temperatures above 1000°C, these minerals can develop through high-temperature processes like pyro-metamorphism. These minerals can arise via the recrystallization of clay minerals at high temperatures or from the disintegration of pre-existing minerals like feldspars and pyroxenes. Quartz (SiO2), hematite (Fe2O3), and a variety of high-temperature minerals, such as anorthite (CaAl2Si2O8), diopside (CaMgSi2O6), and gehlenite (Ca2Al2SiO7), make up the majority of the gray clay at 1000°C. When the temperature increases to 1100°C, the diffractogram shows no significant alterations, indicating that anorthite and gehlinite remain in the dominant phases. However, diopside transforms and is converted into augite Ca(Mg,Fe)Si2O6 through the process of Mg substitution by Fe. This change in mineral composition can be attributed to the high-temperature conditions.
Color change and fusion testFig. 7 illustrates how temperature and mineral transformations influence the color of ceramic membranes. The red clay color variations provide insights into the behavior of the illite mineral under different heating conditions. When exposed to 900°C, the illite mineral breaks down, releasing iron oxide [11]. This process results in a brick-red hue that remains even when the temperature is raised to 1000°C. Interestingly, at 1000°C, the iron oxide does not undergo significant changes, and the brick-red hue remains unchanged. However, when the temperature is increased to 1100°C, the iron oxide undergoes an oxidation process, leading to the formation of magnetite. The creation of magnetite gives the material a brown hue distinct from the original brick-red color.
