Aluminate-based endodontic cements are promising in root canal treatments. Calcium aluminates have been used as bone and dental fillers, due to their fast setting time, good workability, and biocompatibility, overcoming some of the disadvantages of mineral trioxide aggregate (MTA) currently available. Similarly, strontium aluminates are of interest, due to their potential as hydraulic cement. They have greater radiopacity than their calcium counterparts and the potential to promote osteoblast differentiation which favors bone regeneration. The present study evaluates the in vitro biological properties of the new endodontic cement CS5C (80/20wt.% of S3A (tri-strontium aluminate (Sr3Al2O6)) and C12A7 (dodeca-calcium hepta-aluminate (Ca12Al14O33))). The in vitro ion release tests showed that Al3+, Ca2+, and Sr2+ ions are responsible for the alkaline pH of the medium, promoting antimicrobial activity against all bacterial strains tested. It also showed biocompatible properties, with promising cellular adhesion and proliferation in vitro. The results revealed that CS5S cement has strong potential for future applications as endodontic cement.
Los cementos endodónticos a base de aluminato son prometedores en el tratamiento de conductos radiculares. Los aluminatos de calcio se han utilizado como rellenos óseos y dentales debido a su rápido tiempo de fraguado, buena trabajabilidad y biocompatibilidad, superando algunas de las desventajas de los agregados de trióxido mineral (MTA) actualmente disponibles. De manera similar, los aluminatos de estroncio son de interés debido a su potencial como cementos hidráulicos. Tienen mayor radiopacidad que sus homólogos de calcio, y potencial para promover la diferenciación de osteoblastos, lo que favorece la regeneración ósea. El presente estudio evalúa las propiedades biológicas in vitro del nuevo cemento endodóntico CS5C (80/20% en peso de S3A [aluminato de tristrontio (Sr3Al2O6)] y C12A7 [heptaaluminato de dodecacalcio (Ca12Al14O33)]). Las pruebas de liberación de iones in vitro mostraron que los iones Al3+, Ca2+ y Sr2+ son responsables del pH alcalino del medio, promoviendo la actividad antimicrobiana contra todas las cepas bacterianas analizadas. También presentó propiedades biocompatibles, con prometedora adhesión y proliferación celular in vitro. Los resultados revelaron que el cemento CS5S tiene un gran potencial para futuras aplicaciones como cemento endodóntico.
In recent years, bioceramic cements for root canal treatment (RCT) received increasing attention in the research community and have been commercialized [1]. The material must promote effective sealing of the root canal [2], have antimicrobial properties, prevent the proliferation of microbes inside the canal [3], in addition to having good radiopacity (so that the cement can be visualized in the root canal) [4] and biocompatibility (to promote cellular proliferation and adhesion) [5].
Cements used in RCT are being studied in an attempt to obtain materials with better physical, chemical, and biological properties, which meet the minimum requirements for the success of the treatment [6,7]. The ability to induce mineralized tissue formation, biocompatibility, and antimicrobial properties are among the biggest challenges in endodontic treatment [8]. To overcome these challenges, certain components that promote an alkaline pH of the medium and consequently antimicrobial action are necessary. In addition, these components need to have the ability to release calcium ions (Ca2+) [9]. Therefore, many researchers are currently studying the development of new cements, combining different materials to obtain the best therapeutic results.
Calcium aluminate cements stand out because they present beneficial physical–chemical characteristics for use in root canal treatment, such as short setting time and better fluidity [10], when compared to mineral trioxide aggregate (MTA). In addition, they can form apatite on their surface after contact with body fluids [6,7,11].
Strontium aluminates also have the potential for use as hydraulic cements. This aluminate has a higher X-ray absorption than calcium aluminate, and therefore higher radiopacity [6]. Acting synergistically with calcium, the strontium aluminate it has the potential to promote osteoblastic differentiation, inhibiting osteoclastic activity and favoring bone regeneration [12–14].
In a previous study, Barbosa et al. [6], developed cements with different concentrations of S3A and C12A7. The results showed that CS5C cement, composed of 80/20wt.% S3A and C12A7, respectively, presented good workability, lower setting temperature, and setting time than those by MTA cement. In addition, the cements showed adequate radiopacity, fulfilling the requirement of the standard ISO 6876:2012 [15]. And bioactive capacity in in vitro studies.
In this sense, the development of endodontic cements based on strontium calcium aluminates may be promising in the treatment of root canals. Therefore, the present study aims to investigate the chemical and biological properties of the new endodontic cement CS5C.
