The use of biopharmaceuticals dates from the 19th century and within 5–10 years, up to 50% of all drugs in development will be biopharmaceuticals. In the 1980s, the biopharmaceutical industry experienced a significant growth in the production and approval of recombinant proteins such as interferons (IFN α, β, and γ) and growth hormones. The production of biopharmaceuticals, known as bioprocess, involves a wide range of techniques. In this review, we discuss the technology involved in the bioprocess and describe the available strategies and main advances in microbial fermentation and purification process to obtain biopharmaceuticals.
Biopharmaceuticals are mostly therapeutic recombinant proteins obtained by biotechnological processes. They are derived from biological sources such as organs and tissues, microorganisms, animal fluids, or genetically modified cells and organisms.1,2 Although several different expression systems may be employed including mammalian cell lines, insects, and plants, new technological advancements are continuously being made to improve microorganism production of biopharmaceuticals. This investment is justified by the well-characterized genomes, versatility of plasmid vectors, availability of different host strains, cost-effectiveness as compared with other expression systems.2,3
Bioprocessing is a crucial part of biotechnology. There is an anticipation that within the next 5 to 10 years, up to 50% of all drugs in development will be biopharmaceuticals. Examples include recombinant proteins obtained through microbial fermentation process.2,3 Bioprocessing for biopharmaceuticals production involves a wide range of techniques. In this review, we describe the main advances in microbial fermentation and purification process to obtain biopharmaceuticals.
Biopharmaceuticals and the pharmaceutical industryDrug development is an extremely complex and expensive process. According to the Tufts Center for the Study of Drug Development4 (http://www.csdd.tufts.edu), it may take approximately 15 years of intense research from the initial idea to the final product and development and costs usually exceed $2 billion. Low-molecular mass molecules are generically named as drugs while high-molecular mass drugs, which are represented by polymers of nucleotides (RNA or DNA) or amino acids (peptides and proteins), are called biopharmaceuticals.5 Biopharmaceuticals based in nucleic acids, such as small interfering RNA (siRNA), DNA vaccines, and gene therapy, are very promising strategies. However, clinical protocols were approved only very recently6 and just a few nucleic acids-based drugs have been therapeutically used to date7 and recent reviews addressed the state of the art of nucleic acids in therapies.8,9 In this review, we focused on peptides and proteins because they represent the major class of biopharmaceuticals.10
The use of proteins as drugs has been highlighted mainly by the high versatility of these biomolecules, which have different physiological roles in the human body including as catalysts, receptors, membrane channels, macromolecule carriers, and cellular defense agents.10,11 Some protein therapies provide high specificity, such as replacement of a patient's defective protein or even fulfill its absence due to genetic defects or immunological complications.10
Biopharmaceuticals: reference, biosimilars, and biobettersIt is worth emphasizing that the same gene product, which encodes the identical amino acid sequence, could be obtained by extraction from an animal tissue or by recombinant DNA techniques. However, the same protein produced by different manufacturers present different characteristics. In order to differentiate the products, the first biopharmaceutical version of the same therapeutic protein is set as the reference medicine, whereas the following ones are denominated biosimilars. Biosimilars may present differences because of post-translational modifications (phosphorylation, glycosylation) and different manufacturing processes. The term biobetter, also named biosuperiors, was recently used to refer to therapeutic macromolecules of the next generation, which present more effective drug delivery system, are modified by chemical methods (e.g., PEGylation) and/or engineered by means of molecular biology techniques to present better pharmacologic properties such as higher activity, enhanced stability, fewer side effects, and lower immunogenicity.12,13 Therefore, while a biosimilar represents a generic version of the original biopharmaceutical, biobetters need original research and development and the costs are significantly higher.14
