Copper mine drainages are restricted environments that have been overlooked as sources of new biocatalysts for bioremediation and organic syntheses. Therefore, this study aimed to determine the enzymatic activities (esterase, epoxide hydrolase and monooxygenase) of 56 heterotrophic bacteria isolated from a neutral copper mine drainage (Sossego Mine, Canaã dos Carajás, Brazil). Hydrolase and monooxygenase activities were detected in 75% and 20% of the evaluated bacteria, respectively. Bacterial strains with good oxidative performance were also evaluated for biotransformation of organic sulfides. Fourteen strains with good enzymatic activity were identified by 16S rRNA gene sequencing, revealing the presence of three genera: Bacillus, Pseudomonas and Stenotrophomonas. The bacterial strains B. megaterium (SO5-4 and SO6-2) and Pseudomonas sp. (SO5-9) efficiently oxidized three different organic sulfides to their corresponding sulfoxides. In conclusion, this study revealed that neutral copper mine drainages are a promising source of biocatalysts for ester hydrolysis and sulfide oxidation/bioremediation. Furthermore, this is a novel biotechnological overview of the heterotrophic bacteria from a copper mine drainage, and this report may support further microbiological monitoring of this type of mine environment.
Brazil is the world's 15th largest producer of copper concentrate.1 The mining company Vale S.A. is responsible for the majority of copper production, having reached 275,000mt of copper concentrate in the first nine months of 2013,2 and operates at the Sossego Mine (Canaã dos Carajás, PA, since 2004) and at the Salobo Mine (Marabá, PA, since 2012). The process used for copper ore concentration involves crushing and subsequent semi-autogenous grinding (SAG), followed by comminution in a ball mill. The material is classified according to the particle size and then passes through a flotation process. The generated waste (a slurry) is deposited in a dam approximately 5200m in length.
The aqueous wastes generated from mining activities are known as mine drainages and are described as restricted environments that often have a high metal content and low organic matter concentration. In addition, water drainage from mine wastes and abandoned mines are often acidic as a result of the extended exposure of sulfidic minerals to water and oxygen. However, the pH of copper mine drainage may drastically differ according to the waste chemical composition, the time of exposure and the microbial community.3
Most microbial studies focus on the mineral-oxidizing prokaryotes from acid mine drainages (AMD), which usually consist of acidophilic bacteria and archaea. These microorganisms are able to oxidize ferrous iron and/or reduced forms of sulfur and accelerate the oxidative dissolution of sulfidic minerals, which may be applied to metal ore processing and concentrates in a biotechnological process known as biomining and bioleaching.4–6
However, the microbial community from copper mine drainages (acidic or neutral) has been overlooked as a source of new biocatalysts for bioremediation and the synthesis of organic compounds. To the best of our knowledge, there are no reports about the use of heterotrophic microorganisms isolated from these restricted environments as biocatalysts applied for bioremediation of organic pollutants or for synthetic organic chemistry.
From a biotechnological point of view, esterases, epoxide hydrolases and monooxygenases are some of the best-studied and most-applied enzymes. These biocatalysts are commonly used for the enantioselective production of value-added compounds, which are important to pharmaceutical and fine chemical industries. In this context, our research group has been working on the detection of enzymatic activity using fluorogenic probes and organic substrates of interest. Our focus has been on new biocatalysts from the Amazon and Atlantic rainforest,7 petroleum oil and formation water,8 human skin,9 and from Brazilian Culture Collections.10,11
The present work aimed to investigate hydrolases and monooxygenases in heterotrophic bacteria isolated from a neutral copper mine drainage, applying fluorescence-based high-throughput screening (HTS) assays and multibioreactions to monitor organic sulfide oxidations. Therefore, this study represents a new biotechnological application of poorly investigated, heterotrophic microbiota.
Materials and methodsGeneral methodsReagents were purchased from Sigma–Aldrich (Steinheim, Germany). Solvents were distilled from technical solvents. Fluorogenic probes and products for HTS assays (2–16) were synthesized by our group.12 A free sample of 2-methyl-4-propyl-1,3-oxathiane (19) from Givaudan (Jaguaré, SP, Brazil) was provided by Natura perfumery and cosmetic industry (Cajamar, SP, Brazil). Ethyl phenyl sulfide (18) and oxidation products of substrates 18, 19 and 20 (sulfoxides 18a, 19a and 20a, respectively) were synthesized as described by Porto et al. (2002).13 All chemical reactions were monitored by silica gel TLC (aluminum foil, 60 F254 Merck), and visualization was obtained using UV or by spraying with p-anisaldehyde/sulfuric acid followed by heating at approximately 120°C. Flash column chromatography was performed using Merck (Whitehouse Station, NJ, USA) silica gel 60 (0.04–0.063mm, 230–400 mesh). NMR spectra were recorded on a Varian Inova 500 (Palo Alto, CA, USA) for 1H (499.88MHz) and 13C (125.69MHz) measurements. Chemical shifts (δ) are given in ppm, and coupling constants (J) are given in Hertz.
