HPV types 16 and 18 were studied in paraffin-fixed cervical biopsy collected in southern Brazil. HPV 16, HPV 18 and co-infection HPV 16/18 were identified in 10/57 (17.5%), 4/57 (7%) and in 43/57 (75.4%) samples, respectively. Southern Brazil has one of the highest prevalence rates of HPV 16/18 reported.
Human papillomavirus (HPV) is a cause of cervical cancer which is the fourth-most common cancer among women in the worldwide. According to the World Health Organization, there were more than 528,000 new cases and 266,000 deaths from cervical cancer in 2012. Furthermore, approximately 85% of cervical cancer cases are diagnosed in less developed regions.1 In Brazil, cervical cancer is the second-most common cancer in women and a particularly high incidence (16.3/100,000) for this disease has been reported in South region of Brazil.2,3
While most HPV infections are characterized by spontaneous viral clearance, some infections are persistent. This condition involves oncogenic or high-risk HPV types (e.g., 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59) and these have been consistently associated with grades 2 and 3 cervical intraepithelial neoplasia (CIN), as well as cases of cervical cancer.4 Among these types, HPV 16 and HPV 18 infections have been present in more than 70% of all examined cervical cancer specimens.5 In Brazil, approximately 5.4% of women in the general population are estimated to harbor a cervical HPV-16/18 infection at any given time, and 68.2% of cervical cancers have been attributed to HPV types 16 or 18.6 In Brazil, three HPV vaccines were licensed (bivalent/quadrivalent/nonavalent), but only the quadrivalent (Gardasil®, Merck Sharp & Dohme, USA) that protects against HPV types 6, 11, 16, and 18 was incorporated into the public health system and it is currently offered to girls 11–13 years of age.7
Determining HPV prevalence and examining the genotype distribution of HPV in premalignant and malignant lesions are important parameters for estimating the impact of screening programs and the efficacy of HPV vaccines, particularly in relation to variations in the prevalent HPV types according to region. The aim of the present study was to evaluate the prevalence of high-risk HPV types, 16 and 18, in archival cervical biopsy samples with high-grade lesions collected in southern Brazil.
In a retrospective cross-sectional study, conducted between 2008 and 2009, a total of 60 paraffin-embedded samples graded as cervical intraepithelial neoplasia I–III (CIN I, CIN II, CIN III) and cervical cancer (stained with hematoxylin and eosin) were collected at the Hospital of Lutheran University of Brazil (ULBRA), Canoas, Rio Grande do Sul (RS) (the southernmost state in Brazil). The city of Canoas is a medium-sized urban center located in the metropolitan area of the capital (Porto Alegre, RS). All samples were classified by a certified pathologist. The study obtained approval from the Ethics Committee of the Lutheran University of Brazil (protocol number 2008-601H) before releasing archival specimens that were kept coded and confidential. Informed consent was not needed because of the retrospective nature of the study.
Ten microtome serial sections of 10μm (approximately 25mg per sample) were sent to the Centro de Desenvolvimento Científico e Tecnológico (CDCT), Porto Alegre, Rio Grande do Sul, Brazil. Excess paraffin was removed with sterile blade, and the remaining material placed in the microtube.
DNA extraction was performed in biopsy specimens using the DNA kit QIAmp® FFPE Tissue (QIAamp DNA FFPE Tissue Handbook, QIAGEN-Hilden, Germany) with minor modifications according to the protocol suggested by Simonato et al.8 and Vilanova-Costa9. In brief, 1000μL of xylene was added to microtubes containing the samples. The tubes were vortexed vigorously for 30s, and centrifuged for 5min at 10,000rpm. To the pellet was added again 1000μL of xylene, vortexed vigorously and incubated at 65°C for 30min. The tubes were centrifuged at 10,000rpm for 5min, and then the pellet was washed twice with 1000μL of 96% ethanol, vortexed for 30s and centrifuged at 10,000rpm for 5min. The tubes were open and incubated at 42°C for 20min for complete evaporation of residual ethanol. After these steps of deparaffinization, the kit protocol was followed as indicated in the manufacturer's manual.