Additionally, while the first biopharmaceuticals were predominantly delivered by injections, biobetters adopt different approaches to drug delivery administration as oral, dermatological and inhaled formulations which are related with different encapsulation approaches aiming to minimize the biologic instability caused by protein aggregation and denaturation as consequence of physicochemical modifications processes of the biodrug as deamination, hydrolysis, oxidation, among others.15 Protein engineering and rational modification is also a very promising area in new biopharmaceuticals and some aspects will be discussed later.
The use of biopharmaceuticals has grown worldwide in the last few years. In 2016, the total number of products approved by the Foods and Drugs Administration (FDA) and European Medicines Agency (EMA) for use in humans reached 1357, of which >130 have different formulations (reference products), 737 are biosimilars, and the remaining 482 are classified as biobetters16 (http://www.biopharma.com). From 2013 to 2016, 73 biopharmaceuticals were approved for use in humans. Among them, high prominence was given to monoclonal antibodies (23 approvals) widely used in several diagnostic procedures, treatment of inflammatory diseases, and neoplastic tumors16 (http://www.biopharma.com).
In addition, the European Medicine Agency (EMA) licensed two new products based on gene therapy (insertion of a corrective gene able to produce a normal protein in the patient's genome to cure a genetic disease) for use in human therapeutic protocols. These products were Glybera, developed by the German company UniQure for the treatment of lipoprotein lipase deficiency, and Strimvelis, developed by GlaxoSmithKline (GSK) for the treatment of adenosine deaminase deficiency.17 Although biopharmaceuticals can be very effective for disease control or cure, treatment costs can reach up to $1 million per patient.18
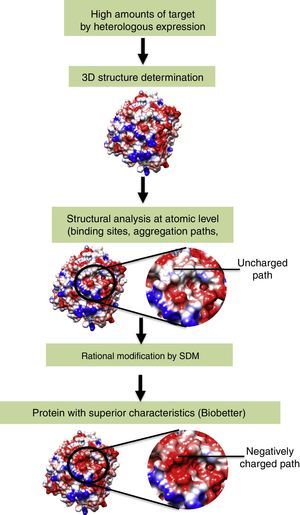
Biobetters based in protein structure engineeringOne of the most promising areas of the biobetters relies in protein structure engineering aiming the development of biodrugs with better pharmacological properties including higher activity, fewer side effects, and lower immunogenicity. The breakthrough in the determination of protein structures and their use as medicines dates from 1980s as a consequence of the advances in recombinant DNA technology. In turn, structural biochemistry has revolutionized our understanding of protein biology and afforded the beginning of protein engineering processes that can create protein drugs that are more effective than wild type proteins. Protein engineering may increase catalytic activity, stability, lower immunogenicity, and susceptibility to proteolytic processes.11,19–21
Protein engineering involves manipulating the protein sequence at the molecular level in order to change its function. The most common manipulations in the protein sequence are base pair cuts and exchanges. However, changes in protein structure caused by oxidation or irreversible reduction of disulfides are also considered. One factor that contributed decisively to protein engineering was the development of techniques that allow the determination of proteins three-dimensional structure at the atomic level. Among these techniques, more emphasis is given to X-ray crystallography because of its high resolution (reaching<1Å). More recently, nuclear magnetic resonance (NMR) and Cryo-electron microscopy (cryo-EM) have also gained space as alternative techniques for solving structures.22
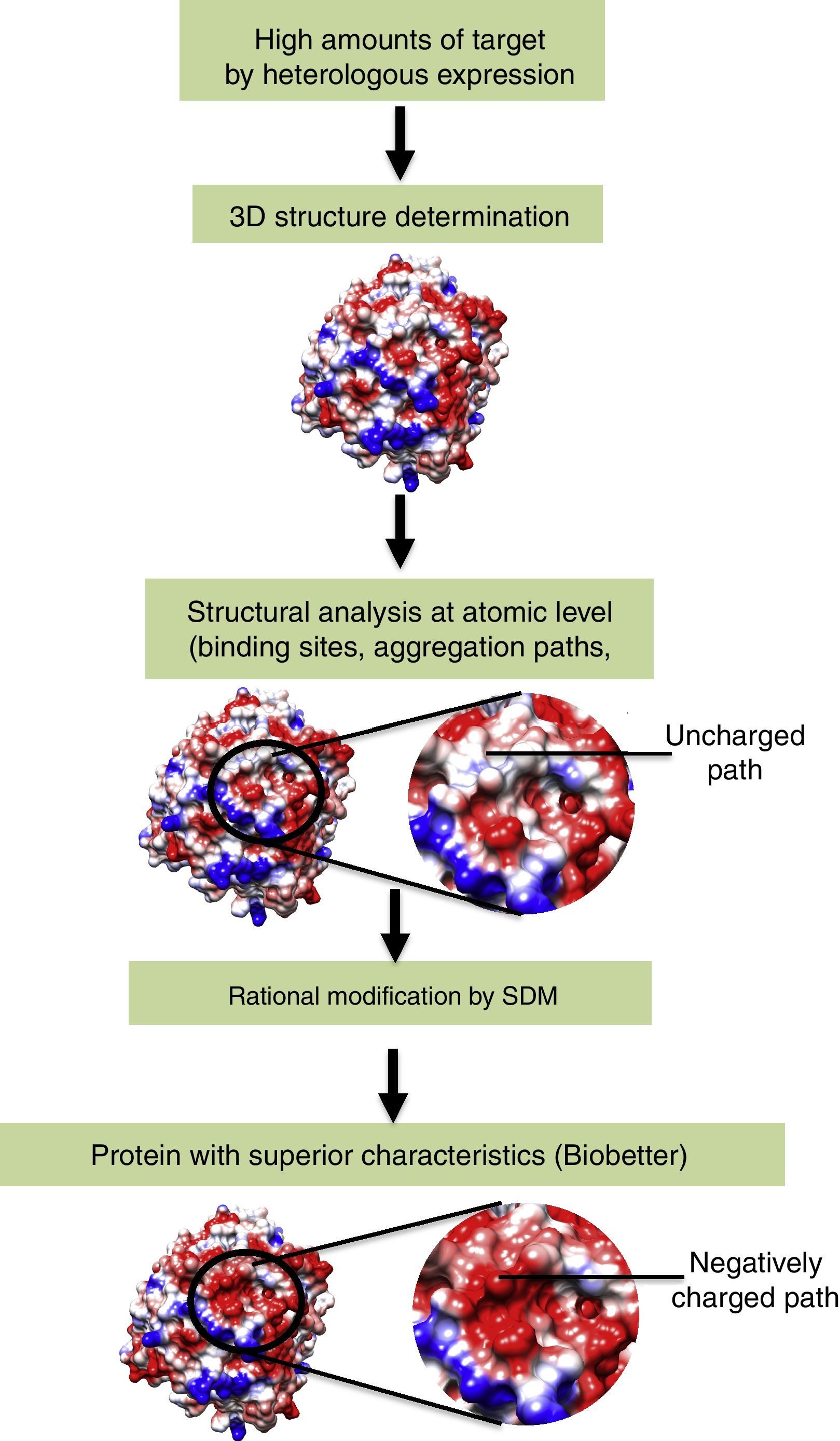
Gene manipulation (e.g., codon replacement) by molecular biology is able to modify protein structure in a specific manner. Among the several techniques used for gene manipulation, we highlight site-directed mutagenesis (SDM). This technique allows rational protein engineering based on its three-dimensional structure.23,24 Using SDM, one can replace, delete, or insert one or more amino acids in the sequence of a protein. Examples include the insertion of post-translational modification sites (glycosylation, acetylation, phosphorylation, etc.), enhancement of kinetic characteristics by modification of the active site environment, and modification of protein aggregation paths.25–28 (Fig. 1) Biobetters generated by protein engineering and gene manipulation may present superior characteristics over the reference biopharmaceutical and represents the major growing class among biopharmaceuticals.
The reference recombinant protein is expressed in high amounts and the molecular structure is determined at atomic levels (crystallography or NMR). Afterwards, the protein is analyzed using bioinformatic tools and regions of interest are identified. After gene manipulation by SDM, the modified recombinant protein (biobetter) is obtained.
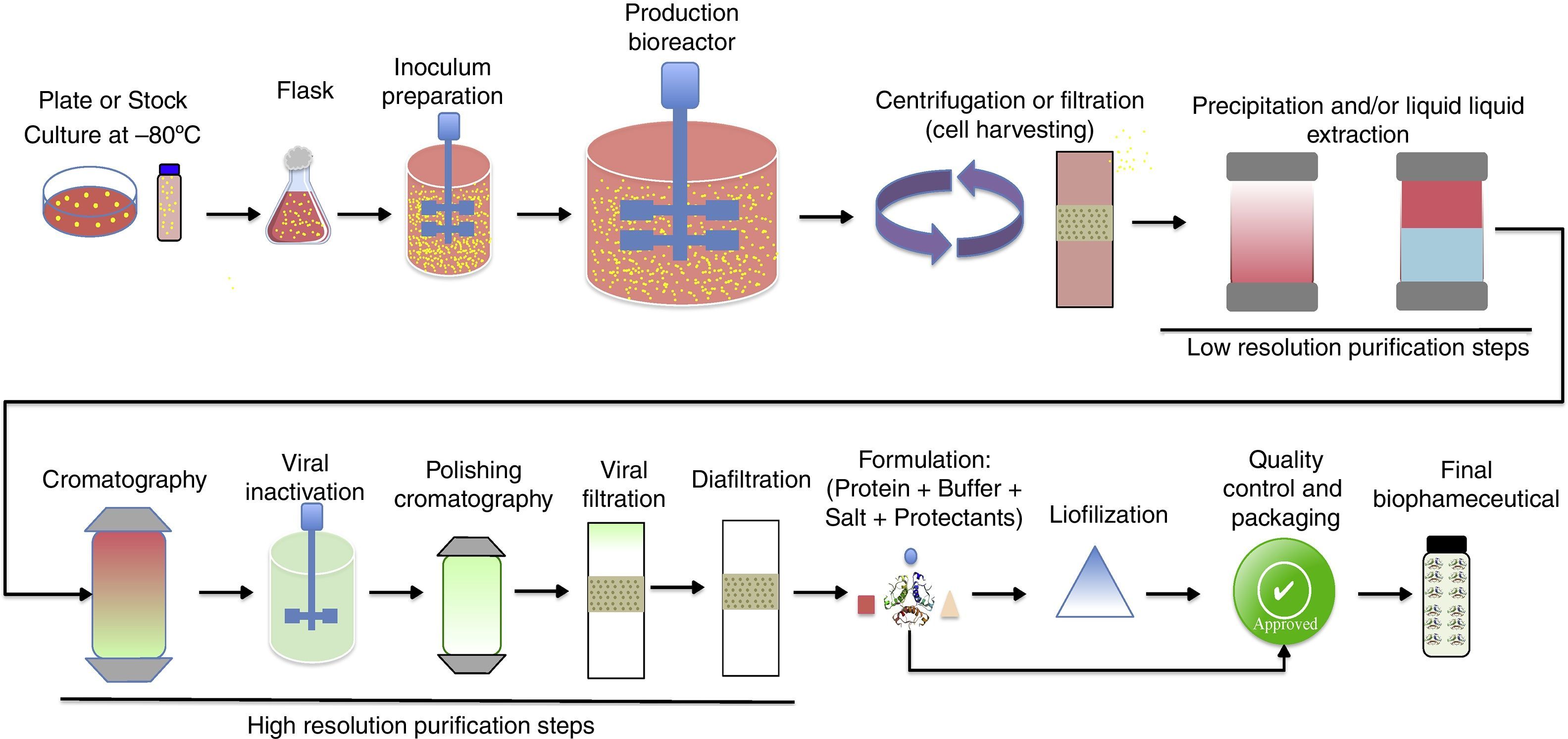
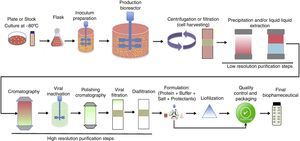