Enzymatic reactions were monitored by GC–MS using an Agilent 7890 gas chromatograph (Santa Clara, CA, USA) coupled with a Hewlett Packard 5975C-MSD (70eV) spectrometer equipped with a fused silica capillary column (HP-5MS, 30m×0.25mm i.d.×0.25μm film thickness). GC–MS analyses were conducted using a 1mLmin−1 He flow, split mode (20:1) and the following temperature program: initial temperature 50°C, increasing at 10°Cmin−1 to 200°C and at 20°Cmin−1 to 300°C, remaining constant for 5min. Enantiomer discrimination was performed on an Agilent 6850 gas chromatograph (Santa Clara, CA, USA) coupled with a flame ionization detector (GC-FID) using a fused silica capillary column Lipodex-E (Macherey-Nagel Inc., Bethlehem, PA, USA) with chiral phase Octakis-(2,6-di-O-pentyl-3-O-butyryl)-γ-cyclodextrin (28m×0.25mm i.d.×0.25μm film thickness). The GC-FID analyses were conducted using a 1mLmin−1 H2 flow, split mode (10:1) and the following temperature program: initial temperature 50°C, increasing at 10°Cmin−1 to 100°C and at 5°Cmin−1 to 180°C, remaining constant for 5min.
Sample collection and isolation of heterotrophic bacteriaSamples from the Sossego copper mine drainage, Canaã dos Carajás, State of Pará, Brazil, were collected in August 2009. The samples were named SO5, SO6 and SO7; collected in sterile disposable flasks; and stored at 4°C. The Sossego mining operation began in 2004. Thus, these are 5-year-old copper mine drainage samples.
The bacteria were isolated according to Eaton and Franson (2005).14 Each sample (1g) was homogenized with sterile water (9mL) and serially diluted. One milliliter of each dilution was transferred to a Petri dish and homogenized with 12mL of Plate Count Agar (PCA). The plates were incubated for 48h at 35°C. Colonies exhibiting a different morphology and color were re-isolated in trypticase soy agar (TSA). The bacteria were stored at −70°C in LB medium containing glycerol.15
Fluorescence-based high-throughput screening assaysBacteria were inoculated into LB solid medium15 and incubated for 16h at 37°C. After growth, cell suspensions (0.2mgmL−1) were prepared in borate buffer (20mmolL−1 pH 7.4).
Assays were performed in flat-bottom polypropylene 96-well microtiter plates, which were incubated at 28°C and 200rpm. Fluorescence intensities were measured using a plate reader spectrophotometer (FlashScan 530 Analytic Jena) at 460nm (excitation wavelength: 390nm).
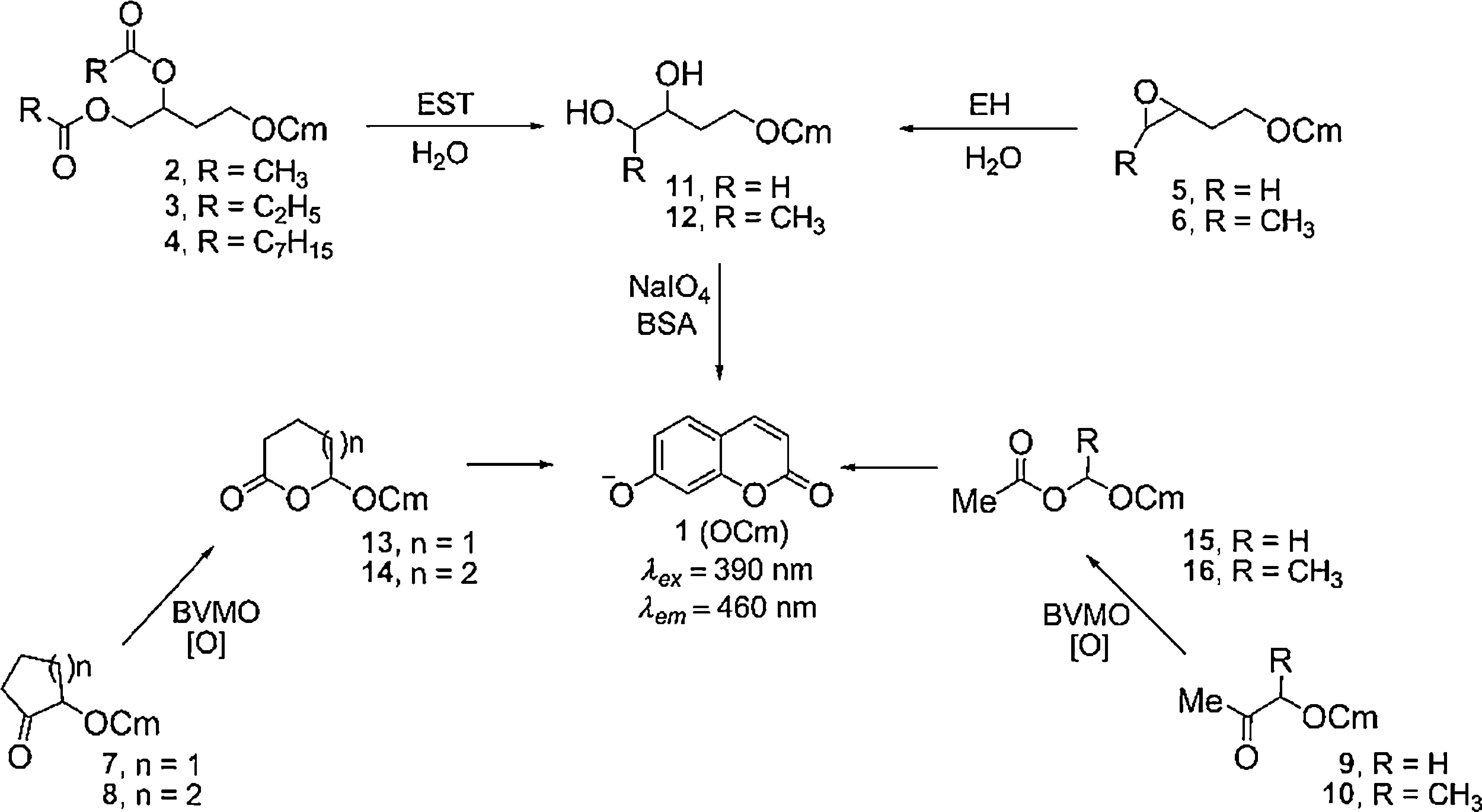
Hydrolase screening was performed using fluorogenic probes 2–6 (Scheme 1, 100μmolL−1), NaIO4 (2mmolL−1), BSA (2mgmL−1) and bacterial cell suspension (0.1mgmL−1) and was monitored up to 24h. Monooxygenases were investigated using probes 7–10 (Scheme 1, 100μmolL−1), BSA (2mgmL−1) and bacterial cell suspension (0.1mgmL−1) and were monitored up to 72h. All enzymatic reactions were followed by positive controls, including hydrolyzed or oxidized fluorogenic products 11–16 instead of probes, and using negative controls developed in the absence of bacterial cells.
