Salinity and alkalinity are major abiotic stresses that limit growth and development of poplar. We investigated biocontrol potential of saline- and alkaline-tolerant mutants of Trichoderma asperellum to mediate the effects of salinity or alkalinity stresses on Populus davidiana×P. alba var. pyramidalis (PdPap poplar) seedlings. A T-DNA insertion mutant library of T. asperellum was constructed using an Agrobacterium tumefaciens mediated transformation system; this process yielded sixty five positive transformants (T1–T65). The salinity tolerant mutant, T59, grew in Potato Dextrose Agar (PDA) containing up to 10% (1709.40mM) NaCl. Under NaCl-rich conditions, T59 was most effective in inhibiting Alternaria alternata (52.00%). The alkalinity tolerant mutants, T3 and T5, grew in PDA containing up to 0.4% (47.62mM) NaHCO3. The ability of the T3 and T5 mutants to inhibit Fusarium oxysporum declined as NaHCO3 concentrations increased. NaHCO3 tolerance of the PdPap seedlings improved following treatment with the spores of the WT, T3, and T5 strains. The salinity tolerant mutant (T59) and two alkalinity tolerant mutants (T3 and T5) generated in this study can be applied to decrease the incidence of pathogenic fungi infection under saline or alkaline stress.
Trichoderma sp. is an important biocontrol agent against pathogenic fungi.1 However, growth and physiological state of Trichoderma sp. is affected by both biotic and abiotic stress factors in the natural environment. For example, salinity and alkalinity are major abiotic stresses that limiting growth of Trichoderma.2,3 Thus, we constructed a Trichoderma sp. mutant library to obtain saline- or alkaline-tolerant mutants and then tested their effectiveness as antifungal agents under both saline and alkaline conditions.
Most existing Trichoderma mutant libraries have been focused on cellulases in efforts to generate commercially applicable cellulolytic strains. For example, Trichoderma reesei mutants MCG77 (ATCC 56764) and QM9414 (ATCC 26921) produce more cellulase than the parent strain.4T. reesei mutant NG-14 has increased filter paper-degrading activity (five times higher) and specific activity (two times higher) compared with T. reesei mutant QM9414.5Trichoderma viride mutant strain QM9123 secretes twice as much cellulase as the parent strain.6T. viride mutant T100-14 has better conversion of cellobiose to glucose than the parent strain.7Trichoderma atroviride mutant TUB F-1505 and T. citrinoviride mutant EB-104 both produce higher levels of cellulases than their respective parent strains.8,9 There are a few previous studies on biocontrol of Trichoderma in regards to isonitrile antibiotic, cyanide hydratase, Cd resistance, and salinity tolerance.10–13 One example is that a T. harzianum mutant can excrete isonitrile antibiotic.11Trichoderma koningii mutant T21 has higher cyanide hydratase activity than the wild type.13 Ten of 200 T. koningii mutants have higher Cd tolerance and can be used to remediate Cd-contaminated soils.14 Four Trichoderma isolates have been identified as stable mutants. Two of these are salt-tolerant mutants (Th50M6 and Th50M11), both of which are significantly more effective than the wild type strain at inhibiting Fusarium oxysporum and improving the yield of tomato plants under saline conditions.3 Seedlings raised from salinity tolerant Trichoderma isolates had higher levels of total phenols and superoxide dismutase activity. Saline tolerant Trichoderma mutants can effectively reduce the physiological damage caused by salt stress10,15; however, there are few studies regarding T. asperellum or alkanity tolerant mutants.
Poplar is a model organism for the application of genetic engineering to forestry. Furthermore, poplar species are widely used for wood production in short-rotation forestry,16 and are cultivated worldwide.17 Popular is also used to elucidate the physiological and molecular mechanisms of stress tolerance in Trees.18 There are many stressors, such as salinity and alkalinity, that can cause damage to poplars and decrease their productivity.19Trichoderma treatments have been shown to alleviate NaCl stress (70, 150 and 240mM NaCl) and significantly increase the length and fresh weight of the shoot and root, number of leaves, leaf area, and chlorophyll content compared with the control.20Trichoderma asperellum Q1 can enhance cucumber growth by inducing physiological protection under salinity stress.21 It is possible that salinity or alkalinity tolerant T. asperellum mutants can improve the resistance of poplar seedlings to saline or alkaline stressors.
In this study, a T-DNA insertional mutant library of T. asperellum was constructed by Agrobacterium tumefaciens mediated transformation system (ATMT). Some salinity or alkalinity tolerant mutants of T. asperellum were obtained. Then, the biocontrol potential of the mutant strains under NaCl or NaHCO3 stress was tested. And, the function of alleviating saline or alkaline stress to the poplar seedlings of the mutant strains under NaCl or NaHCO3 stress was tested. Our results provide theoretical support and a practical reference for the development of Trichoderma strains for alleviating saline or alkaline stress and managing fungal diseases in saline or alkaline soils.
Materials and methodsStrains, plant materials and primersT. asperellum ACCC30536 was obtained from the Agricultural Culture Collection of China. Alternaria alternata CFCC82114 (poplar leaf wither), Rhizoctonia solani CFCC86328, and F. oxysporum CFCC86068 were obtained from the China Forestry Culture Collection Center. A. tumefaciens AGL-1, which contains the binary vector pBHt22 and a hygromycin B-resistance gene (hph), was provided by Prof. Fucong Zheng (South China University of Tropical Agriculture, China).
Populus davidiana×P. alba var. pyramidalis Louche (PdPap poplar) seedlings were cultured aseptically for 15d in Liquid Rooting Medium (MS medium with NAA 0.25mg/L and Sucrose 20g/L) or Differential Medium (MS medium with NAA 0.05mg/L, 6-BA 0.5mg/L, Sucrose 20g/L, and Agar 8g/L) at 25°C23,24; they all had similar heights (about 5cm) and a similar number of leaves (6–8 leaves).24
The primers Hph-L (5′-GAAACCGACGCCCCAGCACT-3′) and Hph-R (5′-AGTGCTGGGGCGTCGGTTTC-3′) were designed to detect the hygromycin B-resistance gene (hph) using the Primer 6.0 software (PREMIER Biosoft, USA) and synthesized by Sangon Biotech (Shanghai, China) Co., Ltd.