Upstream processing on biopharmaceuticals productionThe manufacturing technology for biopharmaceuticals can be divided into up- and downstream processes (Fig. 2). Upstream process is defined as the microbial growth required to produce biopharmaceuticals or other biomolecules and involves a series of events including the selection of cell line, culture media, growth parameters, and process optimization to achieve optimal conditions for cell growth and biopharmaceutical production. The main goal of the upstream process is the transformation of substrates into the desired metabolic products.29 This requires well-controlled conditions and involves the use of large-scale bioreactors. Several factors should be considered such as the type of process (batch, fed-batch, continuous, etc.) temperature, pH, and oxygen supply control, sterilization of materials and equipment employed, and maintenance of the environment to ensure it is free of contaminating microorganisms.30
The use of protein biopharmaceuticals in human health dates from the 19th century with the use of diphtheria antitoxin therapy.31 The antidote consists of immunoglobulins extracted from the serum of immunized animals that recognize and neutralize the toxin (e.g., horse or sheep).31,32 In fact, several antitoxins are available to treat envenomation by snakes, scorpions, and wasps, or infections. However, the use of non-human animal antibodies can cause hypersensitivity of the patient to the animal serum, which is known as serum sickness.33
The 20th century experienced the use of several molecules coming from animal sources such as insulin, growth hormone (GH), glucagon, and asparaginase.34–36 However, the discovery of the prion diseases related to the administration of hGH revealed another potential risk associated with non-human animal proteins. This reinforced the need for the production of protein pharmaceuticals from other sources.37 At this time, the biopharmaceutical industry looked at heterologous expression of protein drugs by means of recombinant DNA techniques in microorganisms.38
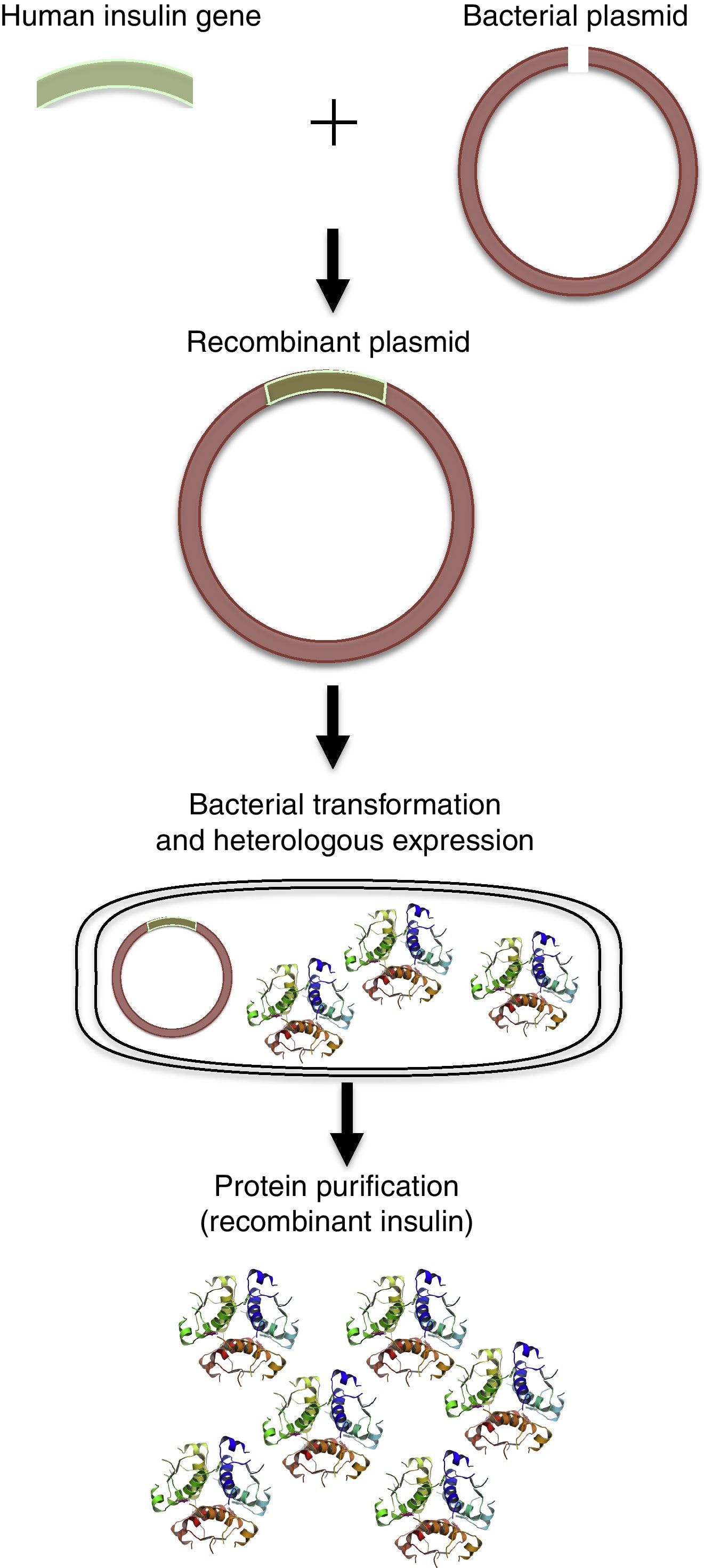
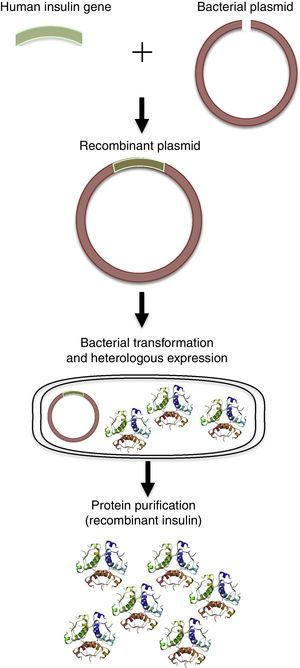
With the advances of molecular biology and recombinant DNA, human proteins could be obtained by heterologous expression using Escherichia coli, as well as other bacteria. The classic example is human insulin, which is used to treat diabetes mellitus types I and II (DMI and DMII). Initially, insulin was purified from the extracts of bovine and porcine pancreas. However, the process was expensive and many cases of immune responses caused by animal insulin in patients were reported10,39 The human insulin gene was then isolated and the human protein could be obtained by heterologous expression using E. coli (Fig. 3).
Filamentous fungiThe great diversity of molecules produced by filamentous fungi justifies the exploitation of these organisms. In particular, the isolation and identification of taxol-producing endophytic fungi is a new and feasible approach to the production of this antineoplastic drug. The development and use of taxol-producing fungi have made significant progress worldwide.40 Taxol was produced by Fusarium oxysporum grown in potato dextrose broth. In addition, the filamentous fungus Aspergillus niger isolated from Taxus cuspidate was found to produce taxol.41
Extracellular enzymes produced by filamentous fungi have also been explored. β-d-galactosidase (lactase – EC. 3.2.1 23) is the enzyme responsible for the catalysis of lactose to glucose and galactose. Global market for lactase has been increasing significantly due to its importance in lactose intolerance treatment. Lactase is marketed in tablet or capsules to be used as a food supplement for individuals intolerant to lactose before the intake of milk or dairy products.42,43 Lactase also participates in the galactooligosaccharides (GOS) synthesis with applications in functional foods such as low-calorie foods and as an additive in fermented dairy products, breads, and drinks. GOS, a group of oligosaccharides, are not digestible and are beneficial to the human or animal body. The benefits of GOS ingestion arise from a population of bifidobacteria in the colon that suppress the activity of putrefactive bacteria and reduce the formation of toxic fermentation products, avoiding intestinal constipation and increasing the production of vitamins B complex.44,45
Another biological drug of importance in fungi is the asparaginase enzyme. This enzyme is used for the treatment of selected types of hematopoietic diseases such as acute lymphoblastic leukemia and non-Hodgkin lymphoma. As tumor cells are dependent on the exogenous supply of asparagine for their proliferation, the presence of the drug, which depletes the bloodstream from asparagine, causes its selective death. However, the drug, which is obtained from E. coli (ELSPAR™) and Erwinia chrysanthemi, causes severe immunological reactions. Thus, the fungi enzyme could provide an alternative to the bacterial enzymes as an anti-tumoral agent as it presents stability and optimum pH near physiological conditions.
