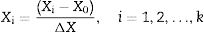Ethanol production from sweet sorghum juice (SSJ) using the thermotolerant Saccharomyces cerevisiae strain DBKKUY-53 immobilized in an alginate-loofah matrix (ALM) was successfully developed. As found in this study, an ALM with dimensions of 20×20×5mm3 is effective for cell immobilization due to its compact structure and long-term stability. The ALM-immobilized cell system exhibited greater ethanol production efficiency than the freely suspended cell system. By using a central composite design (CCD), the optimum conditions for ethanol production from SSJ by ALM-immobilized cells were determined. The maximum ethanol concentration and volumetric ethanol productivity obtained using ALM-immobilized cells under the optimal conditions were 97.54g/L and 1.36g/Lh, respectively. The use of the ALM-immobilized cells was successful for at least six consecutive batches (360h) without any loss of ethanol production efficiency, suggesting their potential application in industrial ethanol production.
The use of alternative energy resources has been increasingly replacing the use of petroleum-based fossil fuels due to a continuously decreasing fossil fuel reservoir and environmental problems caused by the growing consumption of oil and its derivatives.1,2 Bioethanol is one of the high-potential alternative fuel energy sources, since it is clean, renewable, carbon-neutral and environmentally friendly.3–5 Bioethanol can be produced from various renewable resources, such as sugar-based materials (e.g., sugarcane molasses, beet molasses), starch-based materials (e.g., cassava, corn, potato), and lignocellulosic-based materials (e.g., rice straw, sugarcane bagasse, corn stalk, grass, pineapple peel). Apart from sugarcane and cassava, sweet sorghum [(Sorghum bicolor (L.) Moench)] is one of the most promising alternative energy crops for industrial bioethanol production in Thailand.6–8 It is considered a high-potential feedstock for ethanol fuel production because it has high levels of fermentable sugars, such as sucrose, glucose and fructose, and a high yield of green biomass.9 Sweet sorghum is also considered a high-efficiency energy crop because it requires less fertilizer and water usage, has a wide adaptability for cultivation, and has a short growing period of 3–4 months.10,11
Conventional industrial bioethanol production is generally carried out using a free-cell system. The free-cell system has several disadvantages, such as a high operating cost and a low ethanol productivity.12 To improve ethanol production efficiency, cell-immobilization has been proposed. The immobilized-cell system has several advantages, such as a reduced risk of microbial contamination due to high cell densities and fermentation activity, increased substrate uptake rate, increased ethanol productivity and yield, prolonged activity and stability of the cells, the ability to recycle the biocatalysts, increased tolerance to a high substrate concentration, reduced inhibition of end products, protection of the cells from inhibitors, easy product recovery, and minimal production costs.1,13–17 Several techniques for cell immobilization, such as adsorption, entrapment, cross-linking, covalent bonding, and encapsulation, have been reported.16 Among these techniques, entrapment in calcium alginate beads is the most widely used because it is easily prepared, inexpensive and non-toxic.18 However, it also has some disadvantages, e.g., gel degradation, low mechanical strength and severe mass transfer restriction.14,17 In addition, the complex and sophisticated equipment required for the large-scale preparation of calcium alginate beads can lead to high production costs.19 Loofah sponge, a lignocellulosic material from the gourd Luffa cylindrica composed mainly of cellulose (60%), hemicellulose (30%) and lignin (10%),20 is considered as one of the most promising natural carriers for cell immobilization in industrial ethanol production. Loofah sponge has several advantages, such as low cost, abundance, chemical stability, high porosity, high surface area and non-toxicity.17 Ganguly et al.21 reported that the structure and shape of loofah sponges remained unchanged under various pH conditions (1.1–14) and remained stable in high temperatures despite repeated autoclaving at 121°C for 20–40min. The sponge is suitable for cell adhesion because it is composed of highly porous random lattices of small cross sections.15
In recent years, statistical experimental designs have been widely used to optimize ethanol production conditions.22–25 These techniques have several advantages, such as a reduction in time consumption and a reduction in operating costs due to fewer experimental units. The interaction between independent variables can also be evaluated. In addition, the second order polynomial equation can be used to determine the optimum conditions.26,27 Many factors, such as the incubation temperature, the initial yeast cell concentration and the initial sugar concentration, affect the growth and ethanol production of free and immobilized yeast cells.1,17 Although there are a number of studies on ethanol production using immobilized cells,15,17,28,29 little is known about ethanol production using yeast cells immobilized specifically within the alginate-loofah matrix (ALM). Therefore, optimization of ethanol production from sweet sorghum juice (SSJ) using yeast cells entrapped in ALM was performed in this study using a central composite design (CCD). The ethanol production efficiency during repeated batch fermentation using ALM-immobilized cells was also examined.
