The principal objective of this study was to evaluate the kinetics of dihydroxyacetone production by Gluconobacter frateurii CGMCC 5397 under different oxygen volumetric mass transfer coefficient (kLa) conditions in submerged bioreactors using biodiesel-derived crude glycerol as the carbon source. kLa is a key fermentation parameter for the production of dihydroxyacetone. Cultivations were conducted in baffled- and unbaffled-flask cultures (the kLa values were 24.32h−1 and 52.05h−1, respectively) and fed-batch cultures (the kLa values were held at 18.21h−1, 46.03h−1, and 82.14h−1) to achieve high dihydroxyacetone concentration and productivity. The results showed that a high kLa could dramatically increase dihydroxyacetone concentrations and productivities. The baffled-flask culture (with a kLa of 52.05h−1) favored glycerol utilization and dihydroxyacetone production, and a dihydroxyacetone concentration as high as 131.16g/L was achieved. When the kLa was set to 82.14h−1 in the fed-batch culture, the dihydroxyacetone concentration, productivity and yield were 175.44g/L, 7.96g/L/h and 0.89g/g, respectively, all of which were significantly higher than those in previous studies and will benefit dihydroxyacetone industrial production.
maximum cell concentration (g/L)
maximum production (DHA) concentration (g/L)
fermentation time to reach the maximum production of DHA (h)
specific growth rate (h−1)
maximum specific growth rate (h−1)
DHA productivity (g/L/h)
Dihydroxyacetone (abbreviated as DHA) is a commercially important chemical used in the cosmetic industry as a self-tanning agent.1,2 DHA has also been proposed to be involved in weight augmentation and fat loss, antioxidant activity, and increasing endurance capacity,3 and it offers a safe and effective therapeutic option for recalcitrant vitiligo. Additionally, DHA is an important precursor for the synthesis of various fine chemicals and pharmaceuticals and serves as a versatile building block for the organic synthesis of a variety of fine chemicals.4
Crude glycerol, an important chemical, is abundantly available because it is the main byproduct of the conversion of vegetable oils into biodiesel. The progressive increase in biodiesel production has in turn caused a sudden increase in crude glycerol. Approximately 4.53kg of crude glycerol are created for every 45.3kg of biodiesel produced. The purification of crude glycerol to a chemically pure substance results in a valuable industrial chemical. However, purification is costly, and the glycerol market is already saturated. Thus, the price of crude glycerol continues to decline and directly affects biodiesel production costs.5 Therefore, it is essential to discover new applications for crude glycerol that will provide an ideal platform for chemical and pharmaceutical industries. Some value-added chemicals produced from crude glycerol have been developed, such as epichlorohydrin, DHA, 1,3-propanediol, propionic acid, polyhydroxyalkanoate,6 erythritol,7 citric acid,8 hydrogen,9 mannitol,10 lipids,11 mixed acids,12 and eicosapentaenoic acid.13
Since Bertrand first observed the production of DHA from glycerol by bacteria in 1898, various microorganisms used for DHA production have been reported.14Gluconobacter strains produce DHA via the incomplete oxidation of glycerol with membrane-bound glycerol dehydrogenases (GDHs)15 and are the most extensively used microorganisms for DHA production.16,17 During cell growth, oxygen demand is extremely high because Gluconobacter strains prefer a respiratory, rather than a fermentative, mode of growth.18 In addition, GDH also requires oxygen to accomplish the oxidative conversion of glycerol to DHA.19 Thus, the supply of oxygen to Gluconobacter strains is one of the most crucial factors for DHA production in an industrial process.
Various fermentation strategies have been previously reported to enhance oxygen supply during the DHA production process. Hydrogen peroxide and p-benzoquinone have been added to media as an oxygen source and an electron acceptor.20 Oxygen carriers such as hemoglobin, perfluorochemicals, and silicone oils have also been used to enhance oxygen supply in fermentation media.21 Moreover, some conventional techniques to improve the rate of oxygen transfer from the gas phase have been investigated, including increasing the agitation or aeration rate, raising the partial pressure of oxygen in the gas phase or modifying the bioreactor design.4
The effects of different oxygen supply conditions can be measured by the oxygen volumetric mass transfer coefficient (kLa). The oxygen volumetric mass transfer coefficient is generally considered to be a critical parameter in aerobic cultures of microorganisms due to its poor solubility and the need for a constant supply. The selection, design, and scale-up of biochemical reactors and the accurate estimation of mass transfer rates for different scales and different operational conditions are of critical importance in bioprocesses.22 As with any other transport phenomena occurring between different phases, the oxygen transfer rate is profoundly affected by hydrodynamic conditions in the bioreactor, which affect the mass transfer coefficient and the interfacial area. Therefore, variations in the configurations of bioreactors and the physicochemical properties of the culture medium, including density, diffusivity, rheological properties, and surface tension, can attenuate the oxygen transfer capacity, resulting in oxygen depletion.23
Previously, a newly isolated strain, Gluconobacter frateurii CGMCC 5397, was shown to utilize inexpensive biodiesel-derived crude glycerol to produce DHA.24 However, in our previous study, DHA concentration and productivity were not satisfactory. In this study, the effect of the oxygen transfer coefficient on DHA production from biodiesel-derived crude glycerol by Gluconobacter frateurii was investigated to enhance DHA concentration and productivity. Both baffled- and unbaffled-flask cultures were used in the fermentation process, and fed-batch cultures with three different kLa values were also compared.