Li et al. (2015)46 demonstrated the production of a molecule with antifungal activity against a strain of Cytospora chrysosperma by submerged fermentation in a shaker. The active compound was obtained by extraction in organic solvents, liquid chromatography, and thin-layer chromatography. Svahn et al. (2015)47 produced and isolated amphotericin B by using a strain of Penicillium nalgiovense isolated from Antarctica. It was the first time that amphotericin B was isolated from a different organism as it is usually isolated from Streptomyces nodosus. Amphotericin B also showed a minimum inhibitory concentration of 0.125mg/mL against Candida albicans.
Collagenolytic proteases (Kollagenase™) have been directly used in clinical therapy, including wound healing, sciatica in herniated intervertebral discs, retained placenta, and as a pretreatment for enhancing adenovirus-mediated cancer gene therapy.48 Another alkaline protease with collagenolytic activity was produced by A. niger LCF9 and the enzyme hydrolyzed various collagen types without amino acid release and liberated low molecular weight peptides of potential therapeutic use.49
Carrez et al. (1990)50 detected the presence of interleukin-6 up to 25ng/mL in a modified strain of A. nidulans expressing the human interleukin-6. Years later, Yadwad and colleagues (1996)51 produced approximately 54mg/L of interleukin-6 in an air-lift fermenter with a recombinant strain of A. nidulans and a medium supplemented with salts, fructose, and threonine.
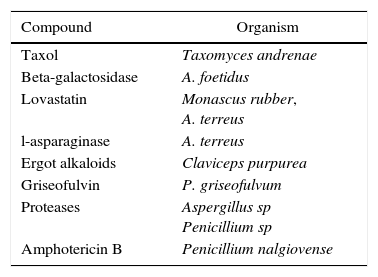
The production of biopharmaceuticals by filamentous fungi is well studied, but the applicability of biomolecules produced by such organisms is still restricted by the high cost of purification of some molecules and by difficulty in filamentous fungal cultivation (Table 1).52 Nonetheless, the use of filamentous fungi for the production of compounds of interest is still an interesting strategy.
Biopharmaceuticals obtained from filamentous fungi.
| Compound | Organism |
|---|---|
| Taxol | Taxomyces andrenae |
| Beta-galactosidase | A. foetidus |
| Lovastatin | Monascus rubber, A. terreus |
| l-asparaginase | A. terreus |
| Ergot alkaloids | Claviceps purpurea |
| Griseofulvin | P. griseofulvum |
| Proteases | Aspergillus sp Penicillium sp |
| Amphotericin B | Penicillium nalgiovense |
Downstream processing includes all steps required to purify a biological product from cell culture broth to final purified product. It involves multiple steps to capture the target biomolecule and to remove host cell related impurities (e.g., host cell proteins, DNA, etc.), process related impurities (e.g., buffers, leached ligands, antifoam, etc.) and product related impurities (e.g., aggregates, fragments, clipped species, etc.). Each purification step is capable of removing one or more classes of impurities.53,54 Downstream processing usually encompasses three main stages, namely (i) initial recovery (extraction or isolation), (ii) purification (removal of most contaminants), and (iii) polishing (removal of specified contaminants and unwanted forms of the target biomolecule that may have formed during isolation and purification).53,55,56
Initial recovery involves the separation between cell and supernatant (broth clarification). For this purpose, the main operations employed are centrifugation, filtration, sedimentation, and flotation. If the target biomolecule is produced extracellularly, the clarified broth is submitted to concentration (e.g., ultrafiltration) followed by purification. For example, secreted and soluble proteins in the culture media of P. pastoris can be directly recovered by centrifugation. Samples can then be concentrated and the target protein purified from the supernatant by processes such as ultrafiltration, precipitation, and/or chromatography.57 For intracellular biomolecules, the cells harvested must be submitted to lysis (e.g., high-pressure homogenizer, sonication, passing through mills, etc.) followed by clarification to remove cell debris. The target biomolecule is purified from the clarified cell homogenate (usually by precipitation and/or chromatography). In cases where proteins are expressed as inclusion bodies (as some recombinants produced by E. coli), an extra step of protein refolding (buffer exchange) is required. These additional steps significantly contribute to increases in production time and costs for intracellular biomolecules.58
Efficient recovery and purification of biopharmaceuticals have been referred as a critical part of the production process. Purification process must be robust, reliable, easily scaled-up, and capable of removing both processes- and product-related impurities to ensure product safety. The achieved purity, the speed of process development, overall recovery yield, and throughput are some of the main key parameters that must be taken into consideration during downstream process development.55 To reach the stringency of purity required in the biopharmaceutical industry, sometimes exceeding 99%, chromatography steps are usually required. Chromatography allows for high resolution and has traditionally been the workhorse for protein purification and polishing.53,56 However, chromatography has also been the major cost center in purification processes, mainly due to media cost and relatively long cycle times. In addition, the biopharmaceutical industry still faces practical limitations in terms of throughput and scalability.55
ChromatographyDifferent strategies based on sequences of classical chromatography have been described for nucleic acids, peptides, and proteins purification. In fact, chromatography is a very effective purification technique with a wide range of industrial applications and currently represents the favorite choice due to its high resolution capacity.56 The separation principle in chromatography is based on the differences in the affinity of the species carried by a fluid mobile phase toward a solid stationary phase. When a sample is introduced and transported by the eluent along the column, some of its components will have more powerful interactions with the stationary phase than others, generating concentration profiles that will percolate the chromatographic column at different speeds. The less retained species will elute earlier from the column than the most retained ones, eventually allowing the collection of the products of interest with a high purity degree.59 Based on the interaction between the solid stationary phase and biomolecules, chromatographic techniques can be summarized into five classes: (i) affinity, (ii) ion-exchange, (iii) hydrophobic interactions, (iv) size exclusion, and (v) mixed-mode chromatography.60
Affinity chromatography simulates and exploits natural biological processes such as molecular recognition for the selective purification of target proteins.61 This class of chromatography is probably the only technique currently available that is capable of addressing key issues in high-throughput proteomics and scale-up.62 The most common example of an affinity process is protein-A chromatography, which has been applied for over a decade in industrial and academic settings for the capture and purification of antibodies.60 Similarly, protein-L may possibly come to play a role in antibody fragments purification.59 Another affinity-based strategy well established for recombinant proteins purification is the use of fusion tags, which are amino acid sequences attached to recombinant proteins with selective and high affinities for a chemical or biological ligand immobilized on a chromatographic column. In particular, the polyhistidine (xHis) tag has been frequently used to purify recombinant proteins due to its binding capacity toward divalent metal cations.60 Despite the fact that affinity methods usually eliminate purification steps, increase yields, and downsize capital equipment, they do present some drawbacks, particularly regulatory ones since complete withdrawal of leached ligands is a requirement.61
Traditional choices in chromatographic set ups include particle-based resins, batch mode operation, and packed columns. In order to address the drawbacks from these standard parameters, some process alternatives are attracting the pharmaceutical industry, especially the chromatographic separations based on simulated moving bed (SMB), expanded bed adsorption (EBA), and single block monolith columns.