The general primers GP5+ and GP6+, which span a region of 140–150-bp from the L1 open reading frame of a broad spectrum of HPV genotypes, were used in the PCR, as described by De Roda Husman et al.,10 with the exception that the GP6+ primer was biotinylated. A negative control (no DNA) was included in each PCR run to ensure that no cross contamination had occurred. The amplification reaction was performed under the following conditions: 95°C for 5min for initial denaturation, followed by 40 cycles of 95°C for 1min, 52°C for 1min, 72°C for 1min, and 72°C for 10min for final extension. Tubes were kept at 4°C for storage of the amplified material. PCR products were electrophoresed on 1.5% agarose gel containing 0.5mg/mL ethidium bromide and visualized under ultraviolet light. As a control of DNA quality, all samples were pre-screened with β-globin primers PCO3 (5′ ACACAACTGTGTTCACTAGC 3′) and PCO4 (5′ CAACTTCATCCACGTTCACC 3′) (110bp fragment).11
Biotinylated PCR products generated by the GP5+/GP6+ PCR were identified using microplate colorimetric hybridization assay (Immobilizer Amino Surface, Nunc, Roskilde, Denmark) developed in our lab.12 Each amplicon was hybridized separately using probes for the two most prevalent HPV types in cervical cancer (HPV 16 and 18).
Data were evaluated using the software Statistical Package for Social Sciences (SPSS) v.16.0. Fisher's exact tests were used to compare the age and the different degrees of cervical lesions. Fisher's exact tests were also used to compare the prevalence of single and combined HPV infection across histological (CIN I vs CIN 2 or worse) strata. p<0.05 was considered significant.
Of the 60 paraffin-embedded cervical biopsies with lesions that were examined, 39 were confirmed to be CIN I (65.0%), 7 were CIN II (11.7%), 11 were CIN III (18.3%), and 3 cervical cancer (5.0%). The distribution of the available biopsy specimens according to patient age were: 15/60 samples (25.0%) were from women 16–25 years of age, 38/60 (63.3%) from women 26–35 years of age, 4/60 (6.7%) from women 36–45 years of age, and 3/60 (5.0%) from women 46–55 years of age. The mean age of the patients was 29.6 years. There was no significant difference between mean patient age and degrees of cervical lesions (p>0.05).
DNA was extracted from each biopsy samples and was subjected to PCR to amplify a β-globin fragment to assess sample integrity. Positive amplification was detected for 57/60 of the cervical biopsy samples. The remaining three samples, two CIN I samples and one CIN II sample, were excluded from further analysis. These 57 samples were subjected to amplification of the HPV L1 region and the amplicons analyzed in agarose gel and using a microplate colorimetric hybridization assay. In the first case, amplification products were observed in 33/57 (57.9%) samples. However, using a microplate colorimetric hybridization assay that employed specific probes to detect HPV 16 and HPV 18, the amplicons were detected in all 57 (100%) samples. Among these samples, HPV 16 was detected in 53/57 (93%) of the samples and HPV 18 was detected in 47/57 (82.5%) of the samples. Regarding single infections versus co-infections, HPV 16 was present in 10/57 samples (17.5%), HPV 18 was present in 4/57 samples (7%), and co-infection of HPV 16/18 was detected in 43/57 samples (75.4%).