Materials and methodsYeast strain, cell preparation and raw materialsSaccharomyces cerevisiae DBKKUY-53, a high-yield ethanol-producing thermotolerant yeast strain,7 was used in this study. It was cultured in a yeast extract malt extract (YM) medium (0.3% yeast extract, 0.3% malt extract, 0.5% peptone and 1% glucose) at 30°C for 2 days and then stored at 4°C as a stock culture. For inoculum preparation, a loopful of the stock culture was transferred to 100mL of the YM broth with an initial pH of 5.0. The preculture was grown in a controlled temperature incubator shaker at 30°C and shaken at 150rpm for 18h. Then, the preculture was transferred into SSJ containing sugar concentration of 100g/L and subsequently incubated at 30°C with shaking at 150rpm for 18h. The cells were collected by centrifugation at 5000rpm for 10min, washed twice with 0.85% (w/v) NaCl solution, and then resuspended in the same solution. The resulting cells were used as a starter culture for cell immobilization and ethanol production.
Concentrated SSJ (75°Bx) obtained from the Department of Agronomy, Faculty of Agriculture, Khon Kaen University, Thailand, was used as a raw material for ethanol production in this study. The juice was stored at −18°C until use.
Cell immobilizationLoofah sponges used in this work were purchased from a local market, Khon Kaen, Thailand. They were cut into small thin square pieces with different dimensions of 10×10×5mm3, 15×15×5mm3 and 20×20×5mm3. S. cerevisiae DBKKUY-53 was immobilized on the alginate-loofah matrices using the entrapment method. The starter culture was inoculated into a sterile 2% (w/v) sodium alginate solution with an initial cell concentration of 2×109cells/mL. Sterilized loofah cubic sponges with different dimensions were immersed in the alginate-cell solution. Then, the alginate-loofah sponges were dropped into a 0.1M CaCl2 solution and gently agitated for 15min. The resulting alginate-loofah matrix or ALM was washed with a sterile distilled water to remove excess Ca2+ ions and unentrapped cells before being used for ethanol production.
Ethanol production by free and immobilized cells using various sizes of ALMSSJ containing sugar concentration of 200g/L was added to a 500-mL air-locked Erlenmeyer flask with a final working volume of 300mL and autoclaved at 110°C for 15min.6 Ethanol production was carried out by transferring the free cells and the cells immobilized in loofah sponges of various dimensions into sterile SSJ with an initial cell concentration of 1×107cells/mL.30 The flasks were statically incubated at 37°C in a controlled temperature incubator. During ethanol fermentation, samples were withdrawn at certain time intervals, and cells, sugar and ethanol concentrations were determined.
Optimization of ethanol production conditions during fermentation by ALM-immobilized cells using a central composite design (CCD)Based on a review of the literature,6,7,14,18 three factors, namely the inoculum size, the initial sugar concentration and the incubation temperature, were selected as parameters for the optimization of ethanol fermentation using a CCD. The codes and actual values of the independent factors for the CCD are shown in additional information section, Table 1. The relationship between the code and actual values is described by the following equation:
where Xi is a coded value of the variable; Xi is the actual value of the variable; X0 is the actual value of the variables at the center point; and ΔX is the step change of the variable.Kinetic parameters of ethanol production from sweet sorghum juice by the free and ALM-immobilized cells of S. cerevisiae DBKKUY-53 at 37°C.