Materials and methodsMicroorganismThe strain Gluconobacter frateurii CGMCC 5397, isolated from rotting fruits using a crude glycerol culture in our laboratory, was employed in this work, stored at 4°C on GY agar slants, and transferred monthly. The medium (GY) consisted of 25g/L glycerol, 5g/L yeast extract, and 20g/L agar.
Crude glycerolCrude glycerol used in this work was obtained from a palm oil-based biodiesel plant in Malaysia operated by Vance Bioenergy [composition: 80.5% (w/w) glycerol, 10.1% (w/w) water, 5.2% (w/w) sodium salts, 0.4% (w/w) potassium salts, 0.3% (w/w) other salts, 0.5% (w/w) methanol, and 2% (w/w) other organics (esters, free fatty acids, soaps, etc.)]. Crude glycerol was used as a carbon source in the culture medium without purification. The purity of the crude glycerol (80.5%, w/w) used in each experiment was taken into consideration when making the appropriate calculations; thus, the initial concentrations of glycerol quoted refer to pure glycerol.
Shake flask culturesA crude glycerol concentration gradient (50g/L, 75g/L, 100g/L, 125g/L and 150g/L) was used in the fermentation media of both the baffled and unbaffled flasks to compare the effects of kLa on glycerol oxidation, cell growth and DHA production. The other fermentation medium components included 24g/L corn steep liquor and 3g/L CaCO3. The seed medium contained 15g/L crude glycerol, 15g/L yeast extract, and 3g/L KH2PO4. The pH of the medium was adjusted to 6.0 with 2M NaOH, and the medium was heat sterilized (20min at 121°C). The seed medium was inoculated with a 2mL stock culture stored at −80°C and incubated at 30°C shaking at 200rpm for 16h. For the fermentation experiments, the medium was inoculated with 5% of the seed culture and incubated shaking at 200rpm at 30°C for 48h. The cells were grown in 250-mL flasks with a working volume of 50mL.
During the cultivation process, 1-mL samples were taken from each flask every 8h to determine the residual glycerol and biomass concentrations. At the end of the fermentation process, 1-ml samples were taken from the flask every 2h, and the residual glycerol concentration was determined. When glycerol was consumed to less than 5g/L in the flasks (except for some unbaffled-flask cultures that were ended earlier because the glycerol could not be exhausted to less than 5g/L due to culture limitations), the DHA concentration and yield were analyzed.
Fed-batch culturesFed-batch cultures were performed in a 5-L bioreactor (Baoxing Bio-Engineering Equipment Co., Ltd, China) with 3L of medium or in a 30-L NBS bioreactor (BioFlo 4500, New Brunswick Scientific, Edison, NJ, USA) with 20L of medium. The fermentation was performed in the same medium as that of the flask cultures except that the initial crude glycerol concentration was 60g/L. The inoculum volume was 5% (v/v) of the initial fermentation medium. A sterile glycerol solution (60%, v/v) was fed with a peristaltic pump. A continuous feedback control strategy was employed in the fed-batch cultures, in which the feeding rate was adjusted every 2h to maintain the residual crude glycerol concentration within 5–25g/L.
During the fermentation process, the pH was automatically maintained at 6.0 with NaOH (2M). The cultivation temperature was kept at 30°C. DO was monitored using a polarographic electrode and was expressed as a percentage of O2 saturation. The foam was controlled automatically by the addition of polyethylene glycol as an antifoaming agent. Samples were withdrawn from the bioreactor every four hours to measure DHA, glycerol and biomass contents. A 50-mL sample was taken every 8h to determine dry cell weight.
The agitation and air-flow rates were set at as follows to achieve different kLa values: 18.21h−1 (300rpm and 0.12m3/h, respectively, in the 5-L bioreactor), 46.03h−1 (450rpm and 0.21m3/h, respectively, in the 5-L bioreactor), and 82.14h−1 (500rpm and 2m3/h, respectively, in the 30-L bioreactor).
Analytical methodsAfter the desired incubation period, the culture was diluted with HCL (0.2M), and the cell concentration (OD560) was determined by measuring the absorbance at 560nm using a spectrophotometer (U-752). The samples were centrifuged at 8000g for 5min, washed twice, and dried at 80°C to a constant weight. The following relation resulted after calibration: dry cell weight [DCW, g/L]=0.3×OD560.