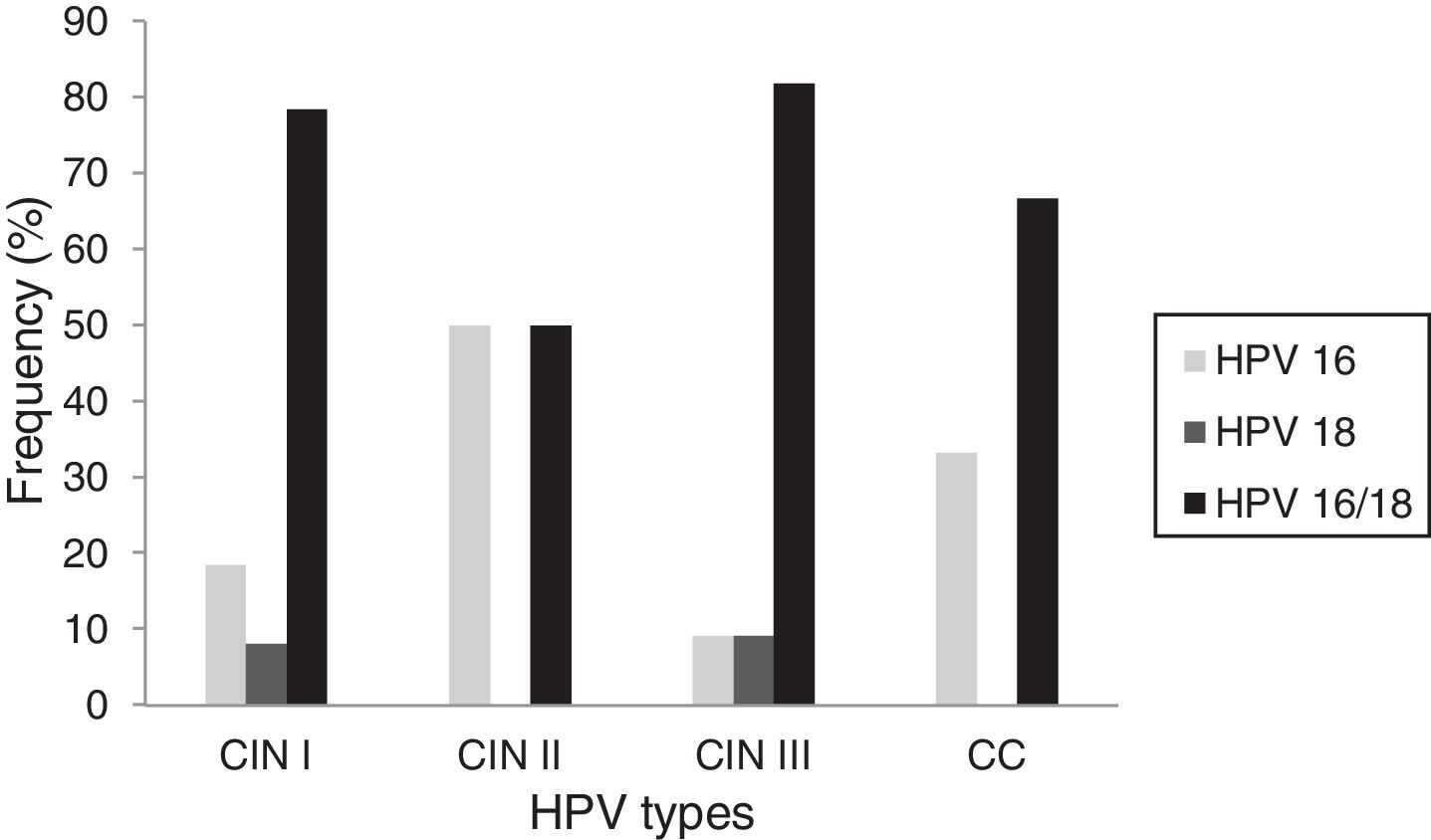
Correlations between the type of HPV present and the histopathological type of the lesions were also examined. Of the samples diagnosed as CIN I, HPV 16 was detected in 5/37 (18.5%) of the samples, HPV 18 was detected in 3/37 (8.1%), and HPV 16/18 was detected in 29/37 (78.4%). Among the samples diagnosed as CIN II, 3/6 (50.0%) presented with HPV 16 and 3/6 (50.0%) were co-infected with HPV 16/18. Among the CIN III samples, HPV 16 was present in 1/11 (9.1%) samples, HPV 18 was present in 1/11 (9.1%) samples, while co-infection of HPV 16/18 was detected in 9/11 (81.8%) samples. Among the cervical cancer samples, HPV 16 was detected in 1/3 (33.3%) samples and 2/3 (66.7%) samples were co-infected with HPV 16/18 (Fig. 1). There were no statistically significant differences between the prevalence of single versus combined HPV infections across the histological strata (p>0.05).