Substrate conversion (%) into product was calculated by comparing the fluorescence intensities of the reaction assay and positive control, considering the latter as 100%. Negative controls were used to monitor spontaneous probe hydrolysis or oxidation. Enzymatic activity was considered positive for conversions greater than 5%.
Multibioreaction assaysBacteria were grown in LB liquid medium for 16h at 37°C and 200rpm. Cells were recovered by centrifugation at 4500×g for 15min. Wet bacterial cells (0.25g) were mixed with Sørensen buffer16 (5mL, 100mmolL−1, pH 7.0) containing 1.5μL of each substrate (18–21) in Erlenmeyer flasks (25mL). The resulting solutions were incubated at 37°C and 200rpm. After 72 and 120h, the reactions were interrupted by saturation with NaCl and extraction with ethyl acetate (2×2mL). The organic phases were combined, dried over anhydrous MgSO4, derivatized with diazomethane, and an internal standard was added (benzophenone; 0.05mgmL−1). All samples were analyzed by GC–MS and chiral GC-FID. Substrate stability and volatility under reaction conditions were monitored by reaction controls in the absence of bacterial cells.
Substrate conversions (%) were calculated by comparing the chromatographic peak areas of the reactants, products and internal standards.
Identification of bacteriaThe bacterial strains displaying hydrolytic activity above 99% or monooxygenases capable of oxidizing organic sulfur compounds were identified by sequencing of 16S rRNA. Genomic DNA was isolated using the Wizard Genomic DNA Purification Kit (Promega), following the manufacturer's instructions. PCR amplification and sequencing of partial 16S rRNA genes were conducted as described by Miqueletto et al. (2011).17 The sequences obtained were compared with sequences available at GenBank (http://www.ncbi.nlm.nih.gov) and the Ribosomal Database Project (http://www.cme.msu.edu/RDP/html/index.html). Multiple alignments of 16S rRNA gene sequences were performed using ClustalW 1.6,18 and evolutionary analyses were conducted in the Molecular Evolutionary Genetics Analysis (MEGA) 6.06 package,19 using the DNA substitution model Kimura 2-parameter.20 A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter=0.5288)).21 The phylogenetic reconstruction was performed using the Maximum Likelihood method with 1000 bootstrapped replicates.
ResultsEnzymatic activity detected by HTSThe 56 heterotrophic bacteria isolated from the three samples of a neutral copper mine drainage were evaluated by fluorescence-based HTS screening using probes 2–10 (Scheme 1).