DHA and glycerol were analyzed using high-performance liquid chromatography (HPLC). Fermentation samples were centrifuged (8000×g, 10min) at 4°C, and the concentrations of DHA and glycerol in the supernatants were quantified using an HPLC system (Waters, Milford, MA) equipped with a refractive index detector, a UV detector (at 270nm), and an Aminex HPX-87H column (300mm×7.8mm, 9μm; Bio-Rad Chemical Division, Richmond, CA). The mobile phase was an 8-mmol/L H2SO4 solution at a flow rate of 0.5mL/min, and the column was operated at 55°C.
DHA yield was defined as the amount of DHA produced from each gram of glycerol and was normalized in accordance with the dilution factor of the base or the crude glycerol solution.
Measurement of the oxygen transfer coefficient (kLa), μmax, and Qp,The sulfite method (Na2SO3 method) was used for estimating the oxygen transfer coefficient, kLa, in the bioreactors.25
The maximum specific growth rate (μmax) was determined by performing exponential regressions on the data points from the exponential phase. To avoid the decrease of volume in the flask cultures, the μmax determination experiments were conducted independently, and 1-mL samples were taken from each flask once an hour during the exponential growth phase.
DHA productivity (Qp) was expressed as DHA concentration per hour.
All the above measurements, including dry cell weight (DCW), glycerol concentration, DHA concentration and kLa, were conducted in triplicate, and the average values with standard deviations were recorded.
ResultsEffects of kLa on shake-flask culturesMany researchers have demonstrated that baffles in flasks enhance agitation and increase the available surface area for oxygen transfer at the air–liquid interface26; thus, baffled flasks have a higher kLa than unbaffled flasks under identical shaking speeds.
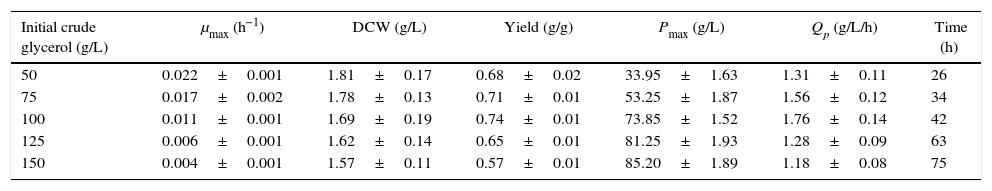
In this work, the kLa values in the unbaffled and baffled flasks were 24.32h−1 and 52.05h−1, respectively. The difference in these kLa values led to differing performances in crude glycerol oxidation, biomass production and DHA accumulation (Tables 1 and 2). In the unbaffled-flask cultures, the final DHA concentration increased significantly as the initial glycerol concentration increased from 50g/L to 100g/L. However, the DHA concentration increased little when the glycerol concentration was over 100g/L, as was previously reported19,24,27; the initially high glycerol concentration exerts an inhibitory effect on DHA production. In our previous work, we have shown that some poisonous coproducts (methanol, chloride salts, metal ion, etc.) in the biodiesel-derived crude glycerol affect DHA production. In the baffled-flask cultures, however, varying initial concentrations of glycerol were all efficiently exhausted to below 5g/L after a relatively short fermentation time, and the incubation times of the unbaffled-flask cultures were 2- to 3-fold longer than those of the baffled-flask cultures with identical glycerol concentrations. As the initial glycerol concentrations increased, the biomass exhibited a continuous decline, and the highest biomass levels (unbaffled-flask cultures: 1.81±0.17g/L; baffled-flask cultures: 1.87±0.10g/L) were obtained when the glycerol concentration was 50g/L.
The effects of different initial carbon concentrations on cell growth and DHA production in unbaffled flasks (kLa, 24.32h−1).
| Initial crude glycerol (g/L) | μmax (h−1) | DCW (g/L) | Yield (g/g) | Pmax (g/L) | Qp (g/L/h) | Time (h) |
|---|---|---|---|---|---|---|
| 50 | 0.022±0.001 | 1.81±0.17 | 0.68±0.02 | 33.95±1.63 | 1.31±0.11 | 26 |
| 75 | 0.017±0.002 | 1.78±0.13 | 0.71±0.01 | 53.25±1.87 | 1.56±0.12 | 34 |
| 100 | 0.011±0.001 | 1.69±0.19 | 0.74±0.01 | 73.85±1.52 | 1.76±0.14 | 42 |
| 125 | 0.006±0.001 | 1.62±0.14 | 0.65±0.01 | 81.25±1.93 | 1.28±0.09 | 63 |
| 150 | 0.004±0.001 | 1.57±0.11 | 0.57±0.01 | 85.20±1.89 | 1.18±0.08 | 75 |
Cells were grown in 250-mL unbaffled flasks for 26–75h with initial crude glycerol concentrations from 50–150g/L, and the corn steep liquor concentration was 24g/L. The data are the average values of triplicate measurements with standard deviations.