Construction of the T-DNA insertional mutant library for T. asperellum and analysis of the mutants genetic stabilityT. asperellum was transformed using the ATMT method.24 200μM of 3′5′-Dimethoxy-4-Hydroxy Acetophenone (AS) was added to the induced and co-cultivated (pH 5. 0) media, and the concentration of T. asperellum was 106 spores/mL.25,26
To determine mitotic stability, 80 putative transformants were selected randomly, cultured five consecutive times on Potato Dextrose Agar (PDA) without hygromycin B, and transferred to PDA containing 300μg/mL hygromycin B.24
The hph gene was assayed using PCR in the putative transformants which had mitotic stability. The sense primer was Hph-L (5′-GAAACCGACGCCCCAGCACT-3′) and the antisense primer was Hph-R (5′-AGTGCTGGGGCGTCGGTTTC-3′).25
Growth conditions of the WT and mutant strains in PDA with differing concentrations of NaCl or NaHCO3The WT and mutant strains were cultured at 28°C for 5d in PDA. Mycelium discs (5mm diameter) were obtained from the edges of the PDA plates with a hole punch, inoculated into new PDA, and cultured at 28°C for 5d. Finally, mycelium discs of 5mm diameter were obtained from the edges of PDA plates, inoculated into the PDA with different concentrations of NaCl or NaHCO3, and cultured at 28°C for 3–5d. The concentrations of NaCl were 2, 4, 6, 8, and 10%, and the concentrations of NaHCO3 were 0.1, 0.2, 0.3, and 0.4%. The colony diameters were recorded every day during culturing. The experiment was completely randomized with three replicates.
The biocontrol potential of the WT and mutant strains in PDA with different concentrations of NaCl or NaHCO3The WT and mutant strains were challenged with the phytopathogens A. alternata, R. solani, and F. oxysporum. PDA with different concentrations of NaCl or NaHCO3 were used in the challenge experiments, and PDA was used as control. The concentrations of NaCl were 2, 4, 6, 8, and 10%, and the concentrations of NaHCO3 were 0.1, 0.2, 0.3, and 0.4%. Symmetrical positions were marked on the plates. Mycelium discs (5mm diameter) were obtained from the same part of the PDA plates, inoculated onto new PDA on the symmetrical positions, and cultured at 28°C for 5–10d. The experiment was completely randomized with three replicates. IR=(DC−DE)/(DC)×100%, where: “IR” is the abbreviation of inhibition rate; “DC” is abbreviation of the diameter of the control group, and; “DE” is abbreviation of the diameter of the experiment group.27
The NaCl or NaHCO3 tolerance of the PdPap seedlings co-cultured with the WT, T3, and T5 strainsThe PdPap seedlings were co-cultured with spores of the WT, T3, and T5 strains under NaCl (1 and 2%) and NaHCO3 (0.05, 0.1 and 0.2%) stress. The cell concentrations were 106 spores/mL. Leaves from the PdPap seedlings were assayed for SOD, CAT, and POD after co-culturing for 0, 1, 3, 5, 7, and 15d using commercially available kits (Nanjing Jiancheng Bioengineering Institute).
ResultsThe T-DNA insertional mutant library of T. asperellum and the genetic stability of the putative transformantsThe transformation efficiency of ATMT is about 60 mutants per 106 spores. To investigate the mitotic stability of the integrated T-DNA, eighty putative transformants with the vector pBHt1 were transferred repeatedly to PDA. After five rounds of growth on PDA, mycelium discs were transferred to selective PDA with 300μg/mL hygromycin B. All transformants that grew under these conditions had high mitotic stability.
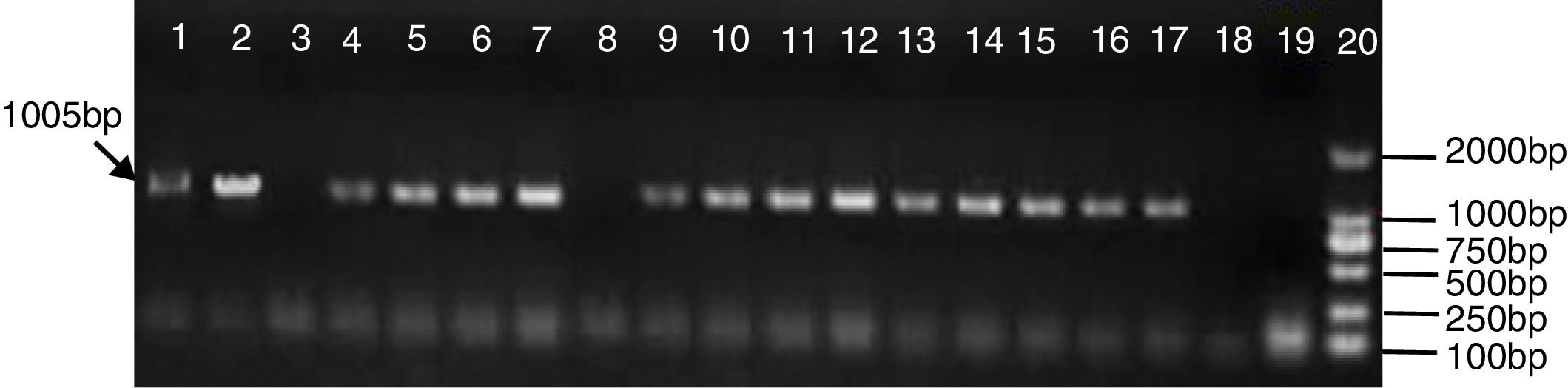
Sixty five positive transformants were obtained. PCR detection of the target gene was performed on the 80 selected putative transformants. Presence of the hph gene in the hygromycin B resistant colonies was confirmed with the Hph-L and Hph-R primers. The 1005bp PCR product was found in all 65 putative transformants; this suggests that the hph gene was successfully integrated into the T. asperellum genome (Fig. 1).