| Conditions | Kinetic parameters | ||||
|---|---|---|---|---|---|
| P (g/L) | Qp (g/Lh) | S (g/L) | Yp/s (g/g) | T (h) | |
| Free cell | 73.64±2.50a | 1.53±0.05a | 31.69±1.53a | 0.44±0.01ab | 48 |
| 10mm×10mm immobilized cell | 75.14±2.74a | 1.57±0.06a | 13.94±0.83b | 0.45±0.03a | 48 |
| 15mm×15mm immobilized cell | 76.75±1.11a | 1.60±0.02a | 14.82±1.94b | 0.39±0.01bc | 48 |
| 20mm×20mm immobilized cell | 76.46±1.63a | 1.59±0.03a | 12.67±0.28b | 0.38±0.01c | 48 |
P, ethanol concentration; Qp, ethanol productivity; S, residual sugar concentration; Yp/s, ethanol yield; and t, fermentation time. Mean values±SD with different letters in the same column are significantly different at p<0.05 based on DMRT analysis.
The experimental data were explained by a second order polynomial function as follows:
where Y is the predicted response; Xi and Xj represent the independent variables; β0 is a constant term; βi represents the linear coefficients; βii represents the squared coefficients; and βij represents the cross product coefficients.The Design-Expert 7.0 Demo version (STAT EASE Inc., Minneapolis, USA) was used for the experimental designs and regression analysis. An analysis of variance (ANOVA) was used to estimate the statistically significant parameters. The quality of the quadratic model equation was expressed as the coefficient of determination (R2). The validation experiment was conducted using optimized conditions determined from the response surface plots.
Ethanol production by repeated batch fermentationRepeated batch fermentation was performed in a 500-mL air-locked Erlenmeyer flask with a final working volume of 300mL. The fermentation conditions were based on the optimization results obtained from the CCD, as previously described. The flasks were statically incubated for 60h, the fermentation broth was withdrawn at 50% of the working volume at the end of each batch, and the cells, sugar and ethanol concentrations were analyzed. Fresh fermentation medium (150mL) was added to the flasks for subsequent batch fermentation.8
Analytical methodsThe viable yeast cell numbers were determined by a direct counting method using a haemacytometer with methylene blue staining technique.31 For immobilized cells, 10g of wet ALM-immobilized cells were dissolved in 0.05M sodium citrate buffer as described by Bangrak et al.,17 After the loofah sponge was removed, viable cells were determined using methylene blue staining as mentioned earlier.31 The fermentation broth was centrifuged at 12,000rpm for 10min, and the supernatant was then analyzed for total sugar concentrations using the phenol-sulphuric acid method.32 The total soluble solids of the fermentation broth were estimated using hand-held refractometer.31 The ethanol concentration (P, g/L) was analyzed by gas chromatography (Shimadzu GC-14B, Japan) using a polyethylene glycol (PEG-20M)-packed column with a flame ionization detector. The temperatures for the injector, detector and column oven were 180°C, 250°C and 150°C, respectively. Nitrogen was used as a carrier gas, and 2-propanol was used as an internal standard.6 The pressure of nitrogen gas was controlled at 200kPa. The volumetric ethanol productivity (Qp, g/Lh) was calculated by the following equation: Qp=P/t, where P is the ethanol concentration (g/L), and t is the fermentation time (h), giving the greatest ethanol concentration. The ethanol yield (Yp/s) was calculated as the actual ethanol produced and was expressed as gram ethanol per gram sugar utilized (g/g). The ethanol fermentation efficiency (Ey, %) was calculated by the following equation: Ey=((Yp/s)/0.511)×100, where Yp/s is the ethanol yield (g/g), and 0.511 is the theoretical maximum ethanol yield per unit of glucose from glycolytic fermentation (g/g).
The Scanning Electron Microscope (SEM, Hitachi model S-3000N, Tokyo, Japan) was used to visualize characteristic of the immobilized cells. The immobilized cells were frozen in vacuum freeze-dried machine (CHAIST, Germany), then they were sputtered with gold (EMITECH model K500X, Kent, UK) and photographed.