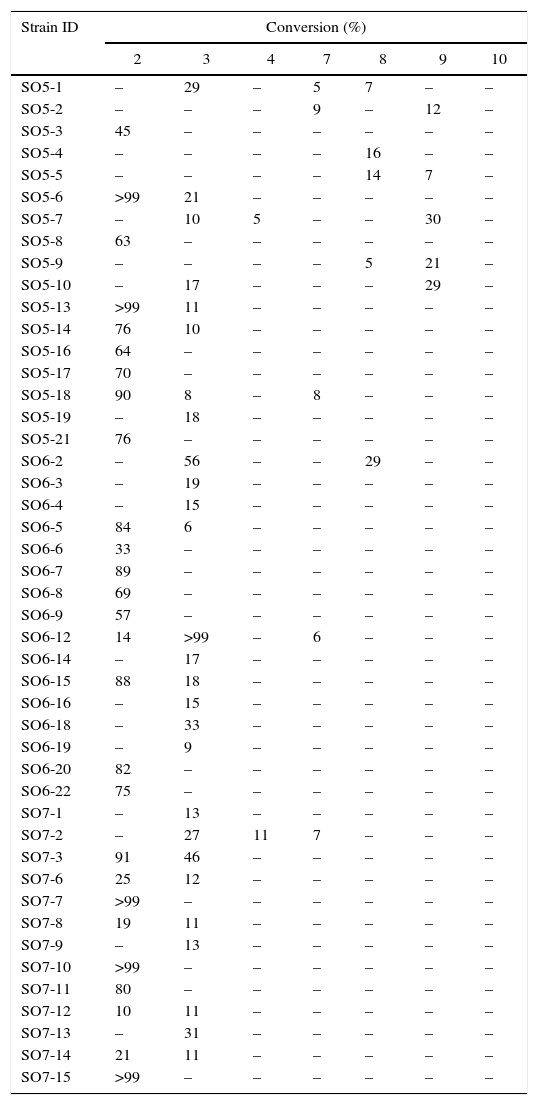
Assays performed in the presence of probes 2 and 3 (Table 1), which were used to detect esterases catalyzing the hydrolysis of short chain esters (acetate and propionate, respectively), indicated that 27 bacteria were able to hydrolyze probe 2, five of which converted more than 99% of the substrate (SO5-6, SO5-13, SO7-7, SO7-10 and SO7-15). Probe 3 was also hydrolyzed by 27 bacteria, and SO6-12 displayed outstanding performance, converting more than 99% of the substrate.
| Strain ID | Conversion (%) | ||||||
|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 7 | 8 | 9 | 10 | |
| SO5-1 | – | 29 | – | 5 | 7 | – | – |
| SO5-2 | – | – | – | 9 | – | 12 | – |
| SO5-3 | 45 | – | – | – | – | – | – |
| SO5-4 | – | – | – | – | 16 | – | – |
| SO5-5 | – | – | – | – | 14 | 7 | – |
| SO5-6 | >99 | 21 | – | – | – | – | – |
| SO5-7 | – | 10 | 5 | – | – | 30 | – |
| SO5-8 | 63 | – | – | – | – | – | – |
| SO5-9 | – | – | – | – | 5 | 21 | – |
| SO5-10 | – | 17 | – | – | – | 29 | – |
| SO5-13 | >99 | 11 | – | – | – | – | – |
| SO5-14 | 76 | 10 | – | – | – | – | – |
| SO5-16 | 64 | – | – | – | – | – | – |
| SO5-17 | 70 | – | – | – | – | – | – |
| SO5-18 | 90 | 8 | – | 8 | – | – | – |
| SO5-19 | – | 18 | – | – | – | – | – |
| SO5-21 | 76 | – | – | – | – | – | – |
| SO6-2 | – | 56 | – | – | 29 | – | – |
| SO6-3 | – | 19 | – | – | – | – | – |
| SO6-4 | – | 15 | – | – | – | – | – |
| SO6-5 | 84 | 6 | – | – | – | – | – |
| SO6-6 | 33 | – | – | – | – | – | – |
| SO6-7 | 89 | – | – | – | – | – | – |
| SO6-8 | 69 | – | – | – | – | – | – |
| SO6-9 | 57 | – | – | – | – | – | – |
| SO6-12 | 14 | >99 | – | 6 | – | – | – |
| SO6-14 | – | 17 | – | – | – | – | – |
| SO6-15 | 88 | 18 | – | – | – | – | – |
| SO6-16 | – | 15 | – | – | – | – | – |
| SO6-18 | – | 33 | – | – | – | – | – |
| SO6-19 | – | 9 | – | – | – | – | – |
| SO6-20 | 82 | – | – | – | – | – | – |
| SO6-22 | 75 | – | – | – | – | – | – |
| SO7-1 | – | 13 | – | – | – | – | – |
| SO7-2 | – | 27 | 11 | 7 | – | – | – |
| SO7-3 | 91 | 46 | – | – | – | – | – |
| SO7-6 | 25 | 12 | – | – | – | – | – |
| SO7-7 | >99 | – | – | – | – | – | – |
| SO7-8 | 19 | 11 | – | – | – | – | – |
| SO7-9 | – | 13 | – | – | – | – | – |
| SO7-10 | >99 | – | – | – | – | – | – |
| SO7-11 | 80 | – | – | – | – | – | – |
| SO7-12 | 10 | 11 | – | – | – | – | – |
| SO7-13 | – | 31 | – | – | – | – | – |
| SO7-14 | 21 | 11 | – | – | – | – | – |
| SO7-15 | >99 | – | – | – | – | – | – |
The results for short-chain ester biohydrolysis can be divided into four distinct groups: (a) 15 bacteria that exclusively catalyzed the hydrolysis of acetate esters (2), (b) 15 bacteria that exclusively hydrolyzed propionate esters (3), (c) 12 bacteria that were not able to discriminate between 2 and 3, and (d) 14 bacteria with no esterase activity, as determined using the tested fluorogenic probes.
