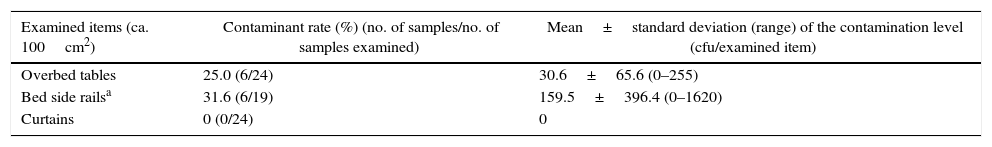
Methicillin-resistant Staphylococcus aureus (MRSA) can contaminate environmental surfaces that are frequently touched by the hands of patients with MRSA colonization/infection. There have been many studies in which the presence or absence of MRSA contamination was determined but no studies in which MRSA contamination levels were also evaluated in detail. We evaluated MRSA contamination of environmental surfaces (overbed tables, bed side rails, and curtains) in the rooms of inpatients from whom MRSA was isolated via clinical specimens. We examined the curtains within 7–14 days after they had been newly hung. The environmental surfaces were wiped using gauze (molded gauze for wiping of surface bacteria; 100% cotton, 4cm×8cm) moistened with sterile physiological saline. The MRSA contamination rate and mean counts (range) were 25.0% (6/24 samples) and 30.6 (0–255)colony-forming units (cfu)/100cm2, respectively, for the overbed tables and 31.6% (6/19 samples) and 159.5 (0–1620)cfu/100cm2, respectively, for the bed side rails. No MRSA was detected in 24 curtain samples. The rate of MRSA contamination of environmental surfaces was high for the overbed tables and bed side rails but low for the curtains. Therefore, at least until the 14th day of use, frequent disinfection of curtains may be not necessary.
To prevent the transmission of methicillin-resistant Staphylococcus aureus (MRSA) infection, maintaining the cleanliness of the hands of medical staff and the hospital environment is important.1–4 In particular, maintaining the cleanliness of environmental surfaces that are frequently touched by patients’ hands (overbed tables, bed side rails, and curtains) is necessary.5,6 There have been many studies in which the presence or absence of MRSA contamination was determined on environmental surfaces that are frequently touched by patients with MRSA colonization/infection, but these studies did not evaluate MRSA contamination levels in detail.7–12 For the establishment of infection, both contaminants and contaminant levels are important. Therefore, we evaluated both the rate and the level of MRSA environmental contamination in the rooms of patients with MRSA colonization/infection using the gauze-wiping method, which is more effective than the swab method for the detection of microorganisms.13
Materials and methodsDuring a 1-month period from August 1 to 31, 2013, in Yamaguchi University Hospital (736 beds), we investigated MRSA contamination of the rooms (curtains, overbed tables, bed side rails) of 24 inpatients (age, 1–85 years) from whom MRSA was isolated via clinical specimens.
MRSA was isolated from the following patient specimens: 8 samples of open pus, 5 expectorated (aspirated) sputum samples, 3 enclosed pus samples, 2 ear discharge samples, 2 pharyngeal mucus samples, 1 tracheotomy swab, 1 nasal discharge swab, 1 vaginal/vulvar swab, and 1 ear swab. In fiscal year of 2012, the rate of MRSA transmission at the hospital was 0.5 nosocomial MRSA cases per 100 hospital admissions.
For sampling, the surfaces of the bed curtains, overbed tables, and bed side rails were wiped using gauze (molded gauze for the wiping of surface bacteria; 100% cotton, 4cm×8cm; Sawada Menko Co., Ltd., Tokyo, Japan) moistened with sterile physiological saline. For the bed curtains, an area of approximately 10cm×10cm near the heads of the patients was examined. In addition, an area of approximately 1cm×100cm of the side rails of the beds was examined, and an area of approximately 10cm×10cm of the overbed tables was examined.
The gauze used for wiping was placed in a tube containing 3mL of nutrient broth. The tube was ultrasonicated (Sine Sonic 100; Ikemoto Rikagaku, Co., Tokyo, Japan) at 36kHz for 5min and swirled for 30s. Each sample was diluted 10-, 100-, and 1000-fold in nutrient broth; 0.5mL of each dilution and of an undiluted sample were plated on salt egg yolk agar plates (Nissui Pharmaceutical, Co., Tokyo, Japan). These salt egg yolk agar plates were incubated at 35°C for 48h. Yellow colonies on the plates with a pearl-ring formation in the surrounding medium were examined by Gram-staining and the coagulase test (for morphology) (Staphylo La Seiken; Denka Seiken, Co., Tokyo, Japan) and by an Api Staph (Analytab Products, Plain View, N.Y., USA) to determine whether they were S. aureus. Staphylo La Seiken is based on the agglutination method and is composed of a latex suspension coated with human fibrinogen and rabbit IgG.