ResultsEthanol production using free- and ALM-immobilized cell systemsThe results of the ethanol production at relatively high temperature (37°C) from SSJ containing 200g/L of sugar using free cells and cells immobilized in loofah sponges of different dimensions are summarized in Table 1. The ethanol concentrations and volumetric ethanol productivities produced by both the free and the ALM-immobilized cells were not significantly different. This might be due to the fact that the initial living cell concentrations of both freely suspended cells and immobilized cells were similar. It should be noted from the current study that cells in the ALM-immobilized system could utilize higher amount of sugars than that in the free-cell system, resulting in less residual sugar concentration in the fermentation broth. As a result, the ALM-immobilized cells exhibited slightly higher ethanol concentrations and volumetric ethanol productivities than the free cells. The highest ethanol concentration (76.46–76.75g/L) and volumetric ethanol productivity (1.59–1.60g/Lh) were achieved by the ALM-immobilized cells with a dimension of 15×15×5mm3 and 20×20×5mm3, which were approximately 3.87% and 4.08% greater than those of the free cells, respectively. With respect to the ethanol yield, the free-cell system exhibited slightly higher ethanol yield than the ALM-immobilized system. The cell concentrations during ethanol fermentation were determined. As observed in this study, the initial living cell concentration of freely suspended cells in the fermentation broth was 1.68×107cells/mL, then it was enumerated and reached the concentration of 1.37×108cells/mL at the end of fermentation (48h). On the other hand, the initial living cell concentrations of ALM-immobilized cells in different dimensions of loofah sponges were similar, ranging from 1×107 to 1.16×107cells/mL. It should be noted from this study that the freely suspended cells were observed apart from the immobilized cells in the ALM-immobilized cells system, resulting in the total living cell concentrations of 8.85×107 to 9.38×107cells/mL after 48h of fermentation, which was slightly lower than that of the free cells system (Additional information section, Table 2). According to the results demonstrated in Table 1 and additional information section, Table 2, it was plausible that both freely suspended cells and immobilized cells contributed in the conversion of sugar into ethanol in the ALM-immobilized cell system.
The central composite design (CCD) matrix for ethanol production from sweet sorghum juice by the ALM-immobilized cells of S. cerevisiae DBKKUY-53.
| Runs | Inoculum (%) | Sugar concentration (g/L) | Temperature (°C) | P (g/L) | Qp (g/Lh) | Yp/s (g/g) |
|---|---|---|---|---|---|---|
| 1 | 10.0 | 260 | 37.5 | 83.13 | 1.73 | 0.42 |
| 2 | 10.0 | 260 | 37.5 | 79.71 | 1.66 | 0.44 |
| 3 | 10.0 | 201 | 37.5 | 84.03 | 1.75 | 0.49 |
| 4 | 7.0 | 295 | 33.0 | 96.64 | 1.61 | 0.45 |
| 5 | 13.0 | 295 | 42.0 | 44.85 | 0.75 | 0.46 |
| 6 | 10.0 | 260 | 37.5 | 77.01 | 1.60 | 0.43 |
| 7 | 7.0 | 225 | 42.0 | 50.15 | 1.04 | 0.43 |
| 8 | 10.0 | 260 | 37.5 | 81.35 | 1.69 | 0.44 |
| 9 | 13.0 | 225 | 33.0 | 96.43 | 1.61 | 0.48 |
| 10 | 5.0 | 260 | 37.5 | 81.58 | 1.36 | 0.43 |
| 11 | 7.0 | 295 | 42.0 | 25.71 | 0.54 | 0.39 |
| 12 | 10.0 | 319 | 37.5 | 78.04 | 1.30 | 0.41 |
| 13 | 10.0 | 260 | 45.0 | 24.36 | 0.51 | 0.39 |
| 14 | 13.0 | 295 | 33.0 | 99.70 | 1.66 | 0.45 |
| 15 | 10.0 | 260 | 30.0 | 93.84 | 1.30 | 0.43 |
| 16 | 7.0 | 225 | 33.0 | 95.39 | 1.59 | 0.45 |
| 17 | 10.0 | 260 | 37.5 | 85.98 | 1.79 | 0.45 |
| 18 | 13.0 | 225 | 42.0 | 49.86 | 1.04 | 0.42 |
| 19 | 15.0 | 260 | 37.5 | 86.06 | 1.79 | 0.42 |
P, ethanol concentration; Qp, ethanol productivity; Yp/s, ethanol yield; Ey, ethanol fermentation efficiency.
It should be noted that the ethanol concentrations and volumetric ethanol productivities obtained from the ALM-immobilized cells in 15×15×5mm3 and 20×20×5mm3 loofah sponges were not significantly different (Table 1). Because the ALM-immobilized cell system using 20×20×5mm3 loofah sponges exhibited more compact structure and its preparation was also easier than the cell system using the 15×15×5mm3 loofah sponges, it was selected as the system for further experiments.