SMB chromatography is the preferred choice for enantiomer separation of synthetic drugs in pharmaceutical industry. However, just recently, its use made a significant rise in biotechnology companies, especially for protein refolding and continuous downstream process.63 The system presents multiple small chromatographic columns sequentially connected and operated with countercurrent flow of fluids. Simulated moving comes from the periodical switch of multiport inlets/outlets from column to column, in the direction of fluid flow, which gives the impression that the column bed is moving. These inlet/outlet valves (feed, desorbent, raffinate, and extract) are positioned in a way that minimize dead zones, allow desorbent recycling, optimize product recovery, and function as semi-continuous mode.64 Especially for refolding, SMB together with the recycling of aggregates lead to a theoretical yield of 100%, excluding the folding equilibrium as a limiting factor for productivity.65 Nevertheless, SMB is more complex to implement and requires a higher investment cost.
EBA chromatography is a 3-in-1 process intended to capture the product directly from the cell suspension, combining clarification, concentration, and initial purification. The bottom feed from the EBA system creates a flow that gradually expands the resin and form a stable particle gradient.66 This gradient consists of particles of different size ranges and different densities, which requires a narrow range of calculated flow rates. All adsorbents in direct contact with the feedstock may bind to cells/cell debris, disrupting the gradient and reducing recovery. This issue is addressed with studies on adsorption pH to identify conditions with maximum product adsorption and minimum cell adhesion.67 Several studies have shown the value of EBA. It efficiently removes precipitates and captures target proteins from refold pools of E. coli-based production68 and it promotes enhanced recovery of Human Epidermal Growth Factor from E. coli homogenate and Pichia pastoris culture medium.67
Particle-based resins rely on mass transfer mainly through diffusion, requiring long times for large biomolecules. On the other hand, the single block monolith column has interconnected channels that transfer mass mainly through convection, which allows for high flow velocity. In addition, monolith does not have the packing step and tolerates the passage of air, reducing costs, and time with packing validation and repacking/replacing solid phase due to air interruption. Other significant advantages are easy scale-up due to flow independent of dynamic binding and compatibility with several organic, polymer-based, and inorganic media.69 The disadvantage of higher buffer consumption can be decreased with the SMB set up, which can also be combined with single use technology. Monoliths are widely applied to the recovery of proteins such as coagulation factor IX (ion exchange)70 and IgG (affinity chromatography)29 from a variety of cell culture including P. pastoris71 and E. coli.69
Alternative separation techniquesWith a burgeoning biotechnology market, there is an ongoing search for new and improved alternatives to chromatography in an effort to lower costs and improve yields, while maintaining high product purity.56 Several promising alternatives have been described in literature including affinity precipitation, high-performance tangential flow filtration, filtration strategies based on thiophilic and affinity interactions, two-phase aqueous systems, high-gradient magnetic fishing, preparative electrophoresis, and isoelectric focusing.53,55,56,58
Magnetic separation with immunocapture supports stands among the techniques used in purification kits, but just recently its application to industrial scale showed viable paths. The initial high costs of the beads from the kits were surpassed with new materials and broader size dispersion and binding capacity, without decreasing batch-to-batch consistency. Sub-micron superparamagnetic particles of coated magnetite crystals can be functionalized according to the desired selectivity. These particles have been used for the purification of enzymes and inclusion bodies.29
Filtration with ion-exchange membranes substitutes flow-through chromatography for polishing steps. They remove host-cell proteins, nucleic acids, and viruses with increased flow rates, reduce buffer consumption and time, when compared to traditional polishing. Hydrophobic interaction membranes can remove dimers and aggregates from monoclonal antibody production and substitute more chromatography steps.72