Experimental procedureSynthesis of powders and preparation of cementRaw materials S3A and C12A7 were obtained from solution combustion synthesis (SCS) for cement production, as reported by Barbosa et al. [6]. The cement used in this study was CS5C [6] composed of 80wt.% S3A and 20wt.% C12A7. The cement paste was prepared with 30wt.% of polyethylene glycol – PEG (average Mn 4000, Sigma-Aldrich) and 70wt.% deionized H2O, in the mixing liquid (liquid/powder 0.39mL/g).
CharacterizationspH analysisThe disks (∅ 7mm; height 5mm) (n=3) were individually immersed in 20mL of simulated body fluid (SBF), prepared according to standard ISO 23317: 2014 [16]. The samples in contact with SBF were kept under constant stirring at 36.5°C on a bench top orbital shaker, model SHKE 6000-7 (Thermo Scientific, Massachusetts, USA). The SBF (n=3) serving as controls were placed in the same conditions without contact with the disks. Values were measured after 1, 3, 7, 14, 21, 28, and 35 days and recorded. The pH meter used was model HI 2221 (HANNA Instruments, Póvoa de Varzim, Portugal).
Ion releaseThe ion release of the cement was evaluated by measuring the concentration of Sr (strontium), Ca (calcium) and Al (aluminum) ions released from the samples immersed in 0.05M Tris–HCl buffer solution (pH 7.4) at 37°C. The disks (∅ 7mm; height 5mm) (n=3) were immersed in Tris–HCl buffer solution for different periods (1, 7, 14, 21, and 28 days) at 37°C. The volume (Vs) of Tris–HCl used was calculated as a function of the apparent surface area (Sa) of the sample in mm2, according to the formula Vs=Sa/10 according to standard ISO 23317: 2014 [16]. After the immersion periods, the samples were removed from the Tris–HCl buffer solution and the extracted liquid was analyzed by inductively coupled plasma-optical emission spectrometry (ICP-OES), model Optima 7300V (PerkinElmer, Massachusetts, USA).
Antimicrobial activitySpecies frequently found in the oral cavity and responsible for endodontic infections are Staphylococcus and Enterococcus. Staphylococcus infections are often overlooked compared to Enterococcus faecalis. Staphylococcus are one of the most persistent species that remain in areas that have not been properly disinfected. Another species that deserves attention is Escherichia coli, which has been frequently discovered in cases of endodontic failure [17].
The antimicrobial activity test was performed by the micro-dilution broth method [18]. The cement samples were handled in a sterile environment using the biological safety cabinet, model Q216F21RA1 (QUIMIS, São Paulo, Brazil). Müeller-Hinton Broth (Kasvi, Paraná, Brazil) and Müeller-Hinton Broth mixed with solidifying medium (BactoAgar – BD) were used for the assay. The Gram-negative strains used were E. coli ATCC 25922 and Salmonella sp. ATCC 14028. Gram-positive strains used Staphylococcus aureus ATCC 25923. Vancomycin was used as negative control and saline solution was used as positive control.
All strains used in the assay were cultured in brain-heart infusion agar (BHI) (accumulated) in a bacteriological oven at 37°C for 24h. The cement samples (n=3) were placed in 2mL of sterile distilled water and kept in an oven at 35°C for 9 days, during which time the release of ions into the solution occurred. After this period, the 24-well plates were filled with 90μL of culture medium, 100μL of the solution, and 10μL of bacterial suspensions prepared at 0.5 on McFarland's scale. Then the plate was incubated in a bacteriological oven at 35±1°C for 48h. After 24h, 10μL were pipetted from each well and plated on solid Müeller-Hinton agar. The plates were then incubated in the bacteriological oven for 24h before the reading was performed. At 48h, the pipetting and plating process was repeated on a solid medium.
Cell adhesionCell adhesion was evaluated after 3, 7, and 14 days of culture. The disks (∅ 7mm; height 5mm) (n=6) were sterilized in an autoclave at 121°C for 20min. Next, cells from the L929 fibroblast cell line were plated directly onto the disks at an initial cell density of 1×105cells/mL using RPMI 1640 culture medium (Gibco™) (Thermo Scientific, Massachusetts, USA) and incubated at 37°C in 5% CO2 using 24-well plates. The cell–material interaction was studied by a morphological investigation using a scanning electron microscope (SEM), model TM 1000 (HITACHI, Tokyo, Japan). The cells were fixed by immersing the matrices in 10% v/v formaldehyde solution (using ultrapure water) and then dehydrated using different ethanol concentrations. To enhance the quality of the images samples were sputter coated with Au.