The color of the sample is gray at room temperature. However, as the sample was heated to 900°C, the color changed to brick red, which was attributed to the release of iron oxide resulting from the decomposition of illite. No significant transformation was observed for iron oxide when the temperature was increased to 1000°C. However, at 1100°C, the sample turned yellow due to the reaction of hematite, resulting in the formation of augite. These results suggest that mineral composition changes can cause significant alterations in a sample's color.
After subjecting red clay with 3.84% CaO and gray clay with 14.16% CaO to heat treatment at 1170°C, noticeable differences were observed in Fig. 8. While the gray clay was completely melted, the red clay remained intact, and the pellet appeared to retain its original shape. This suggests that a higher calcium oxide (CaO) content in the gray clay caused it to melt under the given temperature. In contrast, the red clay, with a lower CaO content, displayed greater resistance to the heat and maintained its form [43].
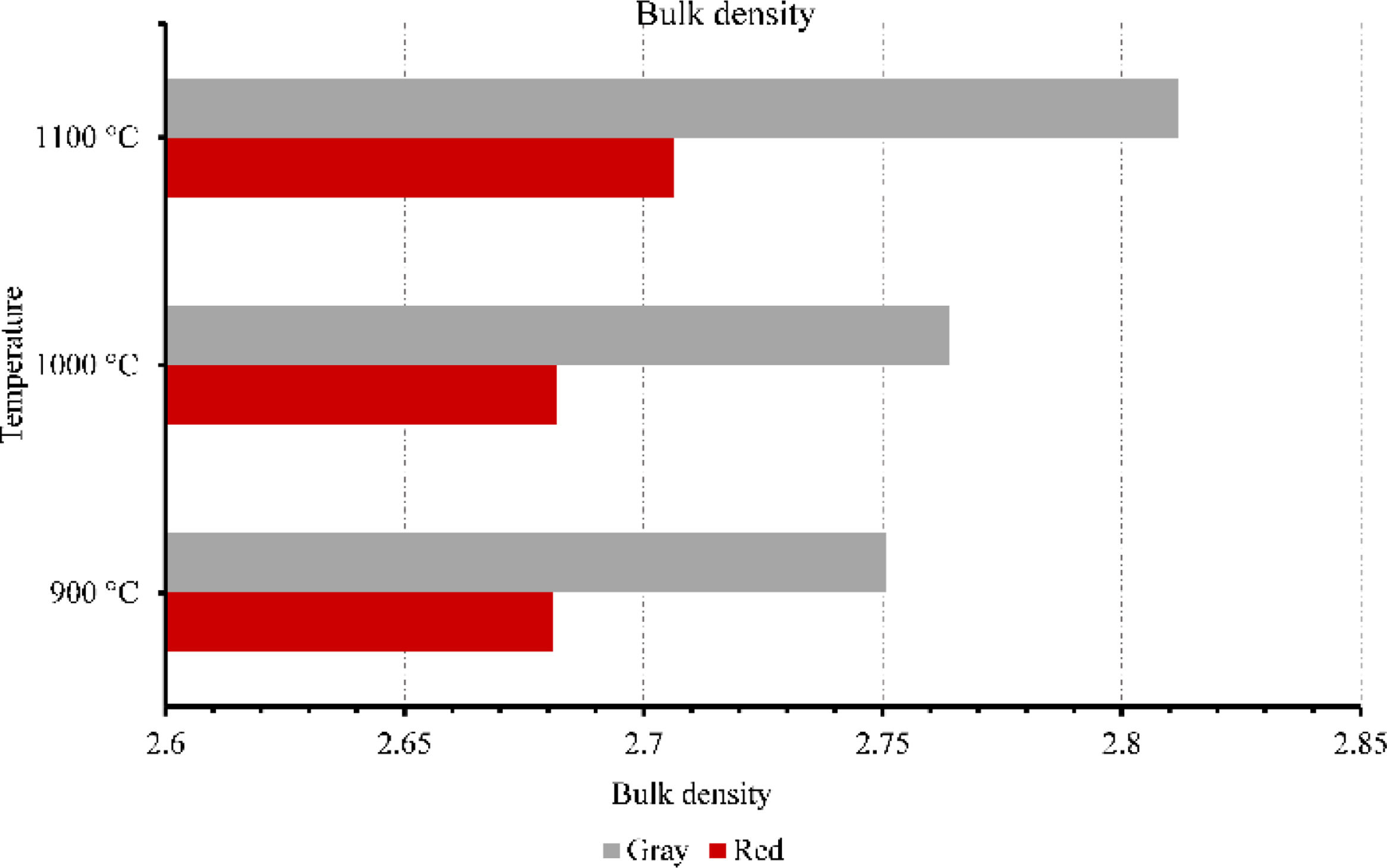
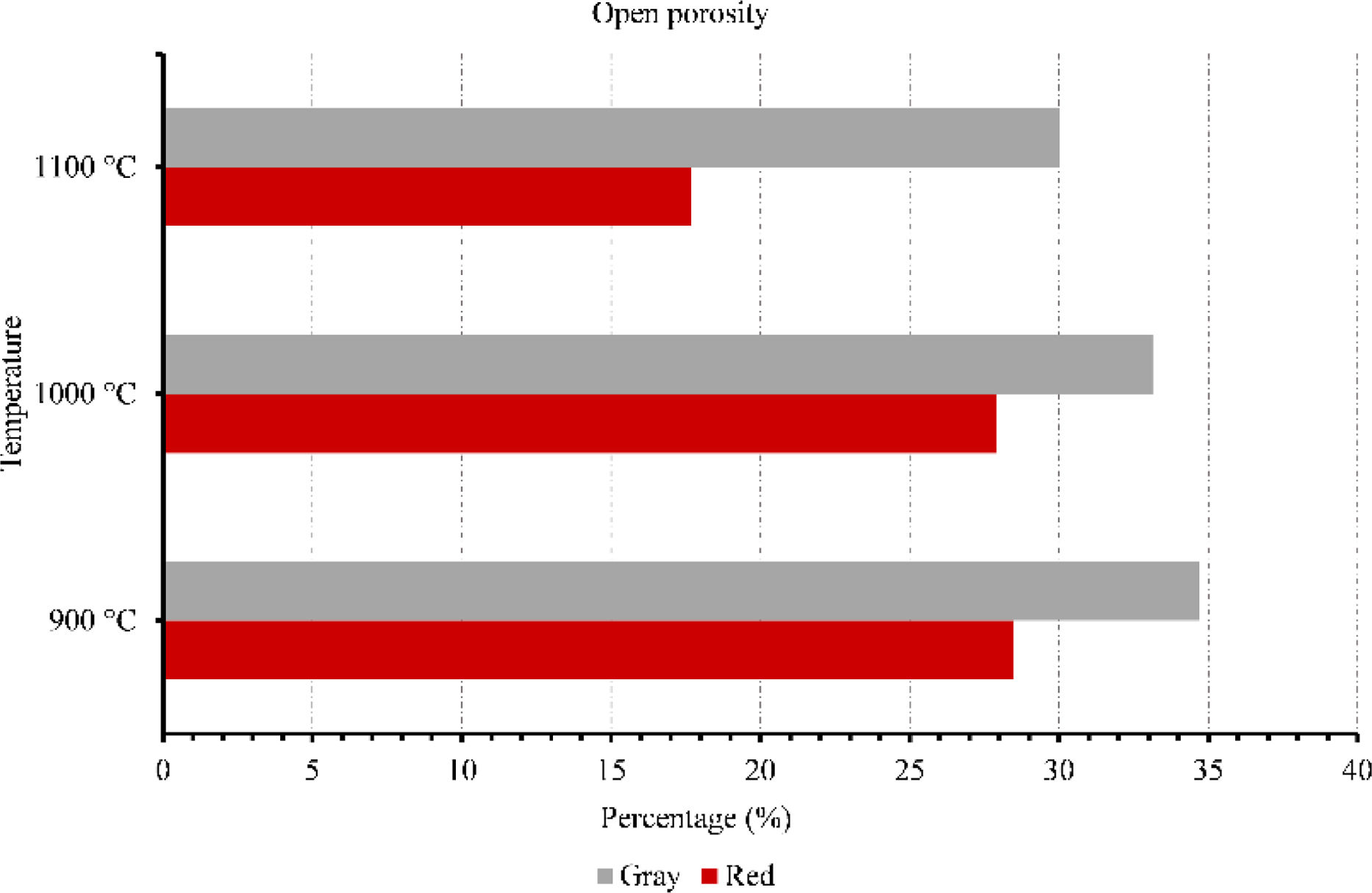
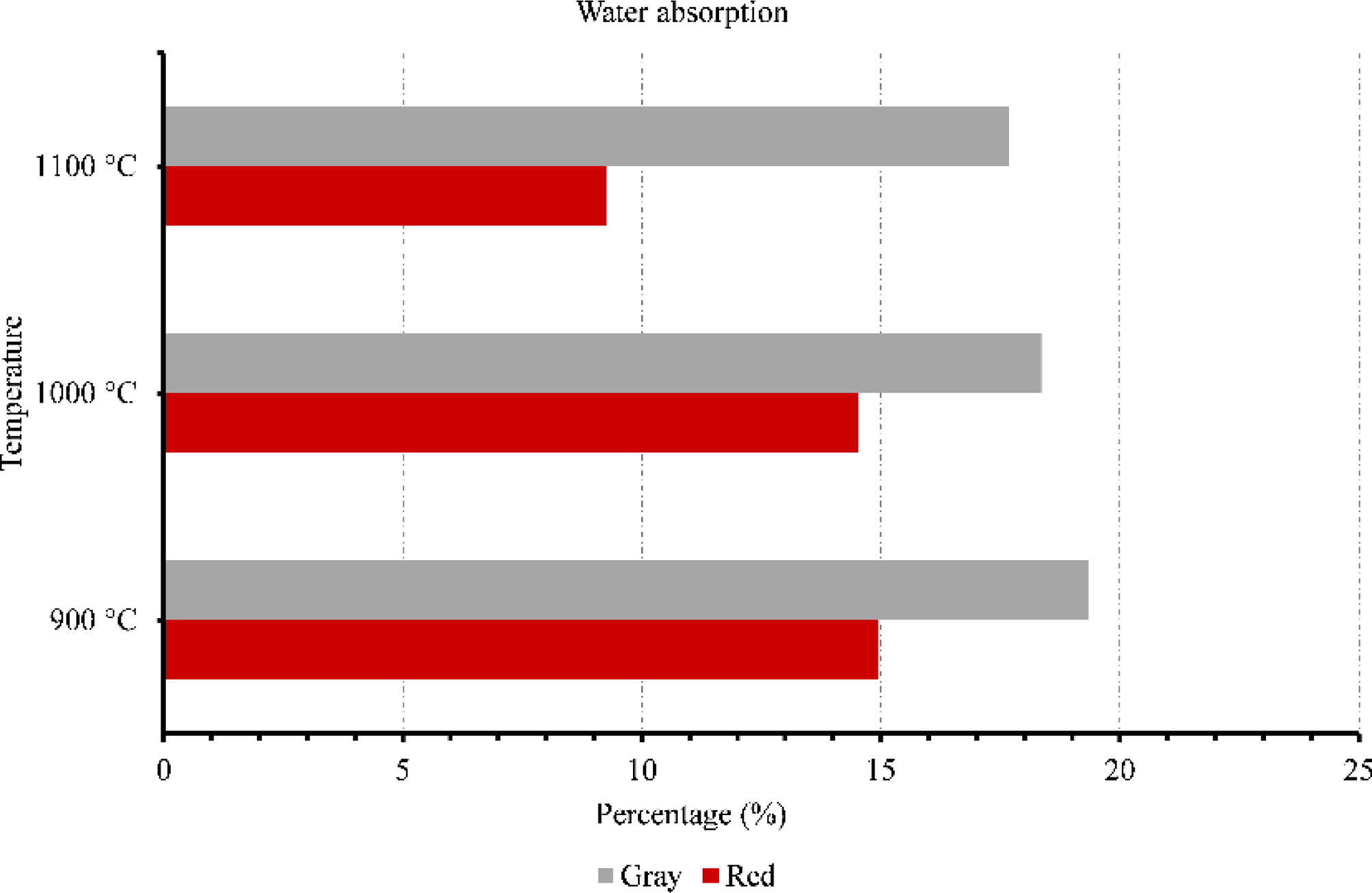
Membranes testsFigs. 9–11 provide data on various properties measured at different temperatures for two types of clays: red and gray. The properties include open porosity, water absorption, and bulk density. The open porosity of both red and gray clays was measured at three different temperatures: 900°C, 1000°C, and 1100°C.