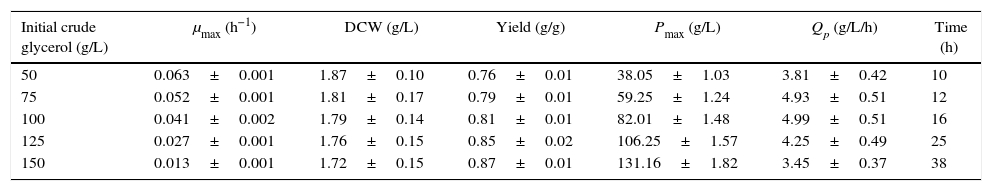
The effects of different initial carbon concentrations on cell growth and DHA production in baffled flasks (kLa, 52.05h−1).
| Initial crude glycerol (g/L) | μmax (h−1) | DCW (g/L) | Yield (g/g) | Pmax (g/L) | Qp (g/L/h) | Time (h) |
|---|---|---|---|---|---|---|
| 50 | 0.063±0.001 | 1.87±0.10 | 0.76±0.01 | 38.05±1.03 | 3.81±0.42 | 10 |
| 75 | 0.052±0.001 | 1.81±0.17 | 0.79±0.01 | 59.25±1.24 | 4.93±0.51 | 12 |
| 100 | 0.041±0.002 | 1.79±0.14 | 0.81±0.01 | 82.01±1.48 | 4.99±0.51 | 16 |
| 125 | 0.027±0.001 | 1.76±0.15 | 0.85±0.02 | 106.25±1.57 | 4.25±0.49 | 25 |
| 150 | 0.013±0.001 | 1.72±0.15 | 0.87±0.01 | 131.16±1.82 | 3.45±0.37 | 38 |
Cells were grown in 250-ml baffled flasks for 10–38h with initial crude glycerol concentrations from 50 to 150g/L, and the corn steep liquor concentration was 24g/L. The data are the average values of triplicate measurements with standard deviations.
In the baffled-flask cultures, the yield increased almost linearly from 0.76g/g to 0.87g/g as the initial glycerol concentrations increased from 50g/L to 150g/L. In contrast, in the unbaffled-flask cultures, the yield increased when the glycerol concentration was lower than 100g/L and then decreased with increasing glycerol concentration; compared with the baffled-flask cultures, the yield was lower under the same conditions. The DHA productivity of the baffled-flask cultures was approximately 3-fold higher than that of the unbaffled-flask cultures with identical glycerol concentrations, which indicates that oxygen was one of the most crucial factors for DHA production, as expected.18,19
For the baffled flask with an initial glycerol concentration of 150g/L, the DHA concentration achieved was as high as 131.16g/L, which was slightly higher than that achieved by Liu et al.24 (125.8g/L in a fed-batch fermentation with a 5-L bioreactor).
Therefore, observing that a high kLa (52.05h−1) in the baffled-flask culture favored glycerol utilization and DHA production by Gluconobacter frateurii CGMCC 5397, we were further inspired to investigate the kLa parameter in larger-scale bioreactors.
Effects of kLa on fed-batch culturesGluconobacter frateurii CGMCC 5397 fermentations were performed at larger scales.
The above results show that substrate inhibition clearly limits DHA production in flask cultures. Fed-batch fermentations with biodiesel-derived crude glycerol at different kLa values were investigated to enhance the production of DHA. Moreover, as reported in our previous work, cell growth and DHA production were not influenced by biodiesel-derived crude glycerol versus pure glycerol fermentation in fed-batch cultures. Therefore, the initial glycerol concentration in the fed-batch cultures was set at 60g/L to prevent inhibition from occurring in bioreactors after the evaluation of various kLa values. The glycerol concentration was controlled at 5–25g/L.