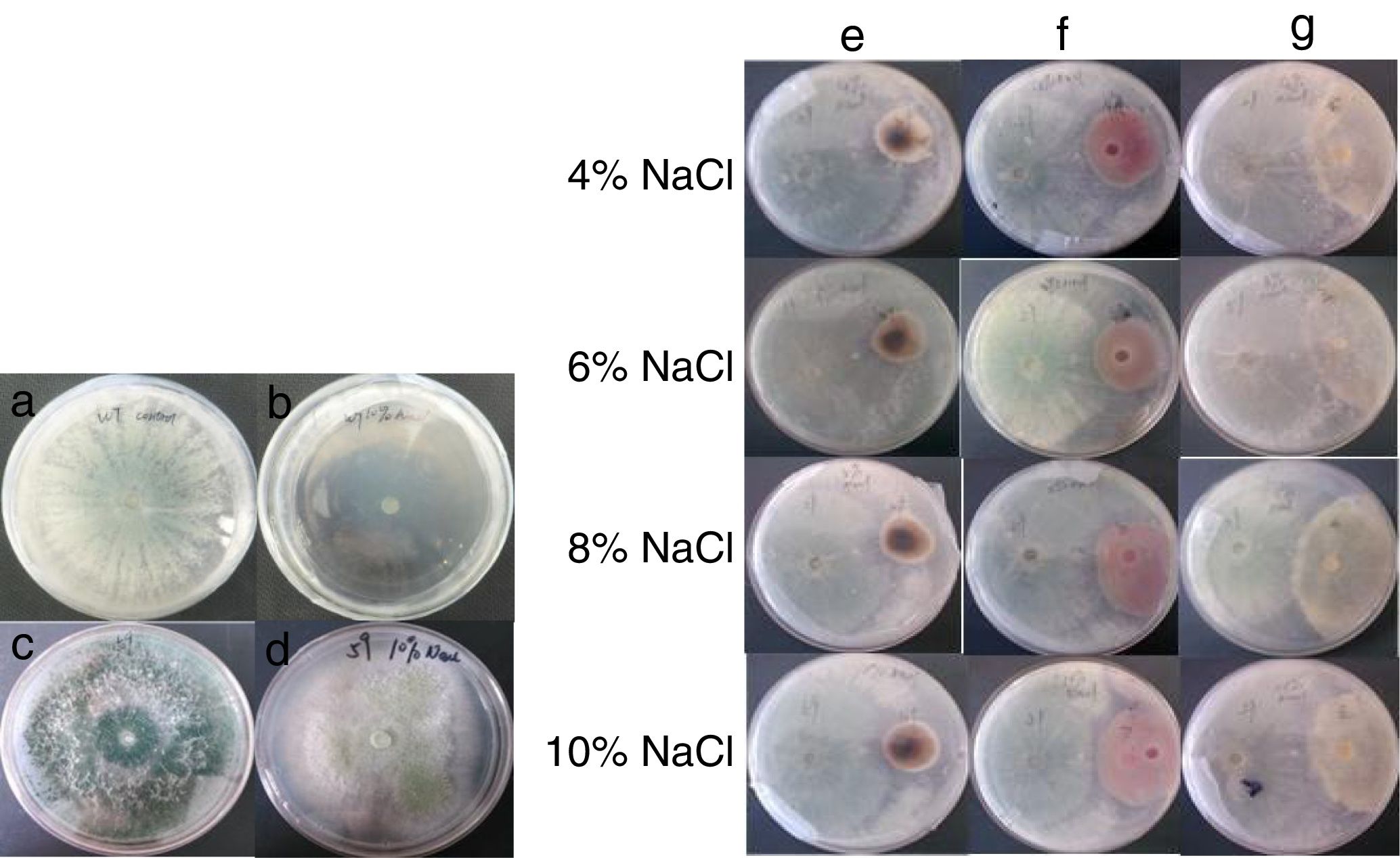
Growth conditions of the WT and mutant strains, as well as their biocontrol potential, under different NaCl concentrationsGrowth of the WT strain can be promoted by 2% NaCl during the early stages of growth. However, in the later stages of growth, this effect gradually decreases and then disappears. The growth of the WT strain was completely inhibited at a NaCl concentration of 10% (Fig. 2).
Biocontrol potential of the T59 mutant under NaCl stress after 6 d: the growth of the WT and T59 strains in PDA (A and C); the growth of the WT and T59 strains in PDA with 10% NaCl (B and D), and; confrontation of the T59 mutant with Alternaria alternata, Fusarium oxysporum, or Rhizoctonia solani under different NaCl concentrations (E–G).
The mutant strains were screened for NaCl tolerance using PDA with 10% NaCl. Of the 65 mutant strains, only one (T59) grew on PDA with 10% NaCl (Fig. 2). Although the T59 mutant grew quickly under different NaCl concentrations, the mycelium growth condition did not change; however, its ability to produce spores was inhibited under NaCl stress. The NaCl tolerance of the T59 strain was higher than that of the WT strain.