The heterotrophic bacteria evaluated in this work clearly prefer short-chain esters as only two strains (SO5-7 and SO7-2) were able to hydrolyze the medium-chain ester 4 (Table 1).
None of the strains were able to hydrolyze 5 and 6, which were specific for epoxide hydrolases, after a 24h reaction period. Activity toward these probes was observed only after 72h (data not shown); however, the conversions were below 10%, and these biocatalysts were not considered any further.
Monooxygenase screening using fluorogenic probes 7–10 indicated that among the 56 strains under evaluation, 11 were active (Table 1). Strains SO5-2, -18, SO6-12 and SO7-2 catalyzed the conversion of cyclic ketone 7 into 13. Strains SO5-1, -4, -5 and SO6-2 catalyzed the oxidation of 8 to lactone 14, and strains SO5-2, -5, -7, -9 and -10 oxidized ketone 9 to ester 15. None of the strains oxidized probe 10.
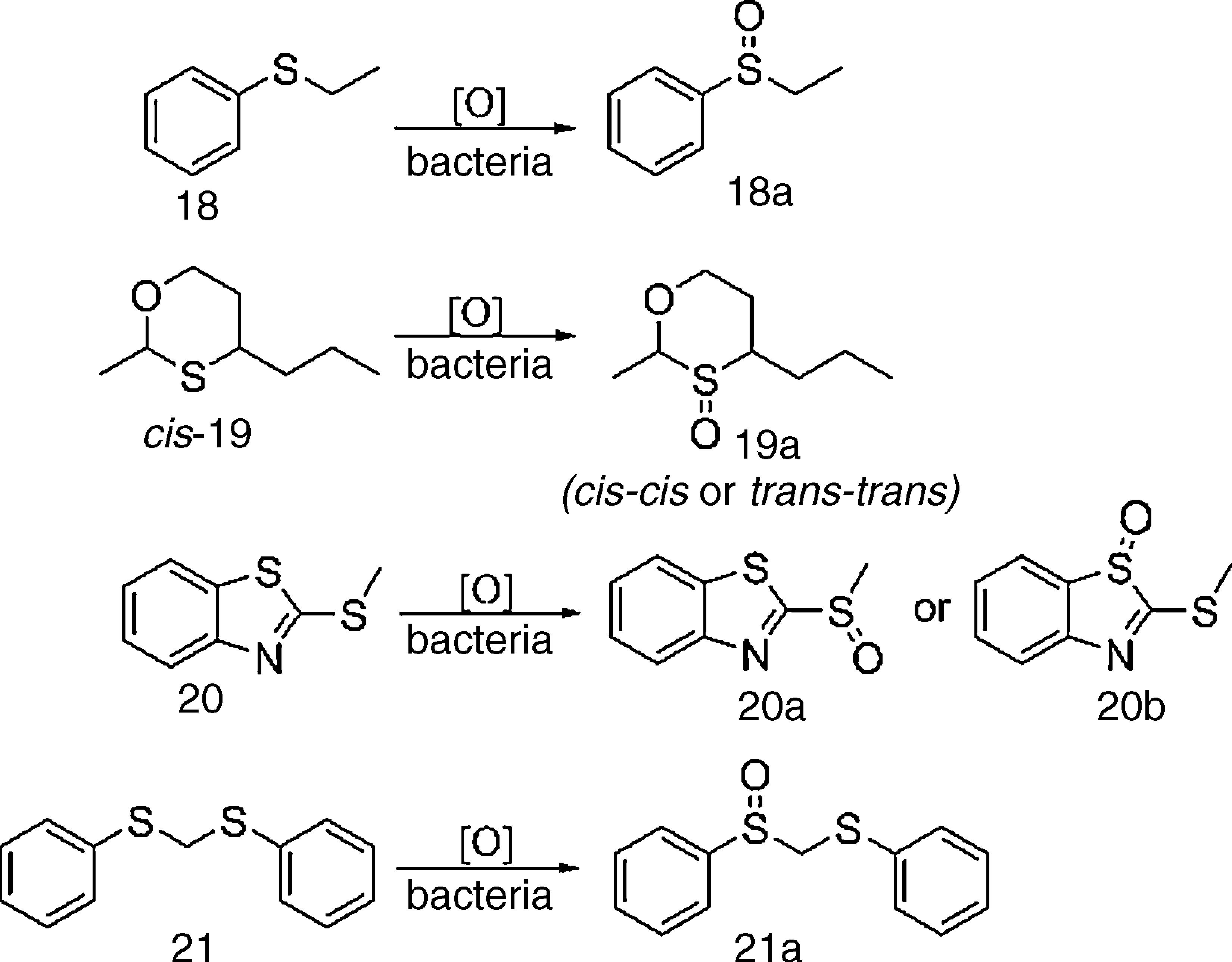
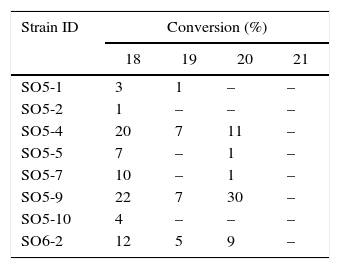
Biocatalytic sulfide oxidation by multibioreaction assaysMultibioreaction experiments (Table 2) revealed that bacteria isolated from a neutral copper mine drainage were readily able to oxidize organic sulfur compounds to their respective sulfoxides (Scheme 2). All tested strains were able to catalyze the oxidation of at least one of the substrates. Eight strains oxidized 18, and three strains oxidized 19 and 20; however, none of the strains oxidized 21. The best results were observed for strains SO5-4, SO5-9 and SO6-2, which were able to oxidize three of the sulfides tested (18, 19 and 20).