General trendsThe aim of downstream process should be to deliver the highest yield of the purest product at the shortest time/cost. However, traditional processes and quality control does not bring the efficiency needed to keep pace with current upstream production. To address current issues, some general trends emerge as most relevant including single use modules, continuous production, process analytical technology, and quality by design.73
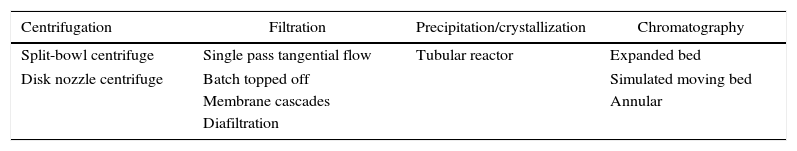
The disposable units are compatible with continuous mode and bring faster routine operation because no cleaning or cleaning/validation has to be performed.73 Continuous processes generally result in higher productivity, less buffer consumption, and smaller footprint. A general end-to-end continuous process can be accomplished by perfusion cell reactors coupled with a continuous capture step, integrated with some of the downstream technologies described in Table 2. A recent and extensive review on continuous downstream processing of biopharmaceuticals describes and discusses each set up option in detail.64
Unit operations for continuous downstream process.
| Centrifugation | Filtration | Precipitation/crystallization | Chromatography |
|---|---|---|---|
| Split-bowl centrifuge | Single pass tangential flow | Tubular reactor | Expanded bed |
| Disk nozzle centrifuge | Batch topped off | Simulated moving bed | |
| Membrane cascades | Annular | ||
| Diafiltration | |||
Process consistency over time can be assured with the aid of process analytical technology (PAT) and Quality by Design (QbD) concepts described in the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines. QbD preconizes that the product is the process. Therefore, it is essential to know the critical process parameters and link them with critical material attributes to predict and adjust their impact on critical quality attributes of the final product. Processes developed under QbD knowledge contain design spaces instead of single value or extremely narrow parameters; values inside the design space results in good product performance and brings the necessary flexibility to continuous processing.74 However, knowledge of the process requires process analytical technology (PAT) tools that include analytical chemistry and mathematical and statistical modeling/analysis. Among the options, near infrared spectroscopy and principal component analysis are trending choices for analytical and mathematical tools, which can be applied to several steps.75
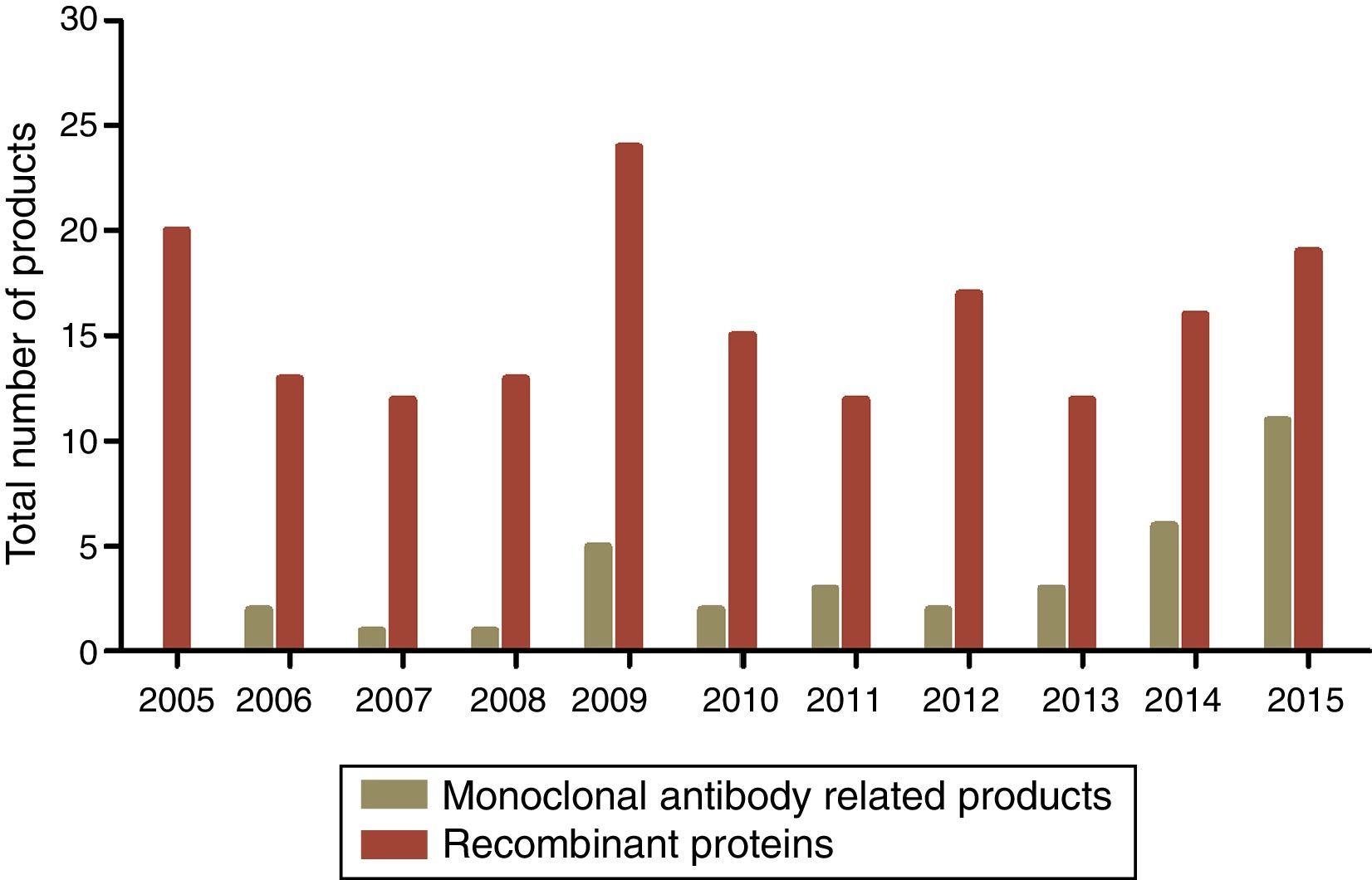
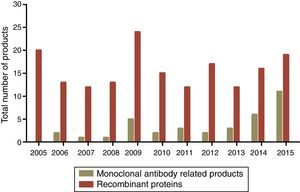
Global consumer market of microbial biopharmaceuticalsIn 1982, human insulin was the first recombinant protein that was FDA approved for use in humans as a biopharmaceutical product.10,39 In the 1980s, the biopharmaceutical industry experienced a significant growth in the production and approval of recombinant proteins including interferons (IFN α, β, and γ) and growth hormones. In the 1990s, the first monoclonal antibodies (MAb) and related products experienced an extraordinary growth, and in 2015, these products represented two-thirds of the products approved for commercial use in the world according to the Biotrack database76 (Fig. 4).
Commercial biopharmaceutical products approved from 2005 to 2015. Dark green bars represent monoclonal antibody related products and non-related total recombinant proteins are represented in red. The data used concerning the number of biopharmaceutical approvals are available at biopharma biopharmaceutical products16 (http://www.biopharma.com/approvals).
Currently, the total market sales from microbial recombinant products reached approximately $50 billion, representing one-third of the total sales of biopharmaceuticals. The choice of microorganism in the production of biopharmaceuticals relies on many factors including low cost production, easy manipulation, and propagation, and molecular biology methods. Some of the most important biopharmaceuticals obtained by natural sources or by heterologous expression are shown in Tables 3–5.
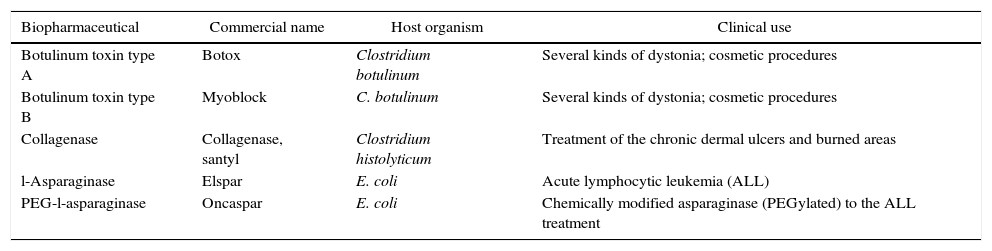
Examples of therapeutic native proteins obtained by purification from natural sources.
| Biopharmaceutical | Commercial name | Host organism | Clinical use |
|---|---|---|---|
| Botulinum toxin type A | Botox | Clostridium botulinum | Several kinds of dystonia; cosmetic procedures |
| Botulinum toxin type B | Myoblock | C. botulinum | Several kinds of dystonia; cosmetic procedures |
| Collagenase | Collagenase, santyl | Clostridium histolyticum | Treatment of the chronic dermal ulcers and burned areas |
| l-Asparaginase | Elspar | E. coli | Acute lymphocytic leukemia (ALL) |
| PEG-l-asparaginase | Oncaspar | E. coli | Chemically modified asparaginase (PEGylated) to the ALL treatment |
* Adapted from Leader et al., 2008.
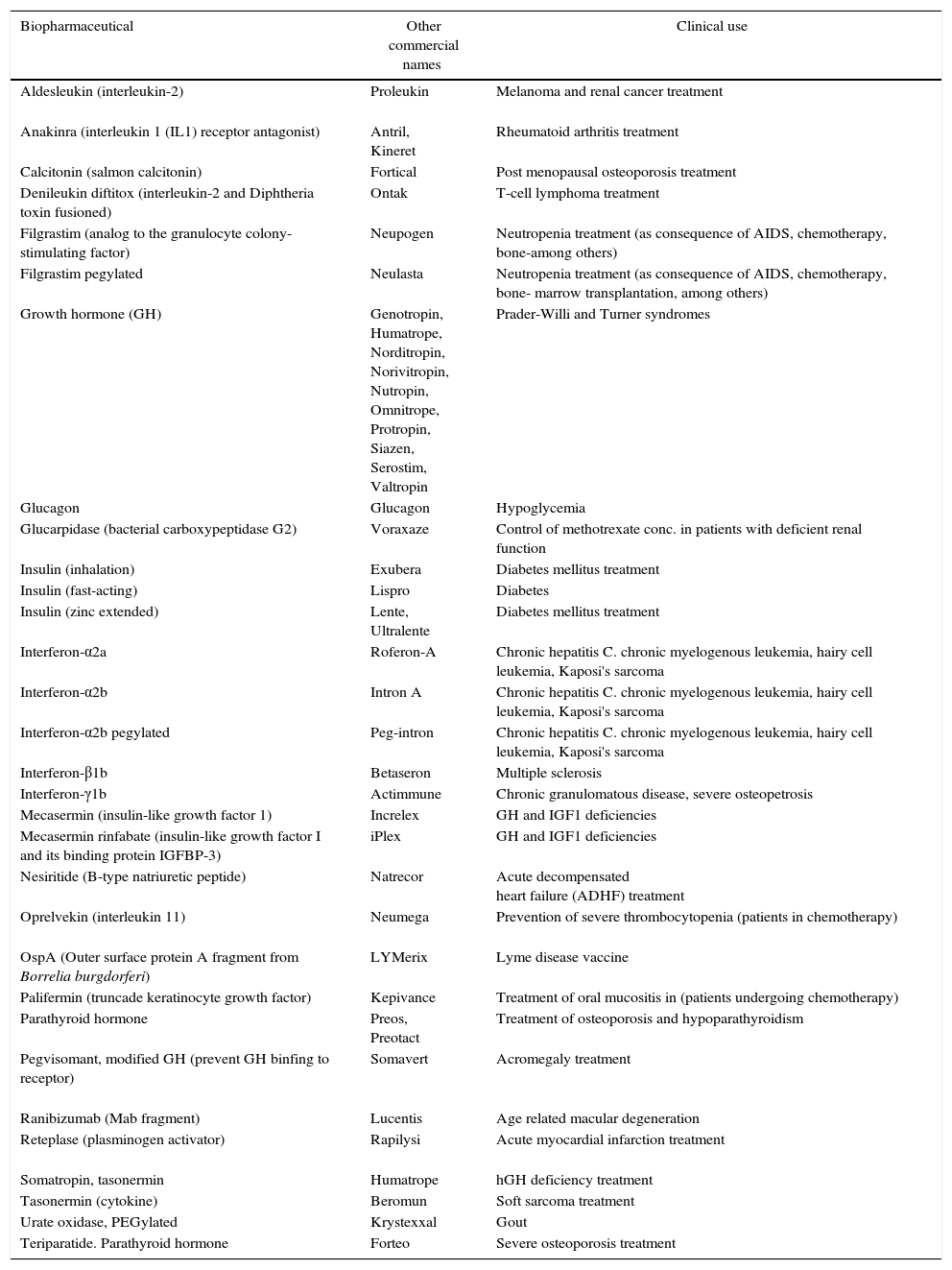
Examples of therapeutic recombinant proteins obtained by heterologous expression in E. coli.