On day three of culture, the T59 strain was exposed to the pathogenic fungi. On the fifth of culture, the T59 mutant totally covered all three fungal phytopathogen fungi (Fig. 2). The T59 strain had the highest inhibition rate (52.00%) against Alternaria alternata under 10% NaCl conditions. Meanwhile, the WT strain grew slowly under NaCl stresses. The T59 strain had a better biocontrol potential than the WT under NaCl stress.
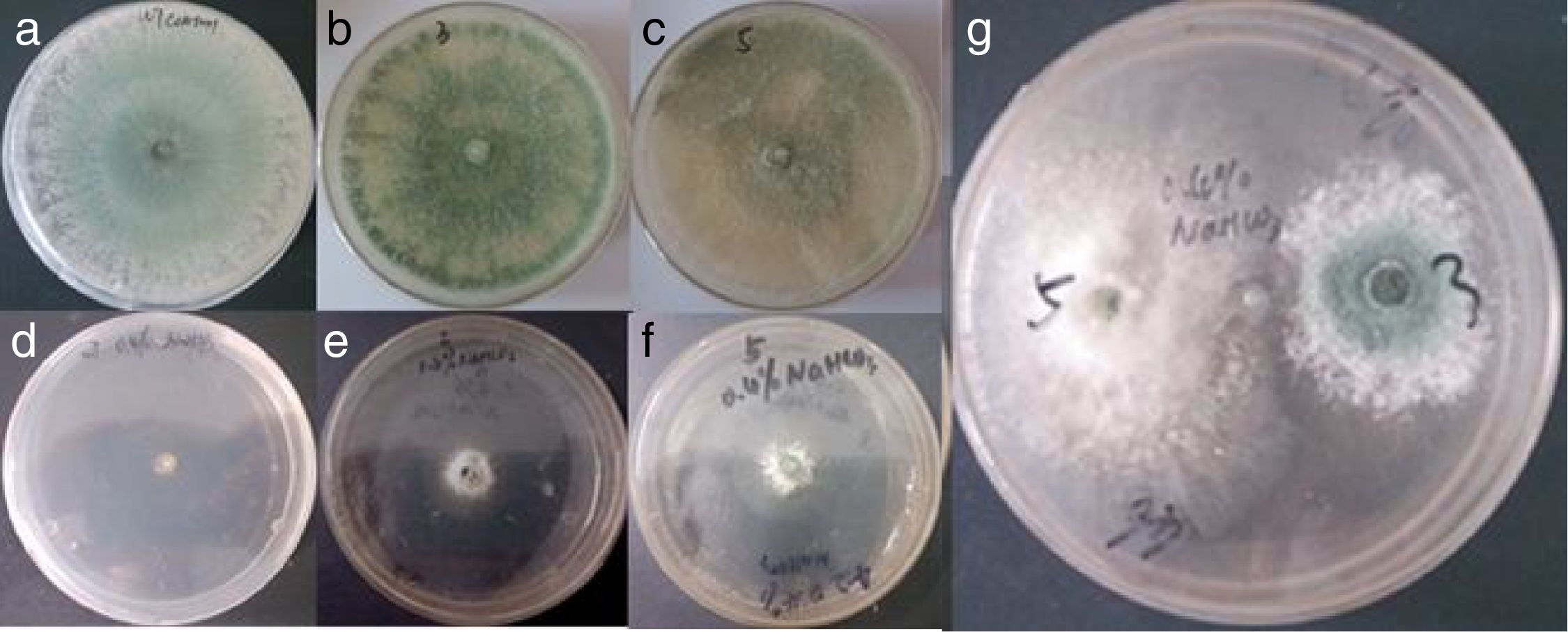
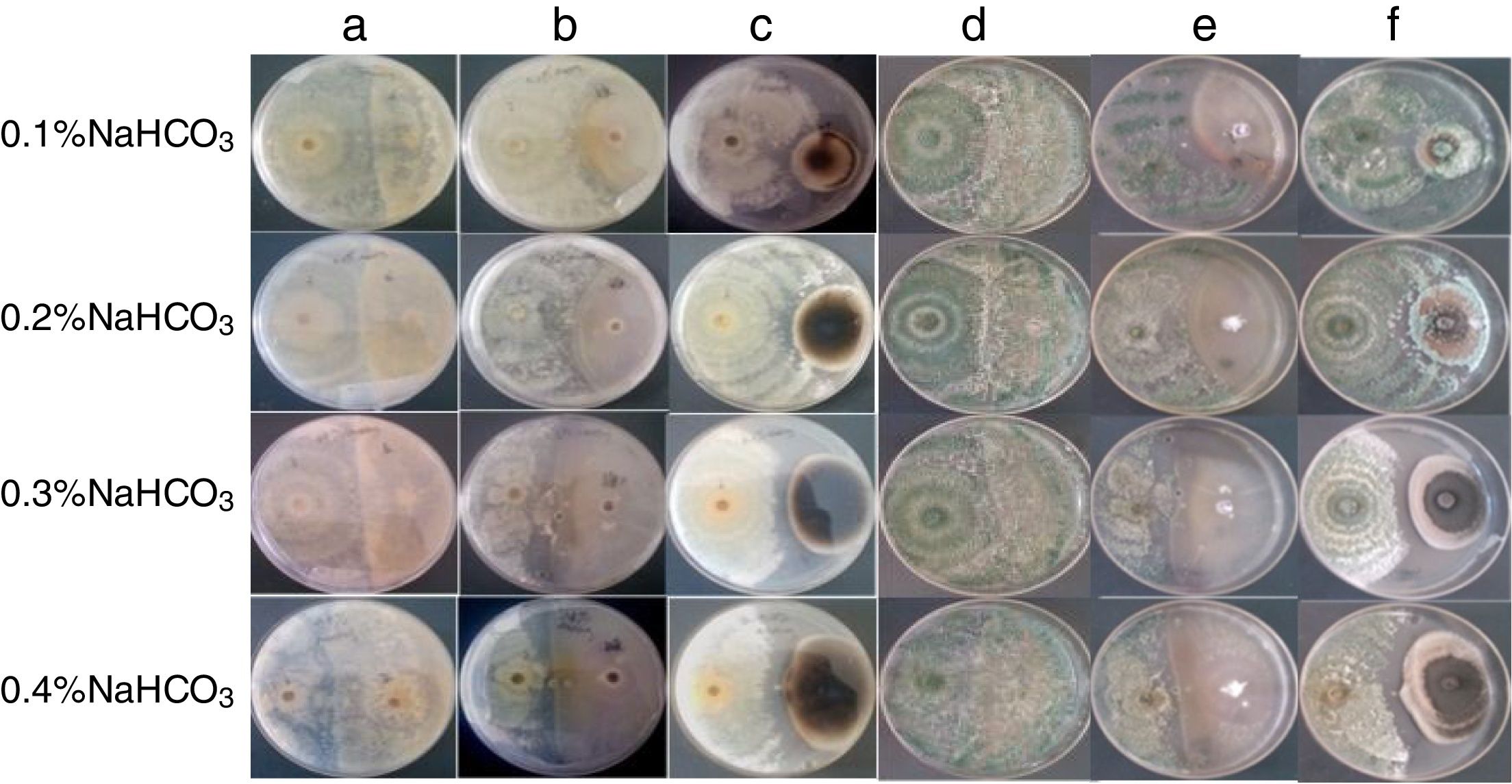
Growth conditions of the WT and mutant strains, as well as their biocontrol potential, under different concentrations of NaHCO3In later stages of growth, the inhibition of the WT strain was weaker under 0.05, 0.1, and 0.2% NaHCO3 concentrations; thus, under these conditions NaHCO3 does not inhibit the growth of the WT strain. The growth of the WT strain was completely inhibited under 0.4% NaHCO3.
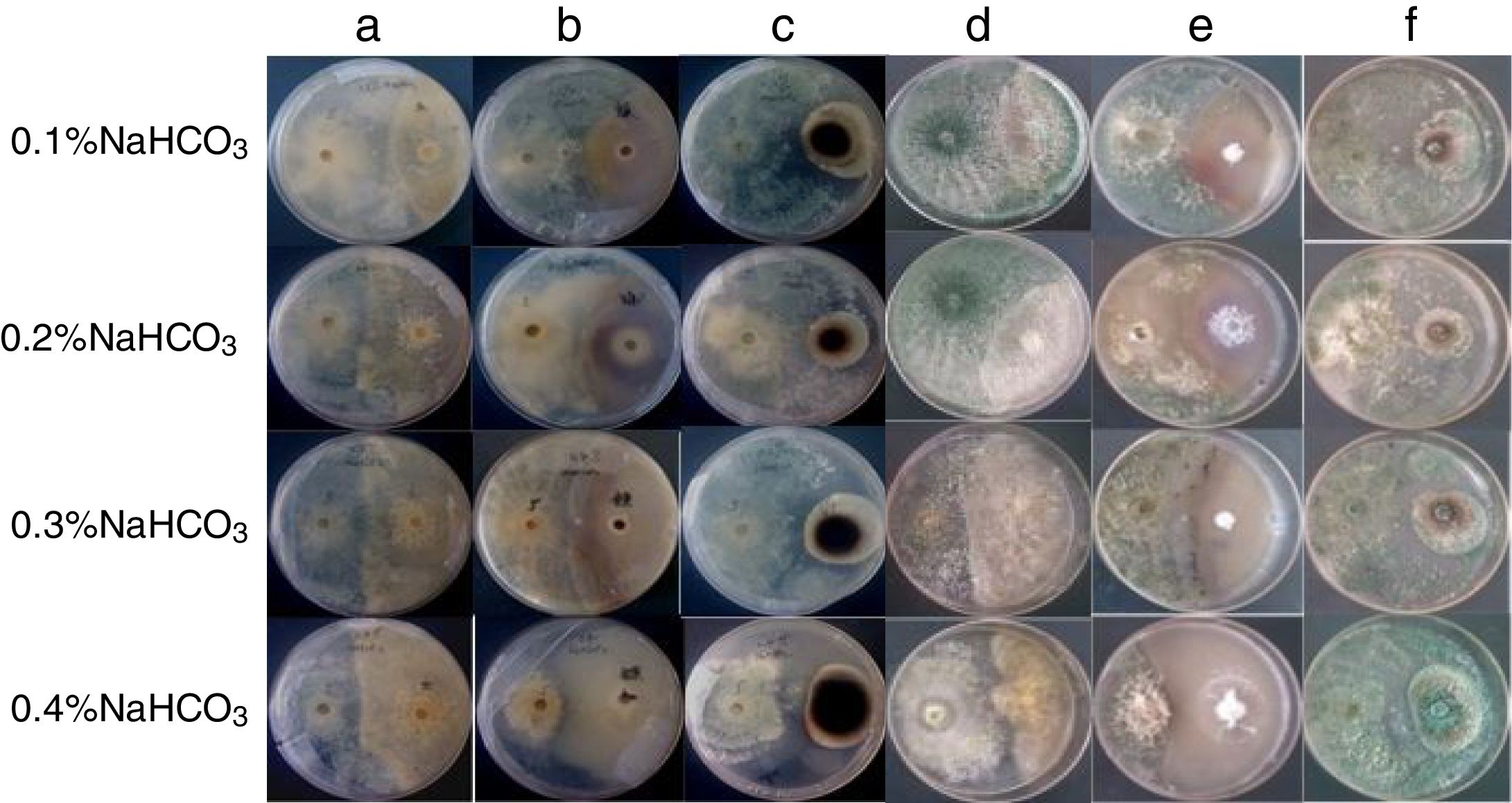
After 3d culturing the T3 and T5 mutants grew on the PDA with 0.4% NaHCO3. The growth of the T5 mutant was faster than that of the T3 mutant (Fig. 3). The NaHCO3 tolerances of the T3 and T5 mutants are higher than that of the WT strain.