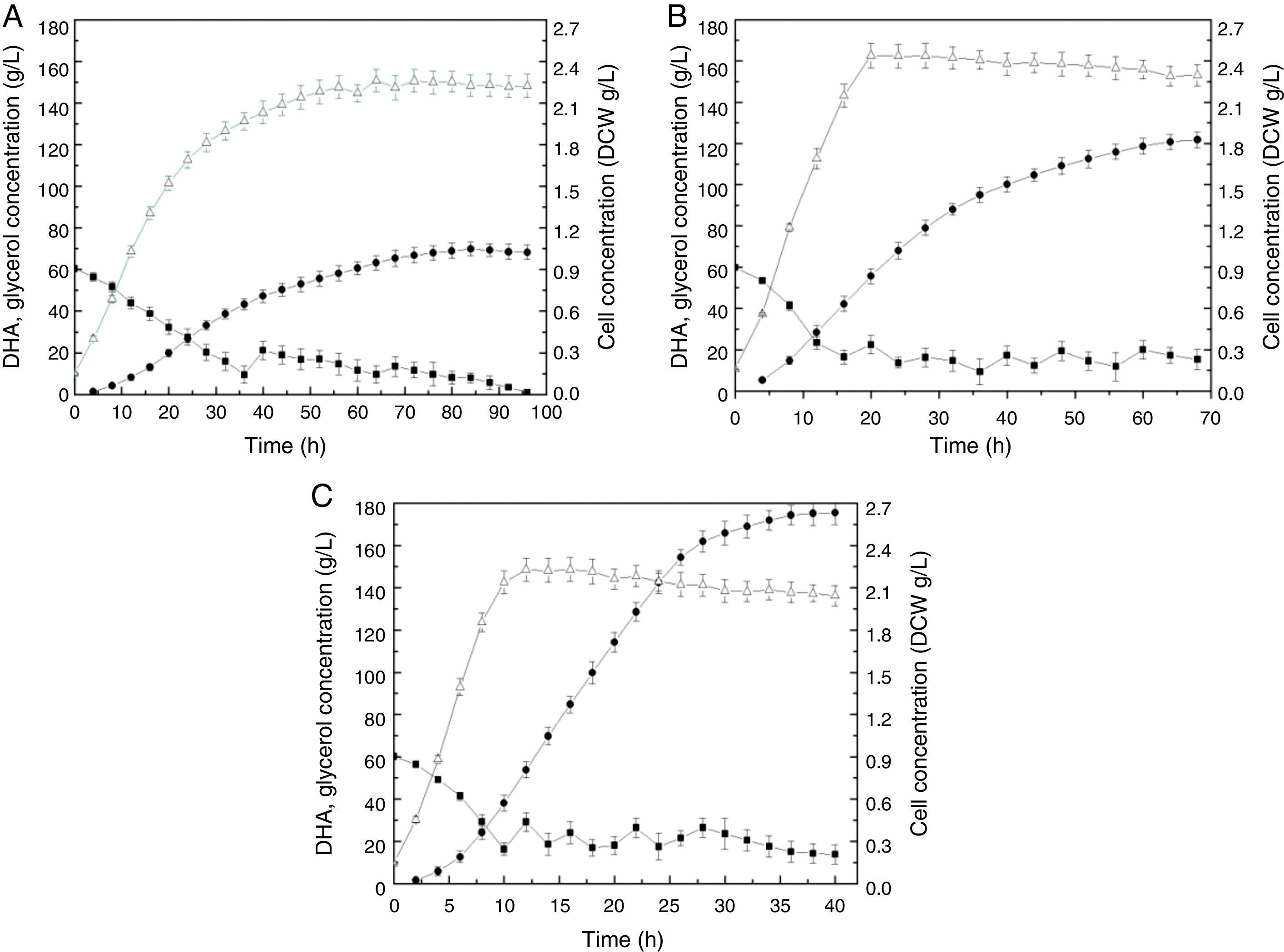
The time courses for cell growth, glycerol consumption, and DHA production by Gluconobacter frateurii CGMCC 5397 at three kLa values (18.21h−1, 46.03h−1, and 82.14h−1) are depicted in Fig. 1. In the culture with the lowest kLa, 18.21h−1 (Fig. 1A), the rate of glycerol consumption was so low that the fermentation was sustained for 100h, presumably due to the weak metabolic activity of the cells enduring limited oxygen transfer. Consequently, the production of DHA was only 68.27g/L at the end of the fermentation. More glycerol was used as a carbon source for cell growth than for DHA biosynthesis, resulting in low DHA production. At the end of the fermentation, DHA production declined, which indicates that DHA can be used for supplying energy, and the DHA concentration decreased when glycerol in the medium was exhausted.28
Time course of DHA production by Gluconobacter frateurii CGMCC 5397 at different kLa values: (A) 18.21h−1, (B) 46.03h−1 and (C) 82.14h−1. Glycerol, filled squares; DHA, filled circles; DCW, unfilled triangles. The experiments consisted of fed-batch cultures fed crude glycerol in triplicate. The data points are the means of the triplicate measurements.
When the kLa was increased from 18.21h−1 to 46.03h−1 (Fig. 1B), DHA production improved approximately two-fold, which indicates that substrate assimilation was obviously active. The glycerol feed started at 16h, which was earlier than in the low-kLa fermentation (36h), and was 8.13g/L. At 70h, 121.69-g/L DHA production was achieved.