At 900°C, the red clay exhibited an open porosity of 28%, while the gray clay had a slightly higher open porosity of 35%. When the temperature was increased to 1000°C, the open porosity decreased for both materials, with the red clay showing a value of 28% and the gray clay measuring 33%. At the highest temperature of 1100°C, the red clay's open porosity dropped to 18%, while the gray clay maintained a value of 30%. Similarly, the water absorption was assessed under an identical set of three temperatures. When exposed to a temperature of 900°C, the red clay exhibited a water absorption rate of 15%, whereas the gray clay displayed a slightly higher absorption rate of 19%. As the temperature increased to 1000°C, the water absorption of the red clay decreased to 14%, while the gray clay experienced a reduction to 18%. Finally, at the highest temperature of 1100°C, the water absorption of the red clay plummeted to 9%, while the gray clay maintained a value of 17%.
Furthermore, the bulk density was assessed. At 900°C, the red clay exhibited a bulk density of 2.68, whereas the gray clay had a slightly higher value of 2.75. The bulk density remained relatively consistent as the temperature increased to 1000°C, with the red clay maintaining a measurement of 2.68 and the gray clay showing a value of 2.76. However, when the temperature reached 1100°C, both clays experienced a slight increase in bulk density, with the red clay measuring 2.70 and the gray clay measuring 2.81. It can be observed that the gray clay consistently had higher bulk density values compared to the red clay across all three temperatures. At 900°C, the difference was minimal, with the gray clay having a slightly higher bulk density. However, as the temperature increased to 1100°C, the disparity in bulk density between the two clays became more pronounced, with the gray clay exhibiting a significantly higher value of 2.81 compared to the red clay's 2.70. This suggests that the gray clay may have a higher packing density or more excellent compaction at elevated temperatures than the red clay.
Both the mineral composition of the ceramic pastes and the optimum firing temperature play a crucial role in determining the efficiency of filtration membranes. These two factors influence the formation of pores in the membrane structure, which has a direct impact on its porosity and, consequently, on its filtration efficiency. At lower firing temperatures, the presence of carbonates and pore formation are closely linked. Carbonation can break down during firing, releasing gases that create pores in the ceramic matrix. Furthermore, pore-forming mechanisms such as sintering and phase transformations are influenced by firing temperature and mineralogical composition. However, at higher firing temperatures, the behavior changes. As temperature increases, ceramic materials undergo vitrification, where pores can be filled with glassy phases, resulting in a decrease in porosity. This is due to the densification of the ceramic structure during sintering, which leads to pore closure or consolidation.
Overall, these results highlight the variations in bulk density, water absorption, and open porosity between red and gray clays at different temperatures, which indicate differences in their structural characteristics and responses to temperature changes. This result will influence their mechanical strength as well as their filtering capacity.