The T5 mutant was exposed to R. solani, and after 4d the T5 mutant inhibited the growth of R. solani under 0.1% NaHCO3 conditions. The T5 mutant is sensitive to higher NaHCO3 levels; however, R. solani exhibited similar growth under 0.2, 0.3, and 0.4% NaHCO3, which was higher than under 0.1% NaHCO3. The T5 mutant was exposed to R. solani after 6d of exposure to both 0.2% and 0.3% NaHCO3, and exposed to R. solani after 7d of exposure to 0.4% NaHCO3. However, the T5 mutant did not completely inhibit R. solani at 0.4% NaHCO3 after 10d of exposure (Fig. 4A and D).
Confrontation of the T5 mutant with R. solani, F. oxysporum, or A. alternata after 10 d: challenge of the T5 mutant with R. solani, F. oxysporum, or A. alternata under different NaHCO3 stresses (A–C, the reverse side); challenge of the T5 mutant with R. solani, F. oxysporum, or A. alternata under different NaHCO3 stresses (D–F, the obverse side).
The T5 mutant inhibited A. alternata after 5d of growth; however, after 10d of growth the mutant did not completely inhibit A. alternata at 0.1% NaHCO3. At 0.2% NaHCO3, the growth of A. alternata was slower than at other NaHCO3 concentrations. The T5 mutant inhibited A. alternata after 6d of growth; however, after 10d of growth the T5 mutant did not covered inhibit A. alternata. At 0.3% NaHCO3, the T5 mutant inhibited A. alternata after 5d of growth; however, after 10d of growth the T5 mutant did not completely inhibit A. alternata. At 0.4% NaHCO3, the T5 mutant did not completely inhibit A. alternata after 7d or 10d of growth (Fig. 4B and E).
The T5 mutant inhibited F. oxysporum after 5d of growth; however, it did not completely inhibit F. oxysporum after 10d of growth at 0.1% NaHCO3. The growth of F. oxysporum increased with NaHCO3 concentrations in the PDA. It turns out that the growth of F. oxysporum is promoted by NaHCO3 and the T5 mutant was unable to inhibit F. oxysporum at 0.2, 0.3, or 0.4% NaHCO3 (Fig. 4C and F).
The T3 mutant had similar abilities to inhibit the three pathogenic fungi under different NaHCO3 levels. However, since the growth of the T3 mutant was slower than that of the T5 mutant under higher NaHCO3 conditions, the ability of the T3 mutant to inhibit A. alternata at 0.3% and 0.4% NaHCO3 was lower than that of the T5 mutant (Fig. 5).
Confrontation of the T3 mutant with R. solani, F. oxysporum, or A. alternata after 10d: challenge of the T3 mutant with R. solani, F. oxysporum, or A. alternata under different NaHCO3 stresses (A–C, the reverse side); challenge of the T3 mutant with R. solani, F. oxysporum, or A. alternata under different NaHCO3 stresses (D–F, the obverse side).
The PdPap seedlings withered in MS with 1 or 2% NaCl (especially 2% NaCl), and the seedlings died after 4d. In the later stages of culture, the inhibition of the pathogenic fungi was weaker under NaCl stress.
At 0.05% NaHCO3, the leaves, stems, and roots of the PdPap seedlings did not change after 15d of culture. At 0.1% NaHCO3, the roots began to brown after 15d culture. At 0.2% NaHCO3, the roots were brown after 3d of culture, but the leaves and stems were normal. At 0.3% NaHCO3, the roots were brown after 2d of culture, and the leaves and the stems were withered.
NaCl and NaHCO3 tolerance of PdPap seedlings treated with the WT, T59, T3, or T5 sporesAfter 2d of co-culture, the spores of the WT and T59 strains germinated under NaCl stress and gathered around the roots of the PdPap seedlings. The NaCl tolerance of the PdPap seedlings did not change, but the PdPap seedlings withered. Thus, the NaCl tolerance of the PdPap seedlings cannot be improved by treatment with WT or T59 spores.
In the early stage of culture, the PdPap seedlings were seriously damaged at 0.3% NaHCO3; however, following treatment with WT, T3, or T5 spores, the roots, leaves, and stems were normal after 2d. After 5d of culture, the leaves and the stems in the groups treated with spores were in better conditions than those in the groups not treated with spores. Thus, the tolerance of the PdPap seedlings to NaHCO3 can be enhanced by WT, T3, or T5 spores.