When the kLa was elevated to 82.14h−1 (Fig. 1C), the final DHA concentration, productivity and yield reached 175.44g/L, 7.96g/L/h and 0.89g/g, respectively, the values of which were significantly greater than those above, and no inhibitory effects were observed. The glycerol feed started at 10h, and cultivation required only 40h. The DHA concentrations reported in this study are higher than those reported previously within the same fermentation timeframes.18,19
DiscussionKinetic analysis of DHA fermentation at different kLa valuesThe above results (Tables 1 and 2) show that glycerol assimilation and cell growth were inhibited by high concentrations of glycerol, and the μmax decreased with increasing glycerol concentrations. However, compared with the unbaffled-flask cultures, the μmax from the baffled-flask cultures increased significantly, and substrate inhibition was substantially relieved under the same fermentation conditions. The baffles in the flasks enhance agitation and increase the available surface area for oxygen transfer at the air–liquid interface; thus, the baffled flasks have a higher kLa than unbaffled flasks at identical shaking speeds. In the baffled flasks (Table 2), the substrate was exhausted faster than in the unbaffled flasks. Therefore, the results obtained in this study demonstrate that the reason that substrate inhibition appeared in the unbaffled-flask cultures was not only due to high concentrations of glycerol itself but was much more likely caused by the limited oxygen availability that resulted from a low kLa (24.32h−1).
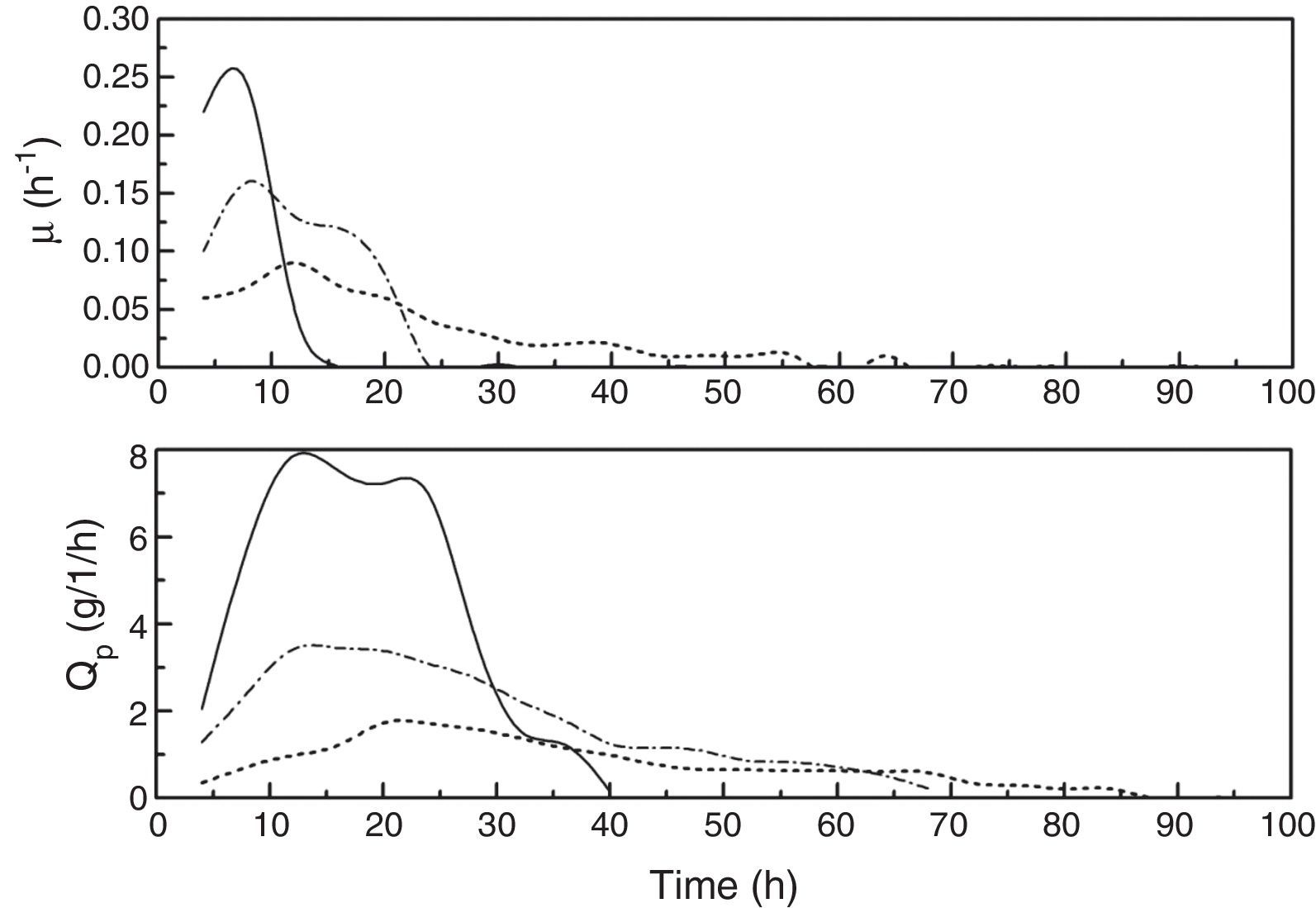
To analyze the kinetic characteristics of the fed-batch fermentation at different kLa values, two kinetic parameters, μ and Qp (DHA productivity), were calculated based on the data in Fig. 1. As shown in Fig. 2, compared with kLa values of 18.21h−1 and 46.03h−1, μ was higher with a kLa of 82.14h−1 early in the DHA fermentation (before 12h) and then fell rapidly later in the fermentation (after 12h). The maximum amounts of biomass produced at the different fermentation kLa values (18.21h−1, 46.03h−1 and 82.14h−1) were 2.28g/L, 2.44g/L, and 2.23g/L, respectively, which indicates that the cells grew best at a kLa value of 46.03h−1. When the fermentation kLa was less than 18.21h−1, the dissolved oxygen was so low that it limited cell growth. However, when the kLa exceeded 82.14h−1, the agitation rate was too high, and the cells were exposed to high shear rates, which caused cell deformation and damage. In addition, high oxygen levels might cause cellular damage as well.21