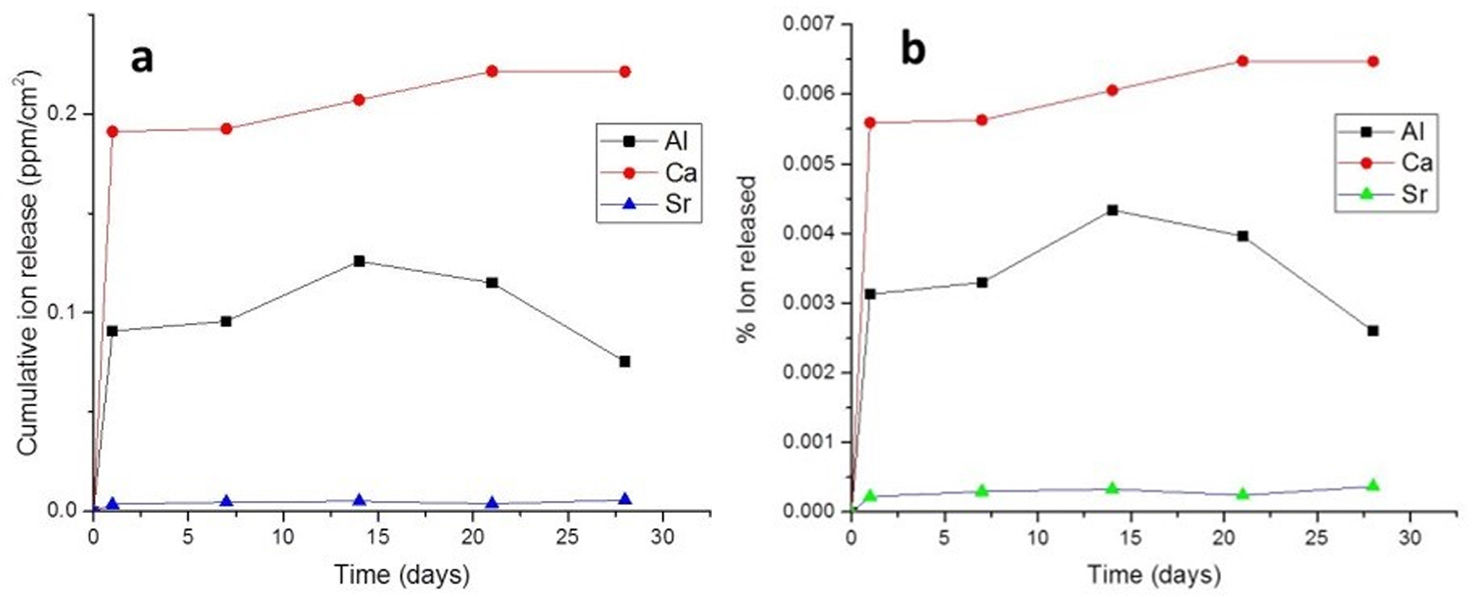
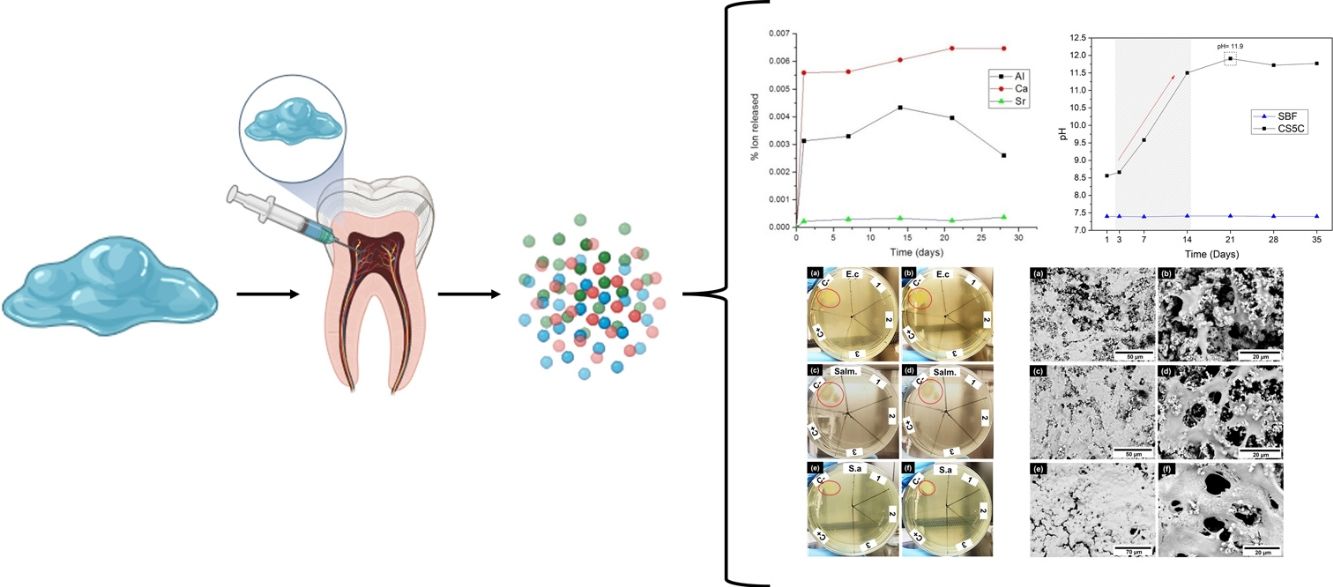
ResultsIon releaseFig. 1a shows the release profiles of Al3+, Ca2+, and Sr2+ ions from the cement after incubation for 28 days in Tris–HCl buffer solution. It is necessary to remark that these measurements have a 10% level of uncertainty.
Ca and Al ions were rapidly released in the first 24h of immersion. For Sr, the amount of ion release was lower compared to Ca and Al over the same periods. Expected behavior, considering that the Sr content in cement is much lower than that of Ca and Al. Also, S3A is less soluble than C12A7.
However, in order to compare the different extraction rates, it would be necessary to see the curves of the percentage of element extracted with respect to the amount of element in the sample. In Fig. 1b we observe this behavior.