Chromatographic conversion (%) of multibioreaction assays for sulfide (18–21) oxidation,a catalyzed by bacteria from a neutral copper mine drainage.
| Strain ID | Conversion (%) | |||
|---|---|---|---|---|
| 18 | 19 | 20 | 21 | |
| SO5-1 | 3 | 1 | – | – |
| SO5-2 | 1 | – | – | – |
| SO5-4 | 20 | 7 | 11 | – |
| SO5-5 | 7 | – | 1 | – |
| SO5-7 | 10 | – | 1 | – |
| SO5-9 | 22 | 7 | 30 | – |
| SO5-10 | 4 | – | – | – |
| SO6-2 | 12 | 5 | 9 | – |
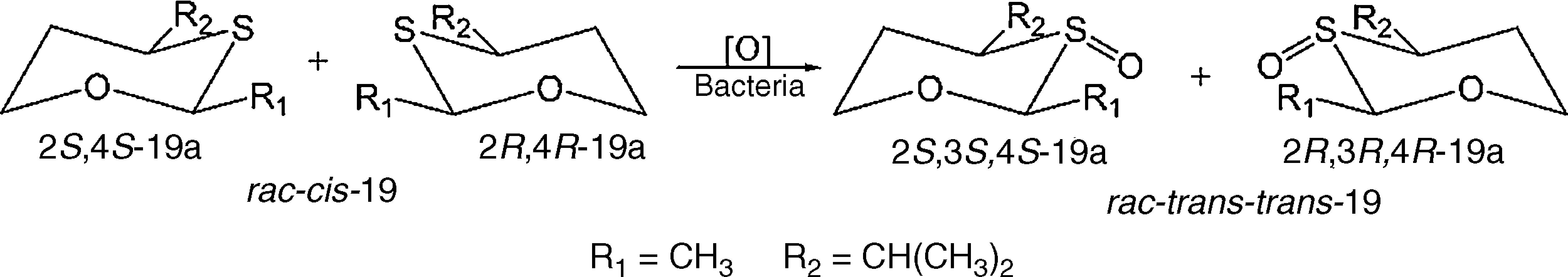
The enantioselectivities of the sulfide biooxidations catalyzed by SO5-4, SO5-9 and SO6-2 were assessed by chiral GC-FID, revealing low enantiomeric excesses (from 5 to 15%, data not shown). Nonetheless, oxidation of cis-19 was highly diastereoselective (over 99% de), providing only a racemic mixture of trans-trans-19a (2S,3S,4S and 2R,3R,4R, Scheme 3).
Isolation and identification of heterotrophic bacteria from mine environmentsAll of the evaluated copper mine drainage samples had a pH between 7 and 8. Thus, specific low pH culture media for isolation of acidophilic bacteria were not used. Heterotrophic bacteria (56 strains) were isolated from the Sossego copper mine drainage samples (18 strains were isolated from sample SO5, 23 from sample SO6 and 15 from sample SO7) using serial dilutions and PCA.