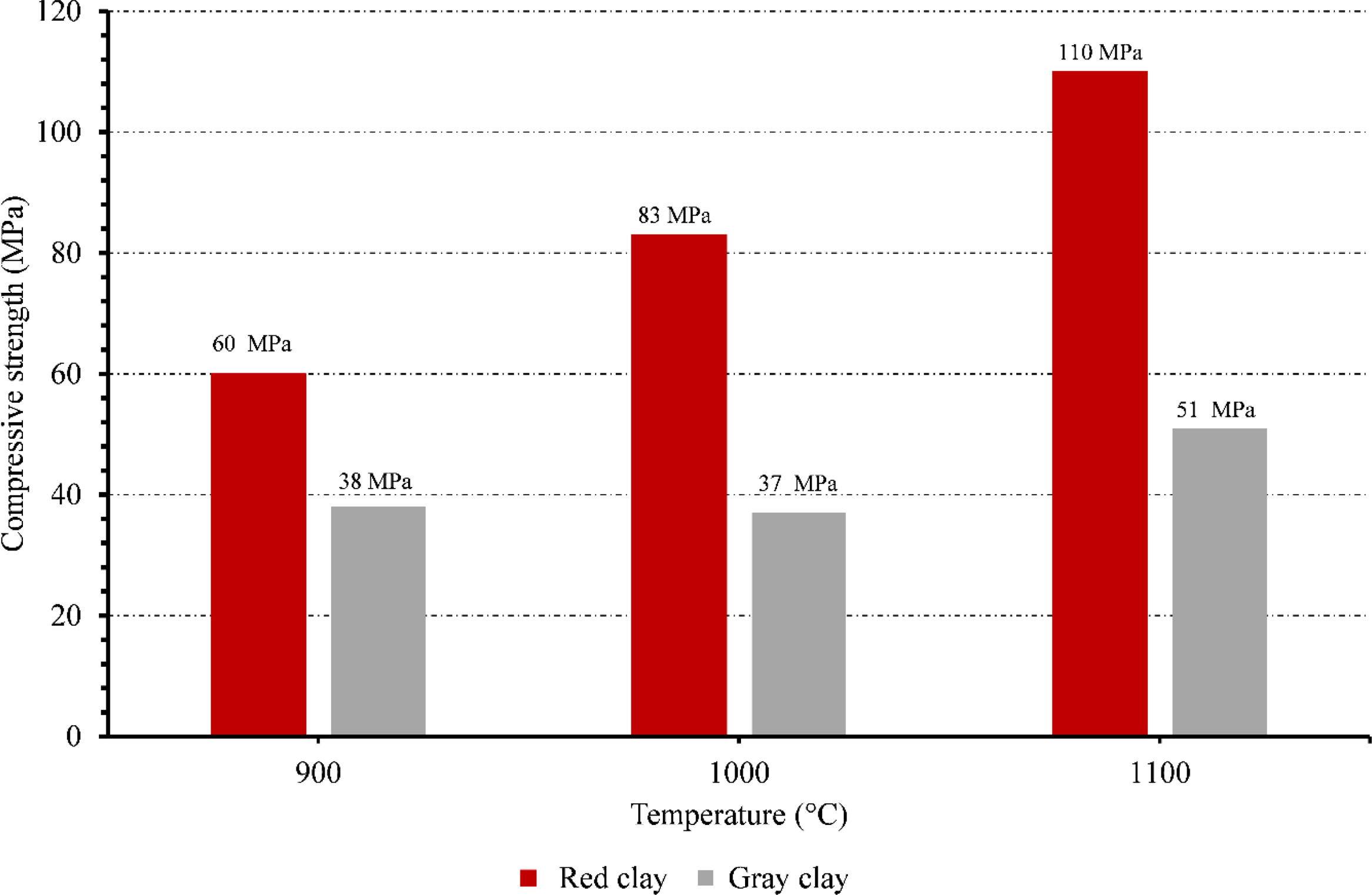
Compressive strengthThe compressive strength of ceramic membranes made from red and gray clay was investigated at different temperatures. The results reveal interesting trends regarding the mechanical properties of these membranes (Fig. 12).