First of all, it must be indicated that the amount of element extracted is in no case greater than 1% of the content of the sample in that element. Secondly, it should be noted that both Ca and Al are extracted in percentages almost an order of magnitude higher than Sr, always with respect to the initial content of the sample in these elements.
Why this behavior? Surely, with this strontium aluminate content, this phase does not reach the limit for percolating, so the calcium aluminate phase acts as a diffusion barrier for Sr. The S3A particles are surrounded by C12A7 particles that act as an additional barrier to its interaction with the fluids and therefore to its leaching.
Also is important to remark that the amount of Ca extracted is much higher than the amount of Al. As the amount of Strontium extracted is small, we can consider that almost Al is coming from de C12A7. But as Ca extracted is much higher from the beginning up to 14 days, we can conclude that the mechanism begins for the extraction of Ca and reach an steady step of dissolving the phase. After 14 days the amount of Al decrease, it must be related to the possible pH change (as observed later) and its precipitation as Al(OH)3 or similar.
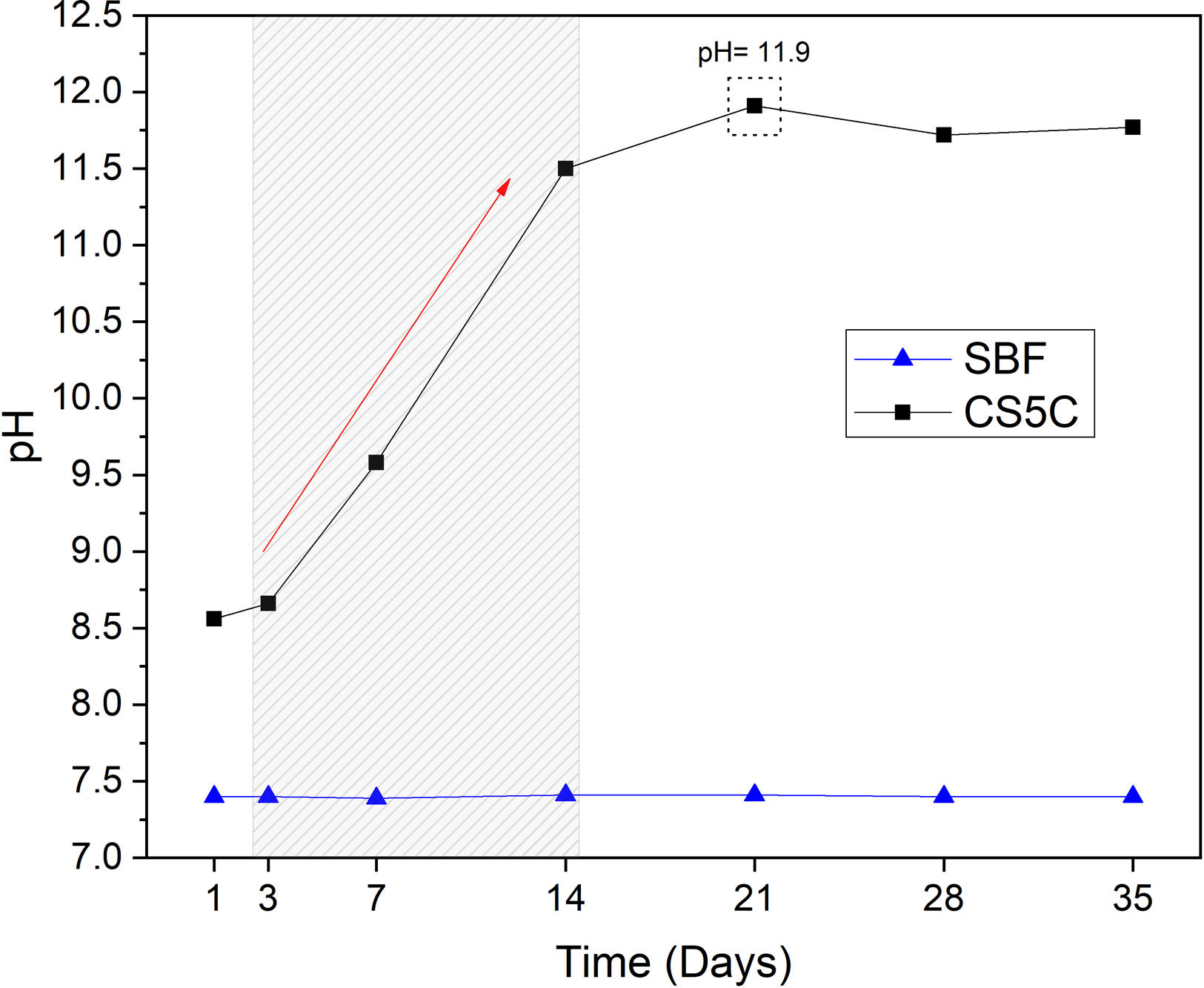
pH analysisThe pH variation of the SBF at 1, 3, 7, 14, 21, 28 and 35 days is presented in Fig. 2. The results show a sharp increase in pH, from 7.4 to 11.5 in the first 14 days, reaching a maximum pH of 11.9 on day 21. After this period the pH of the solution remained constant until the end of the analysis (35 days). The pH of the control SBF remained constant throughout the entire period. As it is well known, the SBF is a buffer solution, but in the experimental conditions, the reactivity of the cement is capable of exceeding the buffering capacity of the SBF, and the pH reaches very high alkaline values (11.9). This is very positive, since it indicates the ability to create an alkaline environment in the environment of its implementation, with the bactericidal capacity that this entails.