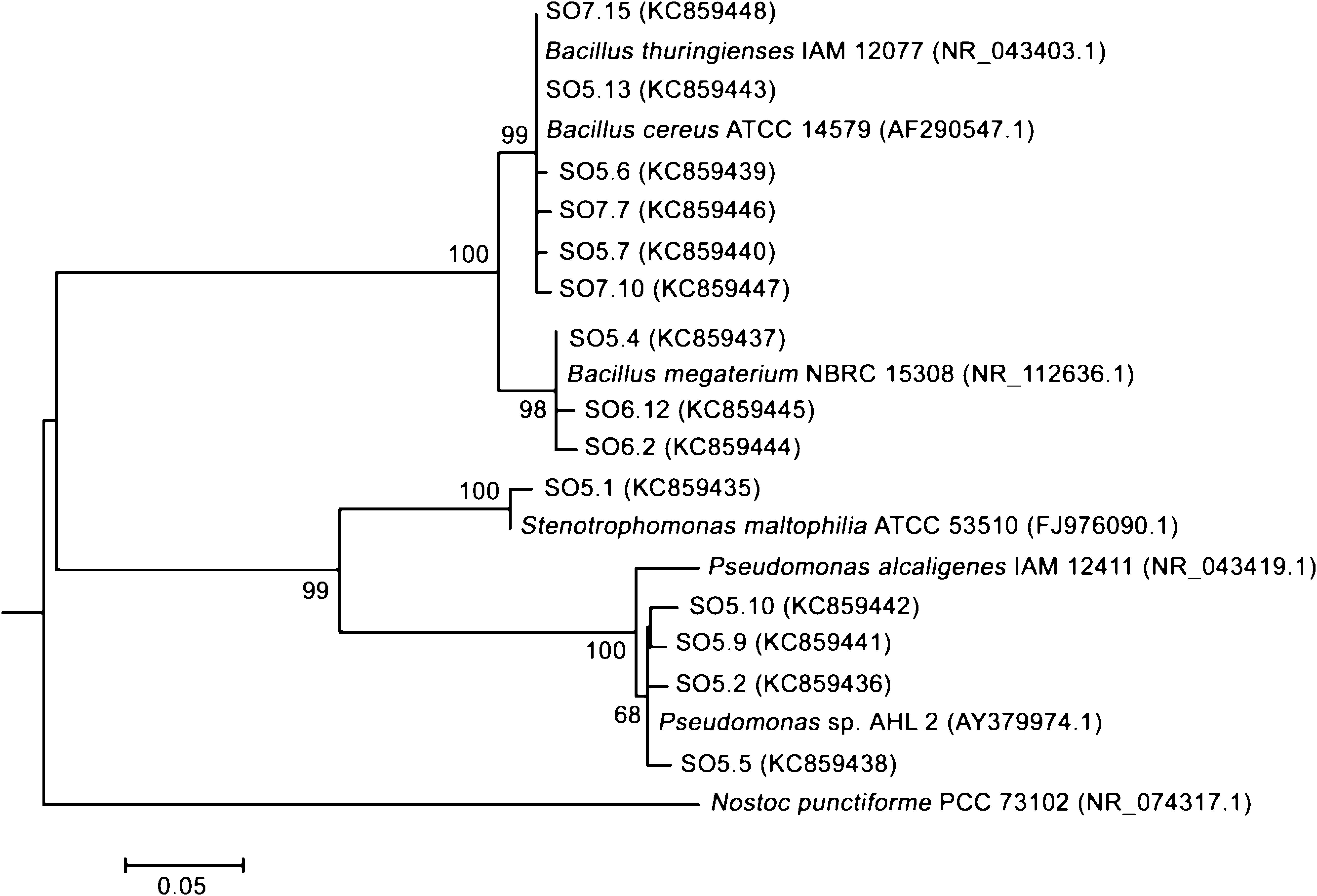
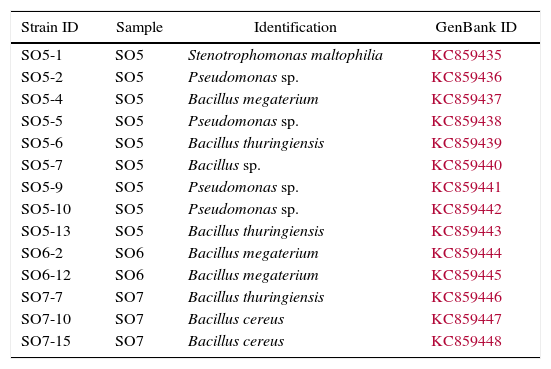
Fourteen strains with outstanding hydrolytic or oxidative activity (above 99% and 10%, respectively) were identified after partial sequencing of the 16S rRNA gene (Table 3, Fig. 1). Nine strains of Bacillus were identified as B. thuringiensis, B. megaterium, B. cereus and Bacillus sp. Other strains recovered were Stenotrophomonas maltophilia and Pseudomonas sp.
Identification of heterotrophic bacterial strains isolated from a neutral copper mine drainage by sequencing of the 16S rRNA gene.
| Strain ID | Sample | Identification | GenBank ID |
|---|---|---|---|
| SO5-1 | SO5 | Stenotrophomonas maltophilia | KC859435 |
| SO5-2 | SO5 | Pseudomonas sp. | KC859436 |
| SO5-4 | SO5 | Bacillus megaterium | KC859437 |
| SO5-5 | SO5 | Pseudomonas sp. | KC859438 |
| SO5-6 | SO5 | Bacillus thuringiensis | KC859439 |
| SO5-7 | SO5 | Bacillus sp. | KC859440 |
| SO5-9 | SO5 | Pseudomonas sp. | KC859441 |
| SO5-10 | SO5 | Pseudomonas sp. | KC859442 |
| SO5-13 | SO5 | Bacillus thuringiensis | KC859443 |
| SO6-2 | SO6 | Bacillus megaterium | KC859444 |
| SO6-12 | SO6 | Bacillus megaterium | KC859445 |
| SO7-7 | SO7 | Bacillus thuringiensis | KC859446 |
| SO7-10 | SO7 | Bacillus cereus | KC859447 |
| SO7-15 | SO7 | Bacillus cereus | KC859448 |
Phylogenetic analysis based on partial 16 rRNA gene sequences of the identified bacterial strains and related microorganisms. The evolutionary history was inferred using the Maximum Likelihood method based on the Kimura 2-parameter model. The numbers at nodes indicate bootstrap percent values from the Maximum Likelihood analysis, based on 1000 resampled data sets. Only significant bootstrap values greater than 65% are shown near each node. Bars represent sequence divergence, and the sequence accession numbers are indicated in parenthesis. Nostoc punctiforme was used as the outgroup.