A comparison of the Qp values in all the experiments indicates that the Qp increased with increasing kLa. However, late in the high-kLa fermentation (after 40h), the synthesis of DHA ceased. Previous studies have shown that high DHA concentrations can inhibit DHA production.29 When the DHA concentration in the culture medium reached 108g/L, glycerol conversion ceased and no more DHA was produced. It was also reported that high DHA concentrations might cause irreversible cell damage.29,30 However, in our work, no inhibitory effects were observed when the DHA concentration was lower than 150g/L, and the maximum DHA concentration reached was 175.44g/L.
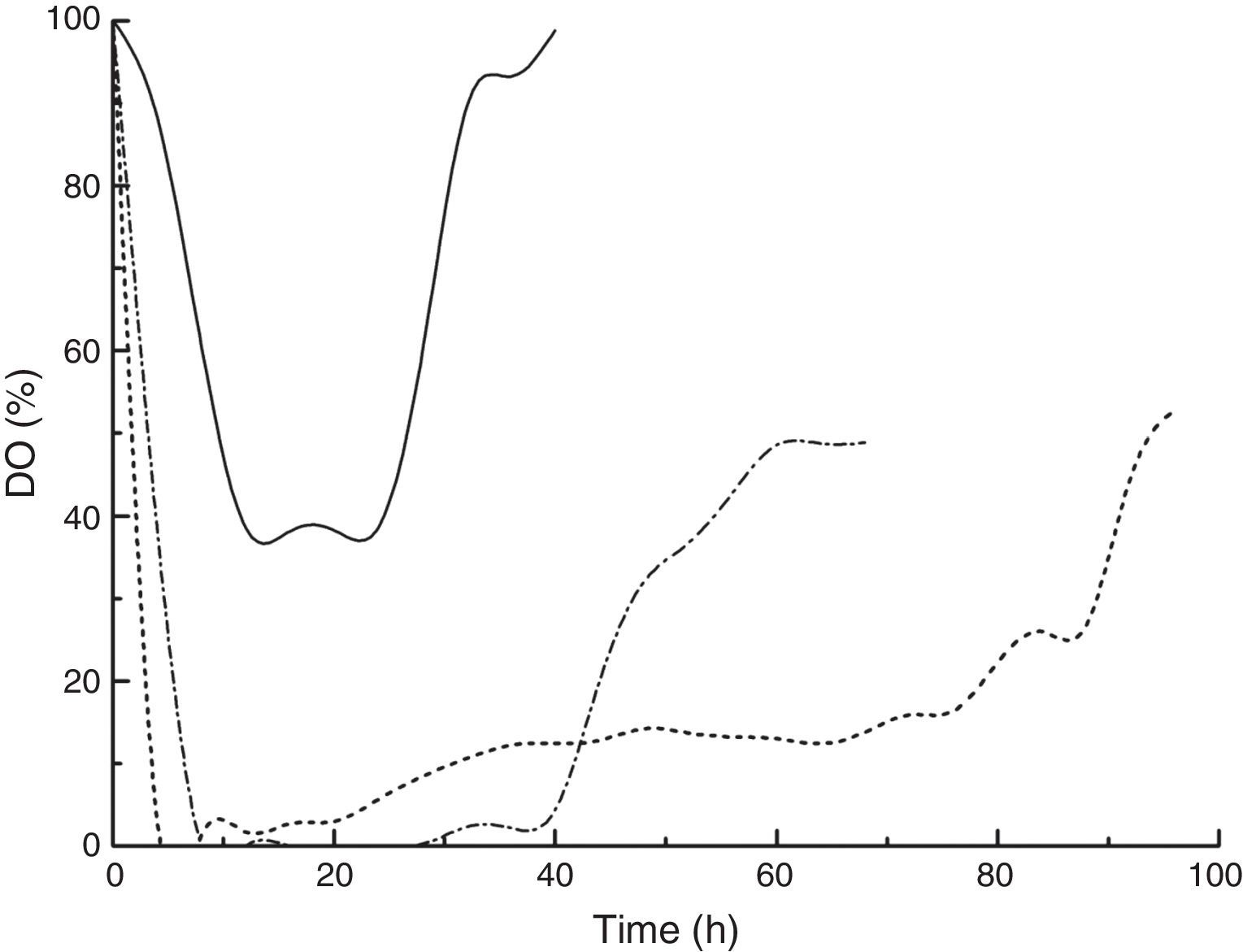
A consistently high kLa during DHA production led to high dissolved oxygen (DO) concentrations (Fig. 3). For cultures with kLa values of 18.21 and 46.03h−1, the DO decreased quickly, reaching zero at 4 and 8h of cultivation, respectively. The dissolved oxygen was unable to meet cell depletion, and DHA synthesis was insufficient. When the kLa was elevated to 82.14h−1, the DO decreased in the first 12h and stabilized at approximately 40%, which was suitable for DHA production.31 The effects of DO concentration on DHA production by Gluconobacter strains and the application of a DO control strategy to DHA production have been studied by several research groups.22,23,31 Flickinger and Perlman found that DO concentration played a vital role in the microbial production of DHA from glycerol. However, DO control is impractical in industrialization because DO varies with agitation speed, cell concentration, carbon source feeding rate and concentration, bioreactor size, etc. Alternatively, kLa control can be considered a promising strategy for improving the supply and transfer of oxygen in vivo because it has been correlated with the combination of stirrer speed, superficial gas velocity and liquid effective viscosity, which are measurable scale-up parameters.32