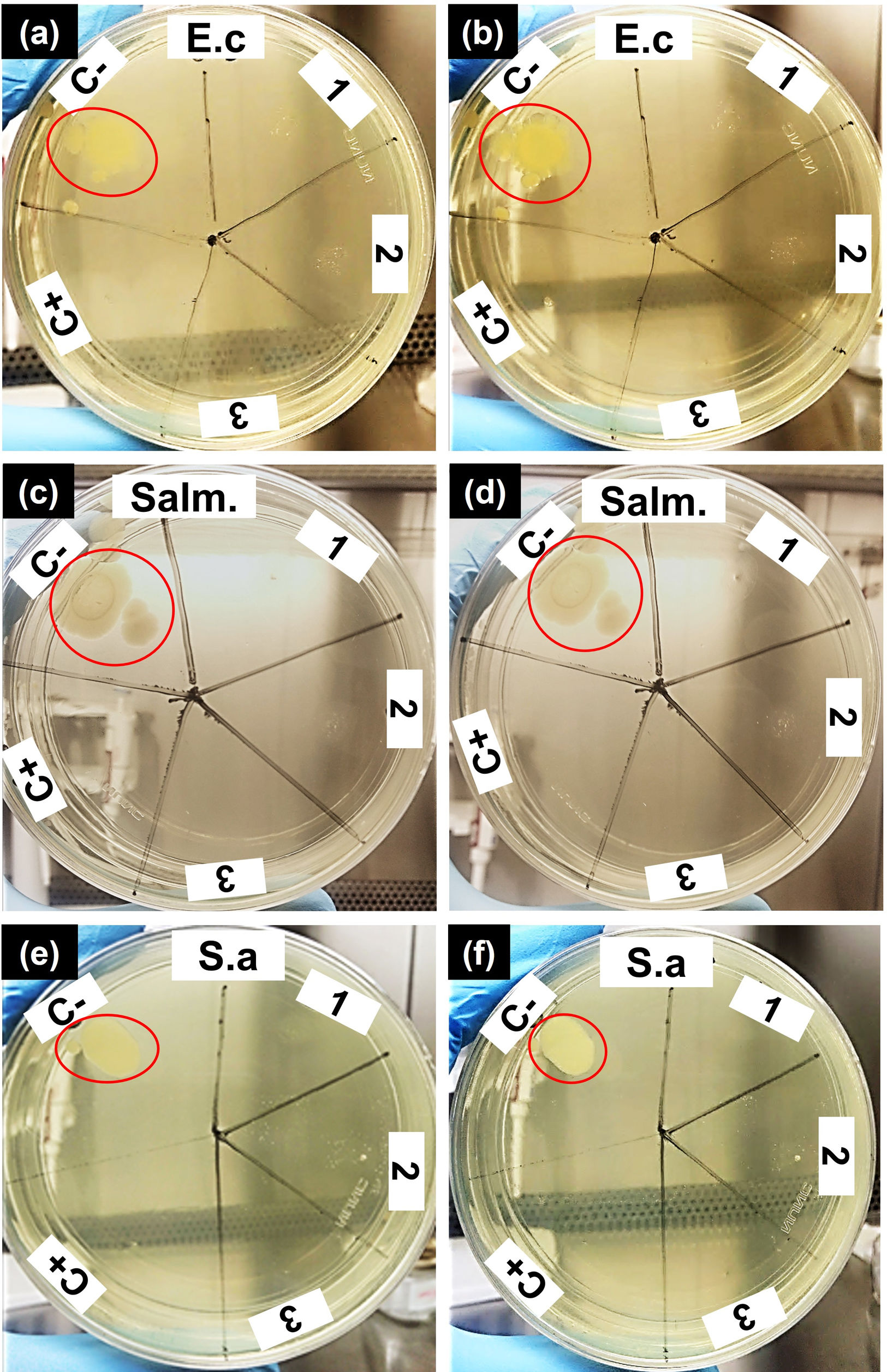
Antimicrobial activityThe results of the antimicrobial activity evaluation of the extract of the CS5C cement are shown in Fig. 3. The assay was performed in triplicate with independent sample preparations, evidenced by numbers 1, 2 and 3 in the plates of Fig. 3. In the first 24h of incubation, the extract of the CS5C cement exhibited antimicrobial activity against all strains studied and, in all samples analyzed. After 48h of incubation, the cement continued presenting a clear inhibitory effect with no record of microbial growth, which was also the case in the positive control (C+) vancomycin. On the other hand, the negative control (C−) exhibited growth zones for all bacterial strains tested (red circles) in 24h and increased colony growth in 48h of incubation.