The methicillin sensitivity of the cultured S. aureus was determined using MRSA screening agar containing 6μg/mL oxacillin (Nippon Becton Dickinson, Co., Tokyo, Japan). Positive growth of MRSA was confirmed on the screening agar. When 10 or more colony forming units (cfu) of S. aureus cultured on salt egg yolk agar were detected, 10 colonies were randomly selected, and their methicillin sensitivity was determined. The MRSA or methicillin-sensitive S. aureus (MSSA) count per gauze wipe was quantified from the ratio of methicillin-resistant to methicillin-sensitive colonies.14S. aureus 209P was used as quality control.
In Yamaguchi University Hospital, environmental surfaces in the rooms of patients from whom MRSA was isolated via clinical specimens are disinfected by nurses by wiping with an 80vol% ethanol solution once a day. The present environmental contamination survey was performed immediately before this routine wiping. Although the 100% polyester curtains (King Run, Kingrun Co., Ltd., Tokyo, Japan) are not routinely disinfected, we examined them within 7–14 days after they had been newly hung.
ResultsTable 1 shows the rates and levels of MRSA contamination of the environmental surfaces (overbed tables, bed side rails, and curtains) in the rooms of 24 patients in whom MRSA was detected. MRSA contamination was observed in 6 (25%) of 24 overbed table samples and 6 (31.6%) of 19 bed side rail samples but was not observed in 24 curtain samples. The mean MRSA counts (range)/100cm2 were 30.6 (0–255)cfu in the overbed table samples and 159.5 (0–1620)cfu in the bed side rail samples. The MRSA contamination rates were therefore high for the overbed tables and bed side rails but low for the curtains. In addition, the bed side rails sometimes showed a MRSA level of 103cfu/100cm2.
Methicillin-resistant Staphylococcus aureus (MRSA) contamination of the environmental surfaces in rooms of 24 inpatients with MRSA-positive body sites.
| Examined items (ca. 100cm2) | Contaminant rate (%) (no. of samples/no. of samples examined) | Mean±standard deviation (range) of the contamination level (cfu/examined item) |
|---|---|---|
| Overbed tables | 25.0 (6/24) | 30.6±65.6 (0–255) |
| Bed side railsa | 31.6 (6/19) | 159.5±396.4 (0–1620) |
| Curtains | 0 (0/24) | 0 |
MRSA can survive for a long period of time in the environment.14 When the hospital room environment is contaminated with MRSA, fingers also tend to become contaminated. MRSA contamination of the environment surrounding patients may play a major role in the transmission of MRSA infection. Therefore, we evaluated MRSA contamination in the environment surrounding patients from whom MRSA was isolated via clinical specimens. In this study, both the contamination rate and the contamination level were evaluated because the latter is also important in the development of infection. We determined that both the MRSA detection rates of the overbed table and bed side rail samples (25.0% and 31.6%, respectively) and the mean contamination levels of these areas (30.6 and 159.5cfu/100cm2, respectively) were high. In the U.K., the proposed criteria for the cleanliness of hospital environments include an indicator organism count of <1cfu/cm2.15 In our study, many bed side rail and overbed table samples showed a MRSA contamination level of >1cfu. Overbed tables and bed side rails surrounding patients from whom MRSA is detected from clinical specimens may frequently be contaminated with a high level of MRSA.
However, none of the curtain samples were contaminated with MRSA in this study. This result was consistent with that of another study that found no MRSA contamination in curtains.5 In contrast, the rate of MRSA contamination in curtains was reported to be 92% (12 of 13 samples) by Ohl et al., 15.5% (31 of 200 samples) by Klakus et al., and 28% (14 of 50 samples) by Trillis et al.16–18 The differences in the contamination rate may have been due to the MRSA detection method used and/or the curtain material; therefore, further studies are necessary to resolve the discrepancies in these results.
Based on the results of this study, disinfection of overbed tables and bed side rails surrounding patients from whom MRSA has been isolated is essential. When patients and medical staff touch such environmental surfaces, it is necessary to regard them as being contaminated and take appropriate countermeasures. However, at least until the 14th day of use, frequent disinfection of curtains may not be necessary. Measures such as cleaning curtains after a patient's discharge from the room may be adequate.
Conflicts of interestThe authors declare no conflicts of interest.