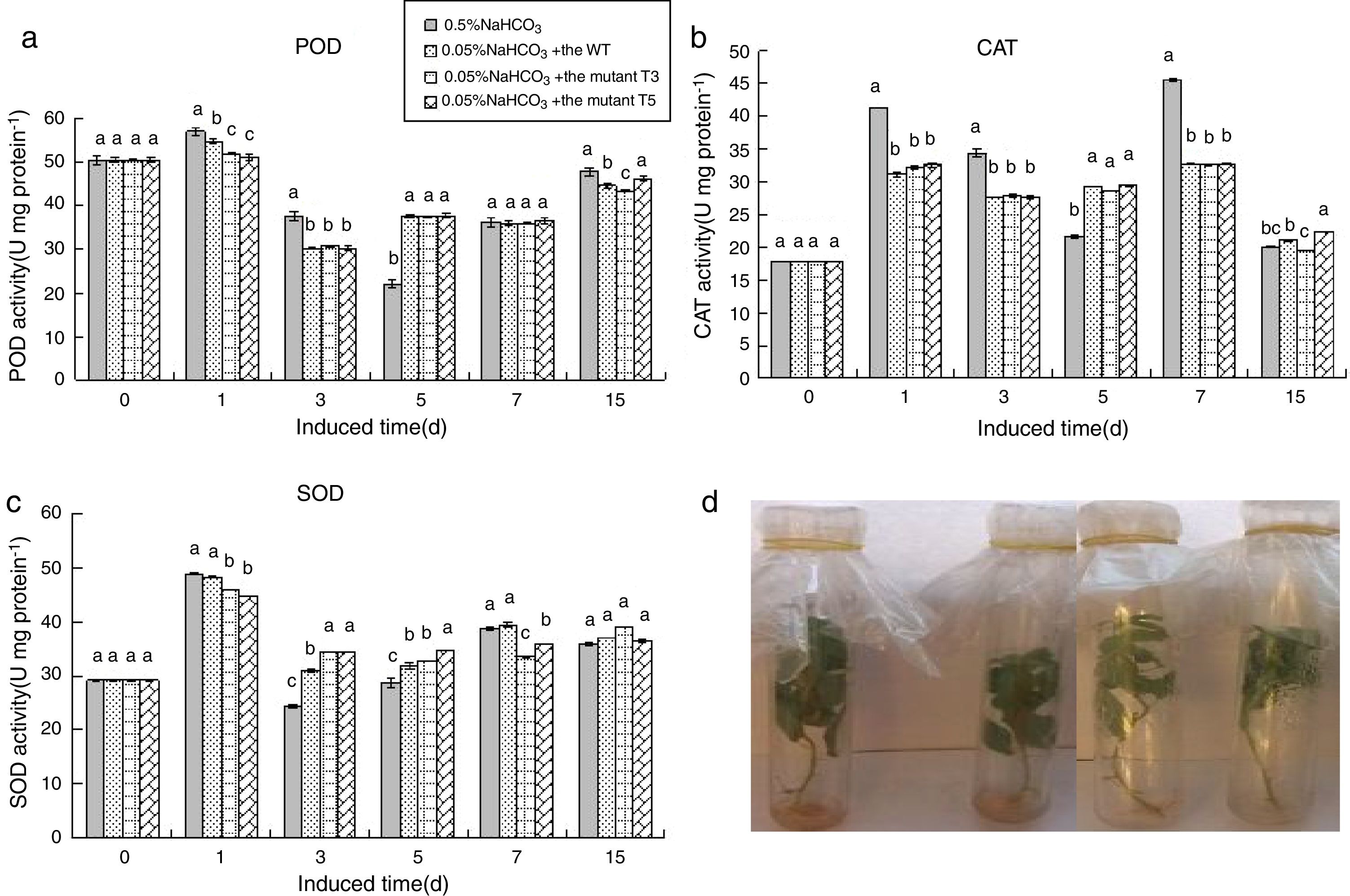
Enzymatic activity of the PdPap seedlings treated with WT, T3, or T5 spores under NaHCO3 conditionsAt 0.05% NaHCO3, the SOD, CAT, and POD activities in the control group increased, decreased, and slightly increased, respectively, compared to the control. The SOD and CAT activities of the PdPap seedlings treated with WT, T3, and T5 spores was maintained during 3–7d of treatment. The SOD activity was increased after treatment, and then remained constant (Fig. 6C). The WT, T3, and T5 spores all had similar impacts on SOD, CAT, and POD activities in the PdPap seedlings (no significant differences at p≤0.05). There were no differences in the conditions of the leaves, stems, and roots of the PdPap seedlings following the four different treatments (Fig. 6D).
Enzymatic activities of the PdPap seedling interacted with the Trichoderma spores and under 0.05% NaHCO3 stress: SOD, CAT, and POD activities of the PdPap seedlings treated with the spores of the WT, T3, and T5 strains under 0.05% NaHCO3 stress (A–C, respectively); PdPap seedlings treated with spores of the WT, T3, and T5 mutants under 0.05% NaHCO3 stress (D). Values in each column followed by the same letter are not significantly different (p≤0.05).
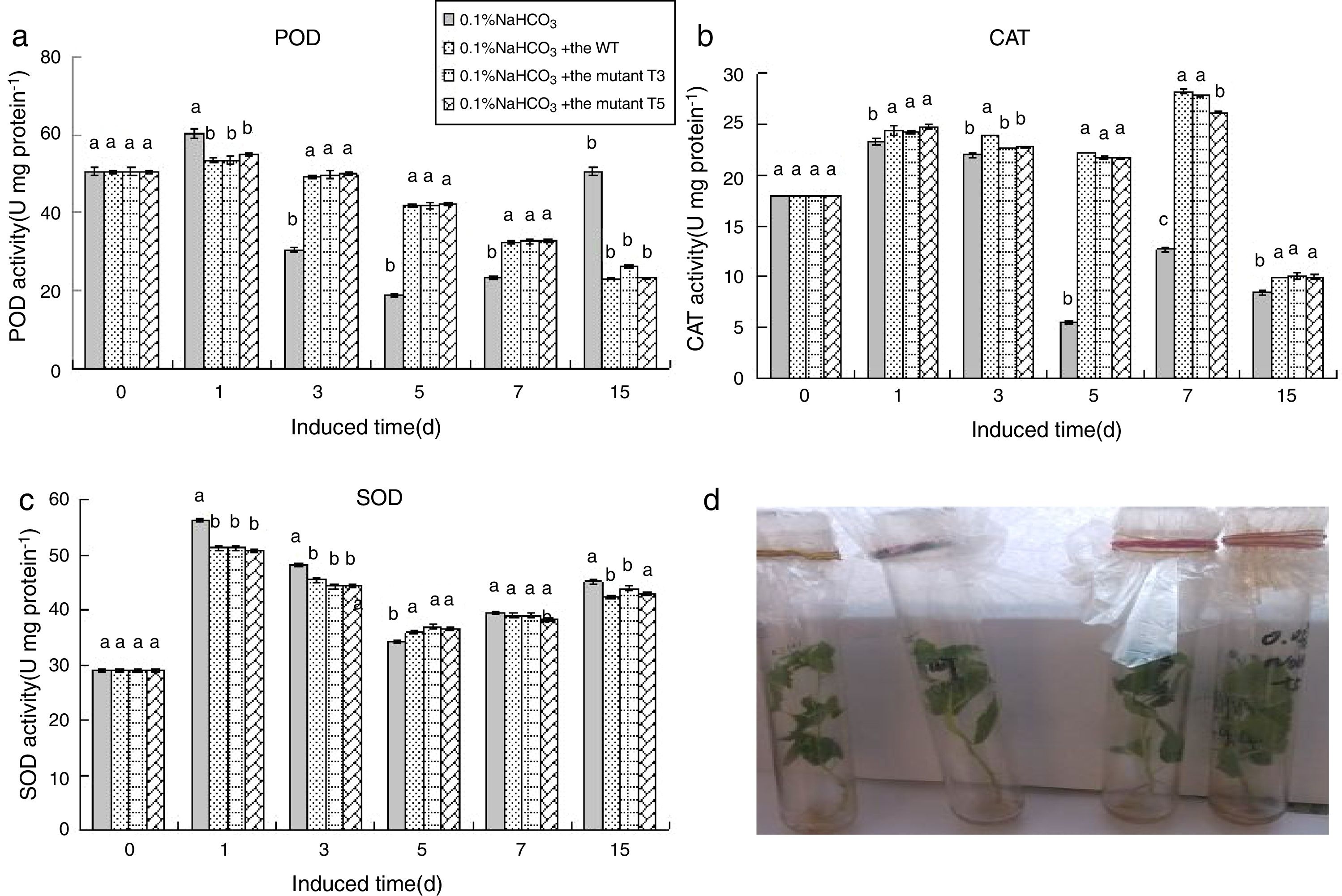
At 0.1% NaHCO3, the SOD and POD activities was increased, decreased, and then increased compared with the control group. The SOD activity of the PdPap seedlings treated with WT, T3, and T5 spores remained constant during 1–15d of culture (Fig. 7C). The POD activity of the PdPap seedlings treated with WT, T3, and T5 spores remained constant during 3–5d of culture and then decreased (Fig. 7A). The CAT activities of the PdPap seedlings treated the WT, T3, and T5 spores remained constant from 3–7d of culture, then decreased rapidly on the 15th day of culture (Fig. 7A). The WT, T3, and T5 spores had similar impacts on the SOD, CAT, and POD activities of the PdPap seedlings, and there were no significant differences (p≤0.05). The biomass of the PdPap seedlings treated with the WT spores were higher than those treated with spores of the other strains (Fig. 7D).