Starting with the red clay ceramic membrane, its compressive strength was 60.97MPa at 900°C. As the temperature increased to 1000°C, there was a notable improvement in the compressive strength, which rose to 83.25MPa. The red clay membrane reached its highest compressive strength at 1100°C, measuring 110.165MPa. These findings indicate that as the temperature increased, the red clay ceramic membrane significantly enhanced its structural integrity and ability to withstand compressive forces. In contrast, the gray clay ceramic membrane displayed a different pattern in terms of compressive strength. At 900°C, its compressive strength was measured at 38.33MPa, lower than that of the red clay membrane. As the temperature increased to 1000°C, the gray clay membrane experienced a slight decrease in compressive strength, measuring 37.925MPa. However, at 1100°C, the gray clay ceramic membrane demonstrated a moderate improvement, reaching a compressive strength of 51.155MPa.
The red clay ceramic membrane consistently outperformed the gray clay membrane in terms of compressive strength throughout all temperature ranges, as can be seen from comparing the two types of membranes. Accordingly, it can be inferred that the red clay ceramic membrane has higher mechanical qualities and is more appropriate for uses requiring excellent resistance to compressive forces.
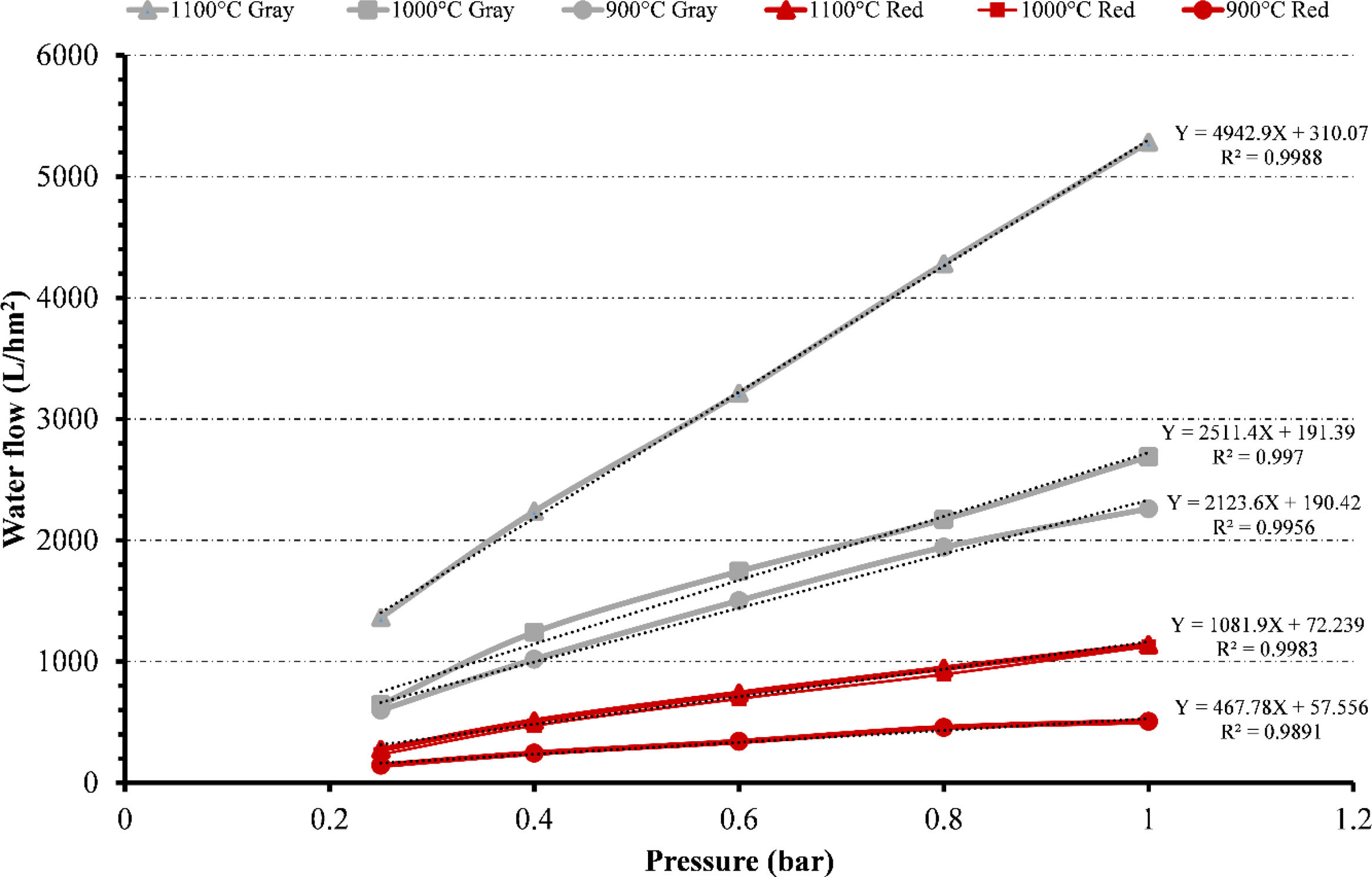
Water fluxFig. 13 summarizes the flux values for gray and red clays at different temperatures (900°C, 1000°C and 1100°C) and pressures (ranging from 0.25 to 1bar). Increased pressure results in higher flux values for both clays at different temperatures. The values obtained for gray clay are always higher than those obtained for red clay.