The 56 heterotrophic bacterial strains isolated from this neutral copper mine drainage were evaluated by fluorescence-based HTS enzymatic screening and displayed distinct enzymatic activity toward the selected probes. Esterases and monooxygenases were found in 75% and 20% of strains, respectively. Hydrolases and oxidoreductases are frequently employed in industrial biocatalyzed processes,22 and the search for novel sources of these enzymes is essential for the development of new processes. The HTS results, therefore, indicate that the restricted environment of a copper mine drainage is a promising source of new biocatalysts.
The best oxidation results were obtained using strains isolated from sample SO5 (Table 1), which were further evaluated by multibioreaction assays assessing sulfide biooxidation.
Reduced organic sulfur compounds, such as sulfides, were chosen as targets because of their presence in industrial aqueous wastes. These organic sulfides are often bad smelling and environmentally damaging. They are classified as air and water pollutants, and their bioremediation is an important ecological issue.23
Sulfur atom oxidation is the first bioremediation step, producing sulfoxides (monooxygenation) or sulfones (dioxygenation), which are less volatile and have a less unpleasant odor. Furthermore, sulfoxides are industrially relevant compounds that have potential as drugs and are usually applied as precursors of aromas and flavors and used in asymmetric organic syntheses.24
Compound 18 is usually employed to detect microbial sulfide oxidation and, in this study, was oxidized by all screened bacteria. Sulfide 19 is a common, fruity odor constituent and was oxidized to sulfoxide 19a by bacteria SO5-4, SO5-9 and SO6-2, which resulted in a change from its original odor to a new aroma valued by fragrance industries.25 Furthermore, oxidation of rac-cis-19 provided only the rac-trans-trans-19a (Scheme 3), with over 99% of diastereomeric excess toward rac-cis-cis-19a, which is chemically expected as both enantiomers have all substituents at equatorial positions. Oxidation of sulfide 20 was also accomplished by SO5-4, SO5-9 and SO6-2, providing sulfoxide rac-20a instead of rac-20b (Scheme 2) because the lone pair of the external sulfur atom of 20 is not part of the aromatic benzothiazole system.
These results are promising and indicate that biooxidation/bioremediation of organic sulfides can be performed by bacteria isolated from the restricted environment of neutral copper mine drainages.
Most isolated bacteria with outstanding enzymatic activities were identified as belonging to the Bacillus genus, which is consistent with the widespread distribution of Bacillus spp., including in mining environments.26–28Bacillus species have displayed broad catabolic abilities and are the major industrial hydrolase producers, responsible for approximately 50% of the total enzyme market production.29
The other genera recovered were Stenotrophomonas and Pseudomonas, identified, respectively, as S. maltophilia and Pseudomonas sp. Both Stenotrophomonas and Pseudomonas have been previously isolated from heavy metal-contaminated soils and other environmental mining samples.30–33 Bacterial species from the Stenotrophomonas and Pseudomonas genera are well known for their metabolic diversity, multidrug resistance and ability to degrade a wide range of compounds, including pollutants.34–36
In this work, the Bacillus strains (SO5-6, SO5-13, SO6-12, SO7-7, SO7-10 and SO7-15) displayed good hydrolase activity (Tables 1 and 3). These strains, as well as the strains identified as Pseudomonas, presented a moderate level of activity for monooxygenases (Tables 1 and 3).
The best biocatalytic performance for sulfide oxidation was accomplished by Pseudomonas sp. (SO5-9), followed by two Bacillus megaterium strains (SO5-4 and SO6-2) (Tables 2 and 3). Mahmood et al. (2009)37 isolated a strain of Pseudomonas stutzeri that was characterized as a chemolithoautotrophic sulfide-oxidizing and nitrite-reducing strain from the sludge of an anoxic sulfide-oxidizing reactor. Lee et al. (2012)38 identified a Pseudomonas sp. strain isolated from a sludge reactor that could successfully convert sulfide to elementary sulfur and thus demonstrated that sulfide oxidation could be applied to electricity generation from sulfide-containing industrial effluent. Therefore, our results indicate the mine strains’ potential application as biocatalysts in organic syntheses and bioremediation, corroborating previous data that demonstrated the use of Pseudomonas and Bacillus in industry.
In conclusion, this study provides a novel overview of the heterotrophic bacteria from a copper mine drainage, which might be useful for future investigations of industrial processes, enzyme expression and bioremediation of contaminated environments. Furthermore, the obtained results will support further monitoring of the microbiological processes occurring in this type of restricted environment.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Pablo dos Santos Pina for the samples from the Sossego mine. We also thank Companhia Ambiental do Estado de São Paulo (CETESB) for the isolation of the bacterial strains, especially Ana Paula G. Christ and Nancy de Castro Stoppe.