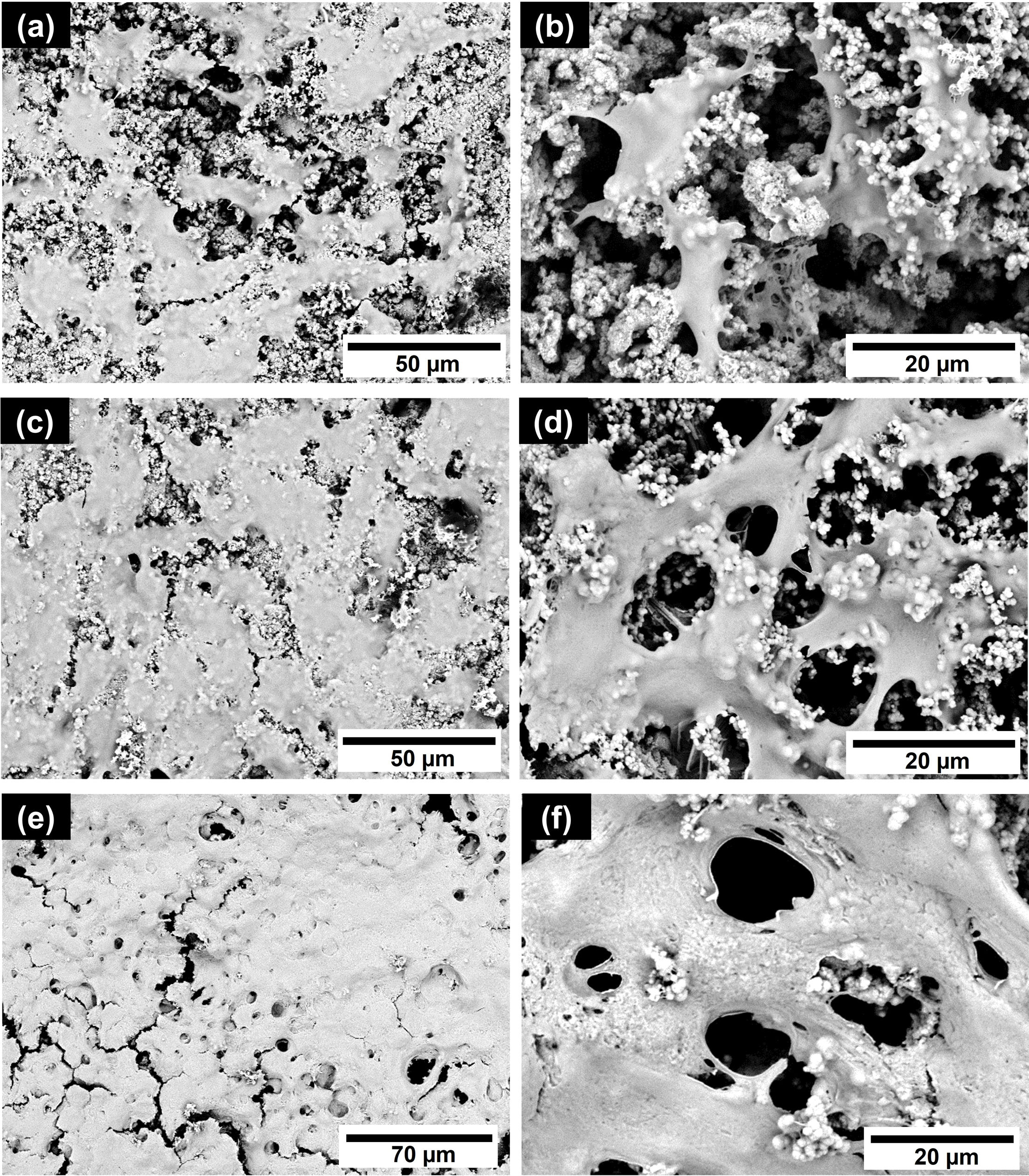
Cell adhesionFig. 4 shows the interaction between fibroblasts and the CS5C cement surface after 3, 7, and 14 days of cell contact. In vitro cell adhesion studies revealed that after 3 days of culture the fibroblast cells were adhered to the entire surface of the CS5C cement (Fig. 4a). The adhered cells showed a flattened and elongated morphology, with cytoplasmic projections (Fig. 4b). On day 7 of exposure to the cells, it is observed that the cells partially cover the sample surface (Fig. 4c), showing well-defined extensions that project between adjacent cells or enter the microporosities of the cement (Fig. 4d). After 14 days, it is observed that the cells proliferated and grew to form a thick cellular mat over the entire surface of the cement (Fig. 4e) with a higher density (Fig. 4f).
DiscussionBioceramic-based cements have been widely studied recently, especially for endodontic applications [19]. Among the beneficial clinical properties of these cements, are their biocompatibility, antibacterial action, endodontic repair capacity, and radiopacity [20].
A new cement [6] based on calcium and strontium aluminates (called CS5C cement), showed potential for endodontics as a root canal filler. This cement was characterized as a paste of adequate consistency and workability with a 60min setting time without excessive heat generation. Its equivalent radiopacity was 3.1mm Al, meeting the requirements of standard ISO 6876: 2012 [15]. With a compressive strength 24h after mixing of 5MPa, it is sufficient for applications in regions where low mechanical stress is expected, increasing to 7.5MPa after aging for 90 days. In addition, it was able to develop an apatite layer on its surface when it was in contact with SBF in vitro, demonstrating that it is bioactive [6].