At 900°C, the gray flux ranges from approximately 598L/hm2 at 0.25bar pressure to 2260L/hm2 at 1bar pressure. The corresponding red flux ranges from 144L/hm2 to 506L/hm2. For 1000°C, the gray flux varies from around 643L/hm2 to 2689L/hm2 as the pressure increases from 0.25 to 1bar. The red flux ranges from 233L/hm2 to 1123L/hm2. At 1100°C, the gray flux ranges from 1362L/hm2 to 5283L/hm2, while the red flux ranges from 274L/hm2 to 1138L/hm2.
Despite the high-temperature heat treatment at 1100°C, the membrane's permeability still increases. This can be attributed to several factors. Firstly, creating larger pores than those formed at 900°C contributes to the increased permeability.
These larger pores allow easier water passage and enhance the membrane's permeability. Secondly, although the overall porosity decreases with the high-temperature treatment, the distribution of pores across the membrane is not uniform. Some areas may have a higher concentration of pores, leading to localized regions of increased permeability. This uneven distribution of pores can contribute to the overall permeability increase despite the decrease in porosity.
In conclusion, the combination of larger pore creation, uneven pore distribution, decreased porosity, and improved compression resistance due to well-sintered ceramic surfaces collectively contribute to the overall increase in permeability despite the high-temperature heat treatment at 1100°C.
The SEM figure provides visual evidence that supports the previously mentioned findings. When clay is sintered at 900°C, it exhibits a homogeneous pore distribution with small pore sizes that do not exceed a few micrometers. On the other hand, when the membrane is sintered at 1100°C, it shows a significantly different pore structure. The pore size increases significantly to approximately 25μm, and the pore distribution becomes uneven, leading to dense and well-compacted zones.
This observation from the SEM image Fig. 14 aligns with the measured permeability results. The smaller pore sizes and homogeneous pore distribution in the clay sintered at 900°C would contribute to a lower permeability, indicating a more restricted flow of substances through the membrane. In contrast, the larger pore sizes and inhomogeneous pore distribution in the membrane sintered at 1100°C would result in higher permeability, allowing for easier passage of substances through the membrane.
Therefore, the SEM image confirms that the observed permeability results are consistent with the pore structures at different sintering temperatures.
ConclusionIn conclusion, our research has demonstrated that the two types of membranes exhibit different thermal behaviors. However, based on the obtained results, it is evident that thermal treatment can strongly influence the filtration efficiency of the membrane. Specifically, gray clay can be utilized for selective filtration at low temperatures and in uniformly sized pores.
This conclusion highlights the importance of considering thermal conditions in designing and utilizing filtration membranes. Modifying the thermal treatment parameters makes it possible to optimize the membrane's efficiency and tailor its filtration behavior to meet specific application requirements.
Our findings offer good insights for developing advanced and efficient filtration membranes. They pave the way for future research to refine thermal treatment processes and further explore the selective capabilities of gray clay-based membranes in other application domains. In summary, our study underscores the significance of considering thermal treatment as a key parameter in optimizing the performance of filtration membranes.
The results reinforce that gray clay can be successfully employed for selective filtration at low temperatures and with uniformly sized pores. These conclusions hold promise for advancing research in filtration membranes and their application in various industrial sectors.
FundingThe authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Competing interestsThe authors have no relevant financial or non-financial interests to disclose.