In the present study, HPV DNA was detected in 100% of the premalignant and malignant lesions that were present in the cervical biopsy specimens analyzed. This level of prevalence is similar to the levels reported by studies conducted in other countries that also examined paraffin-embedded samples graded as CIN I–III and cervical cancer: United States (95%),13 Zambia (94%),14 Ethiopia (93%),15 and Pakistan (88%).16 These findings reinforce the causal role of HPV infections in developing cervical malignancy5 and the importance of archival biopsy samples as a source of material for studies related to the identification of HPV types.
The prevalence rates of HPV types 16 and 18 in the present set of samples were high (93% and 82.5%, respectively), with HPV 16 being the most frequent genotype identified in all of the stages of disease that were analyzed. This predominance of HPV 16 is in agreement with previous studies of various geographical regions of Brazil17 and populations worldwide.18
Concurrent infections with multiple HPV genotypes in high-grade lesions of the cervix are a common finding, and some studies have shown that women with multiple infections, particularly those with co-infections involving oncogenic HPV types, have a significantly higher risks of progressing to cervical cancer compared with women with single infections.19 The following prevalence rates of multiple HPV genotypes in high-grade squamous intraepithelial lesions (HSIL) have been reported: 36.9% in Portugal,20 43.2% in Costa Rica,21 52% in Brazil,22 62.5% in the United States23 and 64.9% in Pakistan.24 To our knowledge, the present study showed one of the highest prevalence rates of HPV co-infection (75.4%) reported, and corroborate with studies that found HPV 16/18 as a frequent combination of HPV infection found in high-grade lesions worldwide.18 In contrast, some studies have shown that other HPV types combinations can be more frequent than HPV 16/18. In Portugal, the rate of co-infections of HPV 16/51 (12.3%) was higher than HPV 16/18 (4.6%). In Campinas (Brazil), co-infections with HPV 16/58 (12.7%) and HPV 16/52 (8%) were more frequent than co-infection with HPV 16/18 (6.7%) in high-grade lesions.22
It is noteworthy to mention that the samples analyzed in the present study is from a region (Canoas city) which the mortality rate (age-standardized) for cervical cancer in 2008 was higher (8.72/100,000) than the Brazilian population (5.12/100,000) in the same year,25 and the global average (6.8/100,000) in 2012.26 Furthermore, from 1979 to 1998, the metropolitan area of Porto Alegre, which includes Canoas, had the highest mean annual mortality rate (9.72/100,000) compared to other meso-regions of the State of RS.27 So, these rates observed in the target population suggest a continuum failure of the cervical cancer screening programs.
In the population screened in the present study, the mean age was 29.6 years. Similarly, another study reported the mean age of women infected with two or more HPV types as 31 years.19 Younger women of this population could be benefited with the prophylactic HPV vaccines, thus reducing the burden of cervical cancer, probably associated to HPV 16 and 18. Future studies like this in CIN I-III/cervical cancer lesions, using larger sample and, after HPV vaccination, could be helpful to characterize the oncogenic HPV types and analyze if HPV 16 and 18 are still the most prevalent in this particular region.
In conclusion, the present study showed one of the highest prevalence of HPV 16 and 18 co-infection in CIN I–III/cervical cancer lesions reported. In this setting, measures to ensure better health for the population are urgently needed which include the advocacy of administration of prophylactic vaccines currently available by these two oncogenic types in Brazil.
Conflicts of interestThe authors have no conflicts of interest.