In the ion release analysis, was observed in the first 24h the release of all ions, Al3+, Ca2+ and Sr2+. After this period, they show a relatively constant behavior. The Ca ion concentration increased after 7 days, reaching a maximum release before remaining stable after 21 days. Meanwhile the Al ion concentration reached its maximum rate of 0.12ppm/cm2 after 14 days. After this period there was a gradual decrease in the concentration of Al ions. For Sr, the rate of ion release was much lower compared to Ca and Al in the same periods. This is expected considering that the strontium aluminate grains should be surrounded by C12A7 grains making it difficult to contact the fluids, in this case the Tris–HCl solution. Sr ion releasing biomaterials are promising in a variety of tissue engineering applications. In addition to their application in endodontic cements, these biomaterials can be applied as scaffolds for tissue regeneration, grafting, and bone cement [21]. According to the ion release analysis, the Ca concentration was higher than the others. The ability to release Ca ions is important for the success of endodontic treatments, due to your action in differentiation of mineralizing cells [22]. In addition, this ion is directly involved in the precipitation of apatite (calcium phosphate) on the cement surface [23].
The amounts of Ca2+ and Al3+ released in the CS5C cement are relatively low, compared for example to the ingestion of foods containing these ions [24,25]. Therefore, they should not be considered a safety concern. In addition, these cements will be implanted in isolated locations (inside the tooth), where the release will be much lower. The Sr2+ concentration is also very low, presenting no toxicity risk [26]. Therefore, CS5C cement can be considered safe for application in endodontics.
The pH increase is mainly related to the ionic interchange related to Ca leaching. The increase in pH is beneficial since it can have a long-lasting antimicrobial effect, eliminating residual microorganisms [27]. Antimicrobial activity is important in cements applied in endodontics since the open root canal is susceptible to bacterial infection during the operating process [28]. Similar results were obtained by Moghanian et al., [29], which evaluated the pH of the SBF solution of bioglasses (composed of SiO2–CaO–P2O5) replacing CaO with SrO. They observed that the ion exchange between Ca2+, Sr2+, and H+ leads to a constant increase in the pH of the SBF solution during the first 7 days of immersion, reaching a pH of approximately 7.8. Also according to Moghanian et al. [29], it was noted that the pH increased at a slower rate until day 14 when it reached a pH of approximately 7.9. Increasing the Sr2+ content in the bioglass resulted in higher pH values of the SBF solution.
The performance of a material depends not only on its dissolution and biocompatibility but also on its antibacterial activity [30]. Therefore, it is also the goal of the endodontic treatment to prevent microbial contamination of the root canal system ensuring clinical success [31]. One of the main causes of root canal treatment failure is the presence of resistant, facultative microbial species from the oral cavity, such as E. faecalis, Candida albicans, and S. aureus[32,33]. In most cases, E. faecalis is the key pathogenic factor in endodontic treatment failure [34], being detected in about 77% of cases [35].
The antibacterial effect of CS5C cement against all tested bacterial strains is mainly caused by the alkaline pH, which was evident in the ion release and pH analyses. These properties corroborate existing studies, which observed the same antimicrobial behavior for bioceramics cements [36–39].
The in vitro cell adhesion results show that as the days passed, the cells proliferated and grew, forming a thick cellular mat across the entire surface of the sample. The adhesion, proliferation, and elongation of cell bodies is a sign of the affinity of cells with the surface, which demonstrates the biocompatibility or even bioactivity of the material [40]. Similar results have been reported by Shahrouzifar and Salahinejad [23], which evaluated the biodegradation, apatite formation capacity, and cell adhesion in vitro on macroporous scaffolds doped with Gentleman et al. [41], reported that supersaturation of body fluids with Sr2+, and Ca2+ ions did not result in any decrease in cell proliferation or increased toxicity against osteoblastic and osteoclastic cells in strontium-incorporated bioactive glasses.
ConclusionsCS5C cement presents Al3+, Ca2+ and Sr2+ ion release in the first days, which is responsible for the alkaline pH. This promotes antimicrobial activity on the cement. The antimicrobial effect is beneficial for reducing the number of remaining microorganisms and for eradicating infection during and after endodontic treatment.
In addition, the CS5C cement is shown to be biocompatible, with a high rate of cell proliferation and adhesion on its surface. These results indicate that the cement is promising for potential applications in endodontics.
However, future studies are needed to explore the mechanisms by which CS5C promotes tissue mineralization.
This work was funded by the Ministry of Economy and Competitiveness of Spain under projects MAT2013-48426-C2-1R and grant PTQ-16-08573, and by the Brazilian Program “Science Without Borders” through the Project No 401220/2014-1.