Enzymatic activities of the PdPap seedling interacted with the Trichoderma spores under 0.1% NaHCO3 stress: SOD, CAT, and POD activities from the PdPap seedlings treated with spores of the WT, T3, and T5 strains under 0.1% NaHCO3 stress (A–C, respectively); PdPap seedlings treated with spores of the WT, T3, and T5 mutants under 0.1% NaHCO3 stress (D). Values in each column followed by the same letter are not significantly different (p≤0.05).
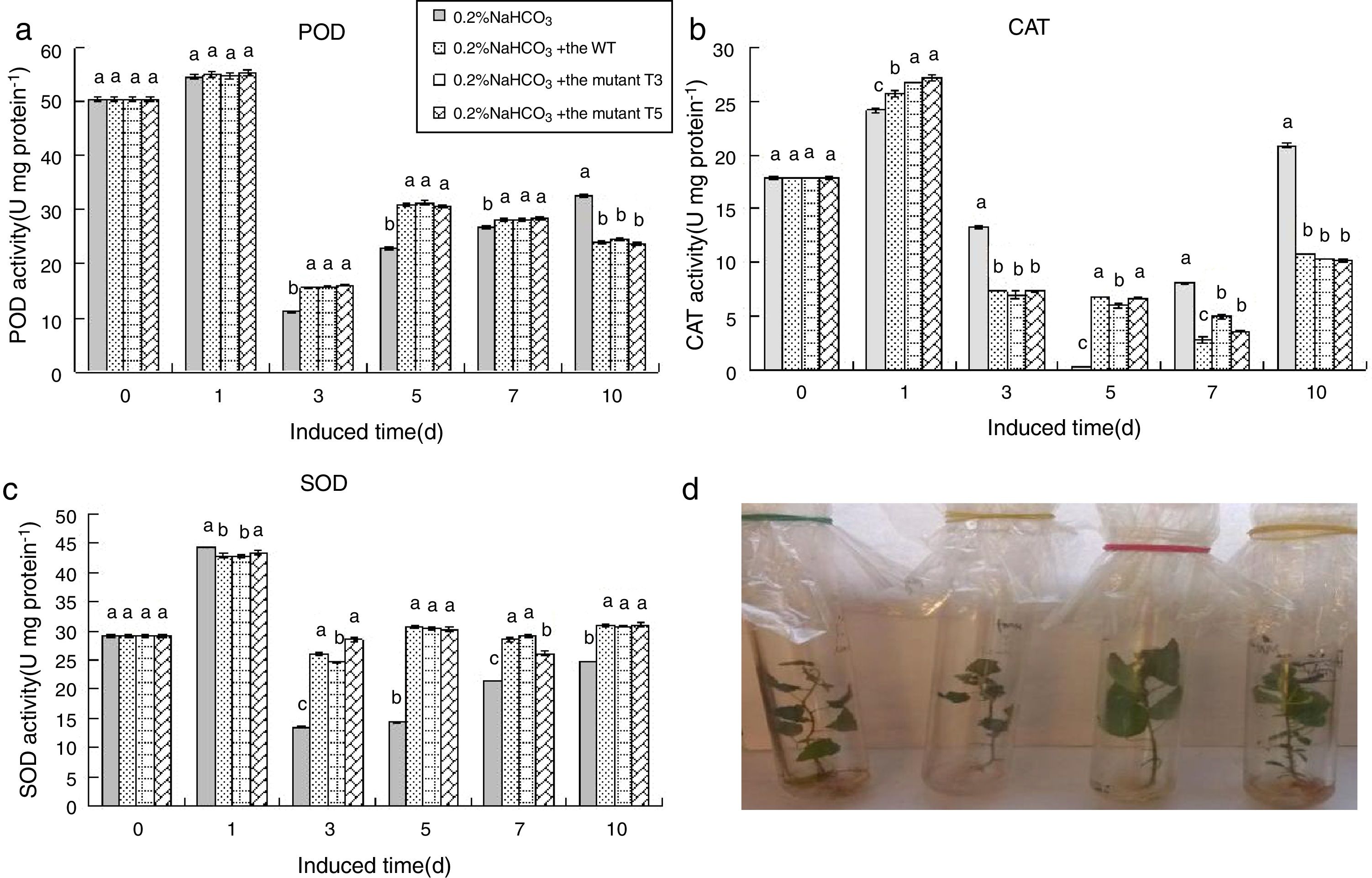
At 0.2% NaHCO3, the SOD and POD activities first increased, then decreased rapidly, and then increased again compared to the control group. The SOD and POD activities of the PdPap seedlings treated with the WT, T3, and T5 spores were the same as those of the control group. However, the SOD activity of the PdPap seedlings treated with the WT, T3, and T5 spores was higher than that of the control group during 3–7d of culture, especially on the 5th day when the SOD and POD activities were 2.14 and 1.37 times greater than in the control group, respectively (Fig. 8A and C). The CAT activity in the PdPap seedlings treated with the WT, T3, and T5 spores was lower than in the control group (Fig. 8B). The WT, T3, and T5 spores had similar impacts on the activities of the SOD, CAT, and POD in the PdPap seedlings (no significant differences at p≤0.05).
The enzymatic activities of the PdPap seedlings interacted with the Trichoderma spores under 0.2% NaHCO3 stress: SOD, CAT, and POD activities from the PdPap seedlings treated with spores of the WT, T3, and T5 strains under 0.2% NaHCO3 stress (A–C, respectively); PdPap seedlings treated with spores of the WT, T3, and T5 mutants under 0.2% NaHCO3 stress (D). Values in each column followed by the same letter are not significantly different (p≤0.05).