The biosynthesis of DHA in Gluconobacter cells is complicated, and there are three possible pathways for DHA production. The enzyme responsible for the oxidative reaction is a membrane-bound, pyrroloquinoline quinine (PQQ)-dependent glycerol dehydrogenase (GDH) that makes use of oxygen as the final electron acceptor without NADH mediation.4,19 In this study, it was found that consistently high kLa values were optimal for DHA production (Table 3). When the fermentation kLa was less than 18.21h−1, the DHA yield was lower than reported in other experiments, which indicates that a greater proportion of carbon resources (glycerol) was used for cell growth and metabolic demand. In our experiments, glycerol was used not only as a substrate but also as carbon source for cellular processes and metabolic demand. When the fermentation kLa was less than 82.14h−1, the proportion of carbon resources (glycerol) used for DHA biosynthesis increased, resulting in a higher DHA yield at the end of the fermentation.
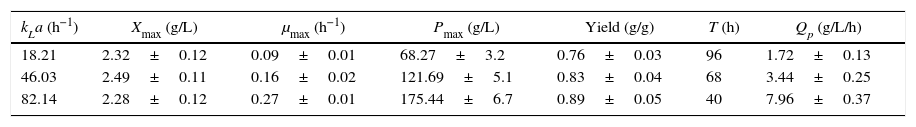
Effects of kLa values on DHA fermentation.
| kLa (h−1) | Xmax (g/L) | μmax (h−1) | Pmax (g/L) | Yield (g/g) | T (h) | Qp (g/L/h) |
|---|---|---|---|---|---|---|
| 18.21 | 2.32±0.12 | 0.09±0.01 | 68.27±3.2 | 0.76±0.03 | 96 | 1.72±0.13 |
| 46.03 | 2.49±0.11 | 0.16±0.02 | 121.69±5.1 | 0.83±0.04 | 68 | 3.44±0.25 |
| 82.14 | 2.28±0.12 | 0.27±0.01 | 175.44±6.7 | 0.89±0.05 | 40 | 7.96±0.37 |
Cells were grown in a bioreactor with 60g/L initial crude glycerol and 24g/L corn steep liquor, and the glycerol concentration was controlled at 5–25g/L. The data are the average values of triplicate measurements with standard deviations.
In conclusion, the results from both the batch and fed-batch cultures at different scales clearly indicate that high kLa values (52.05h−1 for baffled flasks and 82.14h−1 for a 30-L bioreactor) effectively improved DHA concentration, productivity and yield. As kLa is a scale-up parameter and crude glycerol is a relatively inexpensive raw material, the method developed in this paper can be scaled up to industrial processes and applied to other industrial biotechnological processes to achieve both high product concentrations and high productivities.
Conflicts of interestThe authors declare no conflicts of interest.
This research was supported by grants from the Key Project of Science and Technology of Henan Province (No. 132102210387), the Key Project of the Henan Educational Committee (No. 14B180016), and the Foundation for University Young Backbone Teachers of Henan Province (No. 2014GGJS-157).