| Biopharmaceutical | Other commercial names | Clinical use |
|---|---|---|
| Aldesleukin (interleukin-2) | Proleukin | Melanoma and renal cancer treatment |
| Anakinra (interleukin 1 (IL1) receptor antagonist) | Antril, Kineret | Rheumatoid arthritis treatment |
| Calcitonin (salmon calcitonin) | Fortical | Post menopausal osteoporosis treatment |
| Denileukin diftitox (interleukin-2 and Diphtheria toxin fusioned) | Ontak | T-cell lymphoma treatment |
| Filgrastim (analog to the granulocyte colony-stimulating factor) | Neupogen | Neutropenia treatment (as consequence of AIDS, chemotherapy, bone-among others) |
| Filgrastim pegylated | Neulasta | Neutropenia treatment (as consequence of AIDS, chemotherapy, bone- marrow transplantation, among others) |
| Growth hormone (GH) | Genotropin, Humatrope, Norditropin, Norivitropin, Nutropin, Omnitrope, Protropin, Siazen, Serostim, Valtropin | Prader-Willi and Turner syndromes |
| Glucagon | Glucagon | Hypoglycemia |
| Glucarpidase (bacterial carboxypeptidase G2) | Voraxaze | Control of methotrexate conc. in patients with deficient renal function |
| Insulin (inhalation) | Exubera | Diabetes mellitus treatment |
| Insulin (fast-acting) | Lispro | Diabetes |
| Insulin (zinc extended) | Lente, Ultralente | Diabetes mellitus treatment |
| Interferon-α2a | Roferon-A | Chronic hepatitis C. chronic myelogenous leukemia, hairy cell leukemia, Kaposi's sarcoma |
| Interferon-α2b | Intron A | Chronic hepatitis C. chronic myelogenous leukemia, hairy cell leukemia, Kaposi's sarcoma |
| Interferon-α2b pegylated | Peg-intron | Chronic hepatitis C. chronic myelogenous leukemia, hairy cell leukemia, Kaposi's sarcoma |
| Interferon-β1b | Betaseron | Multiple sclerosis |
| Interferon-γ1b | Actimmune | Chronic granulomatous disease, severe osteopetrosis |
| Mecasermin (insulin-like growth factor 1) | Increlex | GH and IGF1 deficiencies |
| Mecasermin rinfabate (insulin-like growth factor I and its binding protein IGFBP-3) | iPlex | GH and IGF1 deficiencies |
| Nesiritide (B-type natriuretic peptide) | Natrecor | Acute decompensated heart failure (ADHF) treatment |
| Oprelvekin (interleukin 11) | Neumega | Prevention of severe thrombocytopenia (patients in chemotherapy) |
| OspA (Outer surface protein A fragment from Borrelia burgdorferi) | LYMerix | Lyme disease vaccine |
| Palifermin (truncade keratinocyte growth factor) | Kepivance | Treatment of oral mucositis in (patients undergoing chemotherapy) |
| Parathyroid hormone | Preos, Preotact | Treatment of osteoporosis and hypoparathyroidism |
| Pegvisomant, modified GH (prevent GH binfing to receptor) | Somavert | Acromegaly treatment |
| Ranibizumab (Mab fragment) | Lucentis | Age related macular degeneration |
| Reteplase (plasminogen activator) | Rapilysi | Acute myocardial infarction treatment |
| Somatropin, tasonermin | Humatrope | hGH deficiency treatment |
| Tasonermin (cytokine) | Beromun | Soft sarcoma treatment |
| Urate oxidase, PEGylated | Krystexxal | Gout |
| Teriparatide. Parathyroid hormone | Forteo | Severe osteoporosis treatment |
The data were obtained from manufacturer pages and from16http://www.biopharma.com.
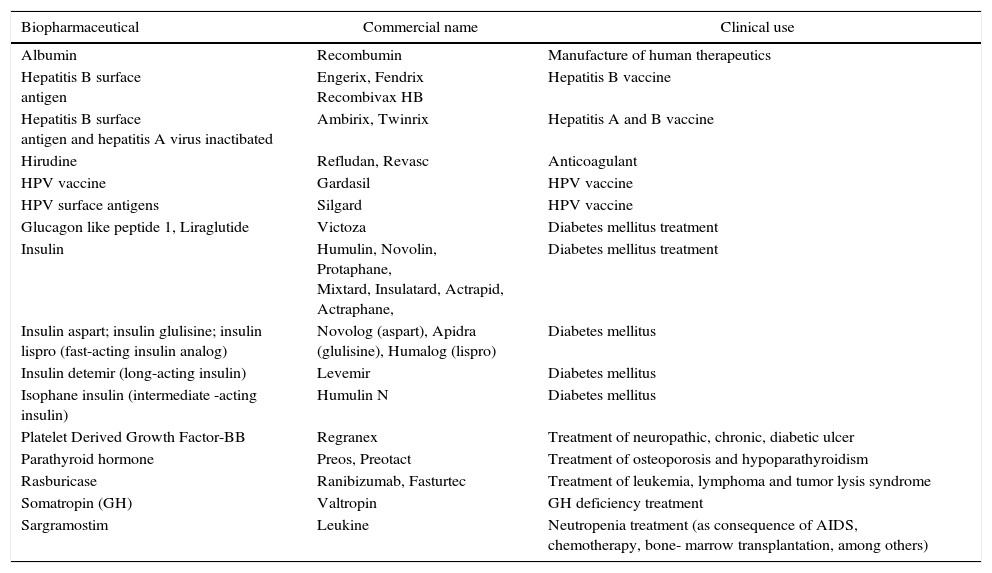
Examples of therapeutic recombinant proteins obtained by heterologous expression in S. cerevisiae.