Soil salinization is a worldwide problem caused by the long-term use of drip irrigation. It has become a major factor limiting agricultural and forestry production.2 We found that the growth of the WT strain of T. asperellum can be promoted by 2% NaCl. However, as incubation time increased the promotion effect gradually weakened and eventually disappeared. The WT strain has powerful NaCl tolerance, but it cannot grow in PDA containing 10% (1709.40mM) NaCl. The T59 mutant had best NaCl tolerance of all the mutants, and can grow in PDA containing 10% (1709.40mM) NaCl. Two T. harzianum salt-tolerant mutants (Th50M6 and Th50M11) which can grow in PDA containing up to 69mM NaCl were obtained.3 Five of forty-five T. harzianum wild-type strains (Th-13, Th-14, Th-19, Th-33, and Th-50) can grow and form spores in growth containing up to 240mM NaCl.20 The T. asperellum wild-type strains (TaDORS3 and TaDOR693) can grow on PDA with 1000mM NaCl.27 Meanwhile, the NaCl tolerance of the T59 mutant is 25 times higher than the Th50M6 and Th50M11 mutants,3 seven times higher than the wild-type strains Th-13, Th-14, Th-19, Th-33, and Th-50,20 and 1.7 times higher than the wild-type strains TaDORS3 and TaDOR693.27
The Trichoderma WT strain was very sensitive to NaHCO3; meanwhile, the T3 and T5 mutants had best NaHCO3 tolerance of all the mutants (T1-T65). There are a few previous studies that report the NaHCO3 tolerance of Trichoderma sp. Under NaCl or NaHCO3 culture conditions, the mutants still exhibited antagonistic abilities towards R. solani, F. oxysporum, or A. alternata. The mutant T59 grows quickly under four different NaCl concentrations, and was more protective against the three kinds of pathogenic fungi than the WT strain. The Th50M6 mutant had better antagonistic activity than the wild-type strain, and had a maximum inhibition rate of 60% against F. oxysporum in saline media.3 The saline-tolerant T. harzianum strain Th-14 significantly reduced the incidence of F. oxysporum-induced wilt disease in chickpea plants.20 The T5 mutant also had antagonistic abilities towards R. solani and A. alternata under NaHCO3 stress. The T5 mutant has a maximum inhibition rate of 45.0% against A. alternata in 0.2% (23.81mM) NaHCO3 conditions. Ragazzi et al. reported that different Fusarium species, including F. oxysporum, were promoted under salt stress conditions.28 Our results agree with this report, as the antagonistic abilities of the T5 mutant against F. oxysporum decreased as NaHCO3 concentrations increased. The antagonistic abilities of the T3 mutant were nearly the same as those of the T5 mutant. The antagonistic abilities of the T3 mutant towards A. alternata under 0.3% (35.71mM) and 0.4% (47.62mM) NaHCO3 culture conditions were weaker than these of the T5 mutant.
Salt represses plant growth and root development.29 In a previous study, tomato plants were able to grow while irrigated daily with a 2800ppm (2.8mg/L) NaCl solution.3 Grey poplar (Populus tremula×alba, syn. Populus canescens) can grow in moderate levels of NaCl (75mM).16 The shoot growth of P. euphratica was not reduced even in media containing 100mM NaCl. However, the shoot growth of Chinese poplars (Populus tomentosa and P. alba cv. pyramidalis) was markedly reduced in 10mM NaCl.30 Photosynthesis, leaf area, height, and growth diameter of poplars declined rapidly as salinity increased. Some plant mortality has been observed after 14 days at 1% (170.94mM) NaCl.31 In this study, the concentration of NaCl in the MS media was 1% (170.94mM) or 2% (341.88mM). The PdPap seedlings became withered under 1% (170.94mM) or 2% (341.88mM) NaCl conditions, and they died after 4d of culture. The NaCl tolerance of the PdPap seedlings was not improved by treatment with WT or T59 spores. The spores grew under NaCl conditions. However, the PdPap seedlings were cultured in MS so there was a difference between the experimental and natural conditions.
Alkaline salts have been shown to have much stronger destructive effects on plants than neutral salts. When a saline soil contains HCO3−, plants are subject to the damaging effects of both salt and alkali stress.32 Of 12 halophytes, most had lower germination percentages in NaHCO3 than in NaCl.33 Saline-alkaline stress can cause an increase antioxidant enzyme activity.34 Superoxide dismutases (SODs) catalyze the dismutation of the superoxide anion to hydrogen and molecular oxygen; this is the first step in active oxygen-scavenging systems. CAT and POD are also very important for oxygen-scavenging systems.35 They play an important role in protecting cells against various environmental stresses, including salinity and alkalinity stress.36 They can be used to evaluate salinity and alkalinity resistance. In this study, the impact of the WT, T3, and T5 strains on SOD, CAT, and POD activities in the PdPap seedlings were similar under NaHCO3 stress (no significant differences at p≤0.05). Under the 0.05% NaHCO3 condition, the SOD activity of PdPap seedlings treated with the WT, T3, and T5 spores increased significantly following 3–5d of co-culturing. This is consistent with previous studies showing that T. asperellum treatment increased SOD activity of ‘XY335’ and ‘JY417’ maize seedlings in saline soil and increased the activity of antioxidant enzymes under NaCl stress in cucumbers.10,37 In this study, CAT activity increased; these differs from a previous report showing that CAT activity was reduced in leaves and roots following T. asperellum treatment.37 POD activity was decreased following treatment. Previous studies have shown that saline–alkaline stress increases the activity of some, but not all, antioxidant enzymes.12,38,39 Under 0.1% NaHCO3 conditions, SOD, CAT, and POD activities in the PdPap seedlings treated with the WT, T3, or T5 spores were lower than from plants under the same conditions but subjected to 0.05% NaHCO3 stress. However, biomass of the PdPap seedlings treated with WT, T3, or T5 spores were higher than those of the PdPap seedlings treated with the spores under 0.05% NaHCO3 stress. Under 0.2% NaHCO3 stress, the SOD and POD activities of the PdPap seedlings treated with WT, T3, and T5 spores was higher than in the control group; however, CAT activity was lower than in the control group. High CAT activity indicates that the plants are under oxidative stress.37 Our results support this conclusion. Efficient antioxidant activity does not necessarily lead to the up-regulation of all antioxidant enzymes.40 The demand of the oxygen-scavenging enzyme decreased under 0.2% NaHCO3 stress. It is possible that the activities of other antioxidant enzymes in the PdPap seedlings were impacted by the spores of the WT, T3, or T5 strains, leading to improvements in the NaHCO3 tolerance of the PdPap seedlings. Thus, the NaHCO3 tolerance of the PdPap seedlings can be improved by treating with spores from the WT, T3, and T5 strains grown under 0.05, 0.1, and 0.2% NaHCO3 stress.
We generated a saline- and two alkaline-tolerant T. asperellum mutants (T59, and T3 and T5, respectively); these mutants had better biocontrol potential under NaCl or NaHCO3 stresses. We introduce a new approach for the biocontrol of T. aspellum by generating saline- or alkaline-tolerant strains by mutation selection. This approach could be useful for enhancing salt and/or alkaline-salt tolerance, as well as the ability to biocontrol for R. solani, A. alternata, and F. oxysporum under saline or alkaline stress conditions.
Ethical approvalThe manuscript does not contain experiments using animal or human studies.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by the National High Technology Research and Development Program (the 13th Five-Year Plan Program) [Grant Number 2016YFC0501505] and the Fundamental Research Funds for the Central Universities, China [Grant Number 2572017AA03].