| Biopharmaceutical | Commercial name | Clinical use |
|---|---|---|
| Albumin | Recombumin | Manufacture of human therapeutics |
| Hepatitis B surface antigen | Engerix, Fendrix Recombivax HB | Hepatitis B vaccine |
| Hepatitis B surface antigen and hepatitis A virus inactibated | Ambirix, Twinrix | Hepatitis A and B vaccine |
| Hirudine | Refludan, Revasc | Anticoagulant |
| HPV vaccine | Gardasil | HPV vaccine |
| HPV surface antigens | Silgard | HPV vaccine |
| Glucagon like peptide 1, Liraglutide | Victoza | Diabetes mellitus treatment |
| Insulin | Humulin, Novolin, Protaphane, Mixtard, Insulatard, Actrapid, Actraphane, | Diabetes mellitus treatment |
| Insulin aspart; insulin glulisine; insulin lispro (fast-acting insulin analog) | Novolog (aspart), Apidra (glulisine), Humalog (lispro) | Diabetes mellitus |
| Insulin detemir (long-acting insulin) | Levemir | Diabetes mellitus |
| Isophane insulin (intermediate -acting insulin) | Humulin N | Diabetes mellitus |
| Platelet Derived Growth Factor-BB | Regranex | Treatment of neuropathic, chronic, diabetic ulcer |
| Parathyroid hormone | Preos, Preotact | Treatment of osteoporosis and hypoparathyroidism |
| Rasburicase | Ranibizumab, Fasturtec | Treatment of leukemia, lymphoma and tumor lysis syndrome |
| Somatropin (GH) | Valtropin | GH deficiency treatment |
| Sargramostim | Leukine | Neutropenia treatment (as consequence of AIDS, chemotherapy, bone- marrow transplantation, among others) |
The data were obtained from manufacturer pages and from16 http://www.biopharma.com.
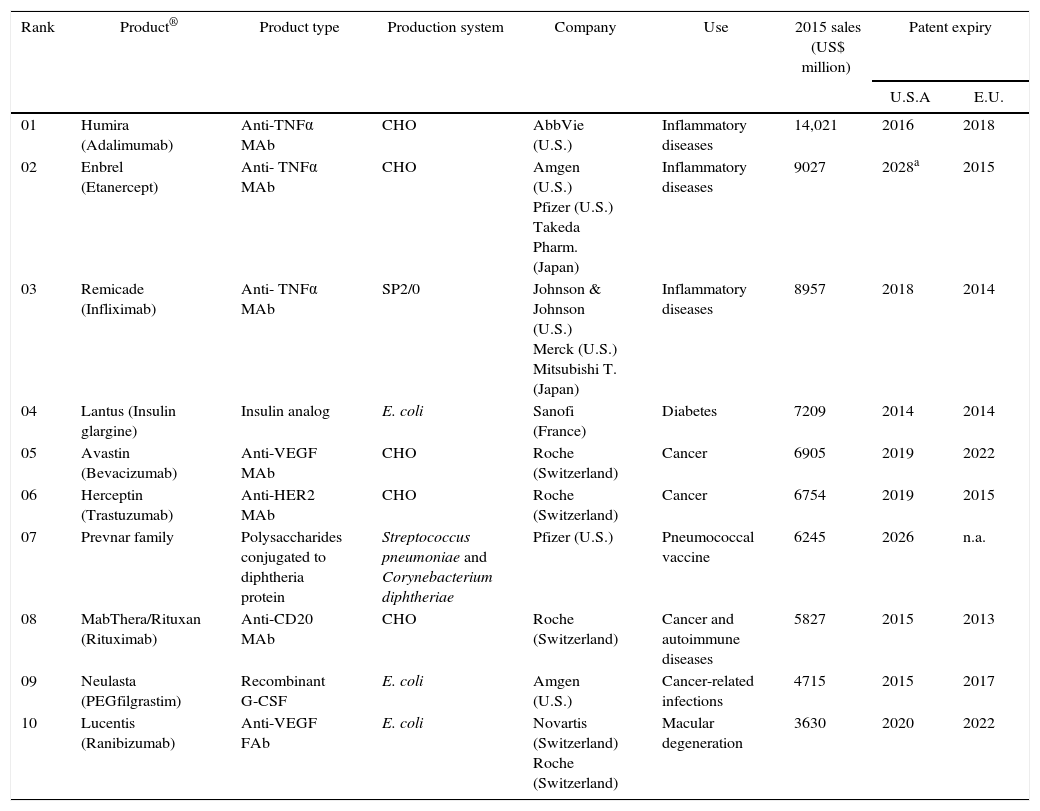
Biopharmaceuticals are revolutionary in the pharmaceutical industry. According to global revenues, 10 biotechnological related products figured among the top-25 best-selling drugs in 2015; 4 of them produced by microorganisms77,78 (Table 6). These biopharmaceuticals are marketed by leading pharmaceutical companies primarily located in U.S.A, Japan, and Europe and comprise a narrow scope of treatment profile, with most drugs for the treatment and management of inflammatory diseases (e.g. rheumatoid arthritis) and cancer.
| Rank | Product® | Product type | Production system | Company | Use | 2015 sales (US$ million) | Patent expiry | |
|---|---|---|---|---|---|---|---|---|
| U.S.A | E.U. | |||||||
| 01 | Humira (Adalimumab) | Anti-TNFα MAb | CHO | AbbVie (U.S.) | Inflammatory diseases | 14,021 | 2016 | 2018 |
| 02 | Enbrel (Etanercept) | Anti- TNFα MAb | CHO | Amgen (U.S.) Pfizer (U.S.) Takeda Pharm. (Japan) | Inflammatory diseases | 9027 | 2028a | 2015 |
| 03 | Remicade (Infliximab) | Anti- TNFα MAb | SP2/0 | Johnson & Johnson (U.S.) Merck (U.S.) Mitsubishi T. (Japan) | Inflammatory diseases | 8957 | 2018 | 2014 |
| 04 | Lantus (Insulin glargine) | Insulin analog | E. coli | Sanofi (France) | Diabetes | 7209 | 2014 | 2014 |
| 05 | Avastin (Bevacizumab) | Anti-VEGF MAb | CHO | Roche (Switzerland) | Cancer | 6905 | 2019 | 2022 |
| 06 | Herceptin (Trastuzumab) | Anti-HER2 MAb | CHO | Roche (Switzerland) | Cancer | 6754 | 2019 | 2015 |
| 07 | Prevnar family | Polysaccharides conjugated to diphtheria protein | Streptococcus pneumoniae and Corynebacterium diphtheriae | Pfizer (U.S.) | Pneumococcal vaccine | 6245 | 2026 | n.a. |
| 08 | MabThera/Rituxan (Rituximab) | Anti-CD20 MAb | CHO | Roche (Switzerland) | Cancer and autoimmune diseases | 5827 | 2015 | 2013 |
| 09 | Neulasta (PEGfilgrastim) | Recombinant G-CSF | E. coli | Amgen (U.S.) | Cancer-related infections | 4715 | 2015 | 2017 |
| 10 | Lucentis (Ranibizumab) | Anti-VEGF FAb | E. coli | Novartis (Switzerland) Roche (Switzerland) | Macular degeneration | 3630 | 2020 | 2022 |
Patents for cloning and production of several original-generation (branded) biopharmaceuticals have expired or will expire within the next years (Table 6). Similar to chemical drugs, once the patent of a biological product is expired the marketing of biosimilars and generics is possible.79 These patent expirations, combined with rising healthcare costs and population aging worldwide are paving the way for the development of biosimilars and biobetters, opening new commercial opportunities.80,81 Many biosimilars are currently under development and these follow-on products will inevitably play substantial competition and an increasing role in healthcare in upcoming years.79,82 In Brazil the scenario is modest, but considering the global panel and recent government incentive for the national biopharmaceutical industry development, we expect to see more patents in the near future and also novel opportunities for biosimilars and biobetters.
Conclusion and future trendsNew technological advancements are continuously made to improve the discovery, rational modification, production, and purification of biopharmaceuticals. Innovative strategies to identify different species of microorganisms from the Brazilian biodiversity must be investigated targeting the discovery of alternative hosts for heterologous expression.
Conflict of interestThe authors declare no conflicts of interest.