Optimization of ethanol production conditions during fermentation by ALM-immobilized cells using a central composite design (CCD)The optimum levels of the inoculum size, the initial sugar concentration and the incubation temperature during ethanol production from SSJ by ALM-immobilized cells were evaluated using a CCD via the Design expert software. The experimental design matrices along with the response variables (ethanol concentration and ethanol productivity) are summarized in Table 2. The ethanol concentrations measured in 19 experiments ranged from 24.36 to 99.70g/L with the ethanol yields ranging from 0.39 to 0.49g/g. The predicted values of the ethanol concentrations in this study were ranged from 15.71 to 100.31g/L (data not shown). A regression analysis was applied to the experimental data shown in Table 2, and the second-order polynomial equation (3) for the prediction of ethanol concentration (P, g/L) as a function of the process parameters including the inoculum size (A, %), initial sugar concentration (B, g/L), and incubation temperature (C, °C), was as follows:
The relatively high coefficient of determination (R2) of the regression of the above model (0.9698) indicates that 96.98% of the ethanol concentration could be explained by the established model. The p-value of the lack-of-fit test was above 0.05 (0.0891), suggesting that the model is reliable. The statistical significance of the regression model of ethanol concentration was evaluated by ANOVA, and the results are summarized in additional information section, Table 3. In this study, the established model was found to be highly significant because the p-value of the experiment was less than 0.05. These results indicated that the incubation temperature (C) and the quadratic terms of the incubation temperature (C2) had significant effects on the ethanol concentration compared with other process parameters.
Kinetic parameters of repeated batch ethanol production from sweet sorghum juice by the ALM-immobilized cells of S. cerevisiae DBKKUY-53.
| Batch | P (g/L) | Qp (g/Lh) | Yp/s (g/g) | X0 (cells/cm3) |
|---|---|---|---|---|
| 1 | 82.54±0.60 | 1.38±0.02 | 0.40±0.01 | 1.40×107 |
| 2 | 84.75±0.87 | 1.41±0.01 | 0.44±0.01 | 6.04×107 |
| 3 | 82.29±1.08 | 1.37±0.02 | 0.42±0.02 | 5.68×107 |
| 4 | 81.50±1.16 | 1.36±0.06 | 0.40±0.01 | 4.84×107 |
| 5 | 80.63±1.15 | 1.34±0.10 | 0.42±0.02 | 3.06×107 |
| 6 | 82.80±1.27 | 1.38±0.12 | 0.43±0.00 | 2.87×107 |
P, ethanol concentration; Qp, ethanol productivity; Yp/s, ethanol yield; X0, initial living cell concentration in each batch.
The 3-D response surface plots (Fig. 1A) showing the effects of the process parameters on ethanol concentration were generated using the data in Eq. (3). Generally, the response surface plots can be used to explain the interaction between two process parameters when another process parameter is fixed at a central level. Based on the response surface plots, the optimum levels of the process parameters needed to obtain the maximum ethanol concentration can be determined.9 As shown in Fig. 1A, increasing the inoculum size increases the ethanol concentration. On the other hand, the ethanol concentration decreases when the initial sugar concentrations and the incubation temperatures are increased.
The regression and the response surface analyses were also evaluated to study the effects of inoculum size, initial sugar concentration and incubation temperature on ethanol productivity. As shown in Table 2, the actual ethanol productivities measured in 19 experiments ranged from 0.51 to 1.79g/Lh, which are reliably close to the predicted values of 0.32–1.76g/Lh. To develop a quadratic polynomial regression model and a second-order polynomial equation (4) for the prediction of the final ethanol productivity (Qp, g/Lh) as a function of the process parameters, including the inoculum size (A, %), the initial sugar concentration (B, g/L), and incubation temperature (C, °C), the ethanol productivities provided in Table 2 were used, and the prediction equation was as follows:
The results of the ANOVA shown in additional information section, Table 4 revealed that this model was highly significant. In addition, the linear terms of the sugar concentration (B), incubation temperature (C), the interaction between sugar concentration and incubation temperature (BC), the quadratic terms of the sugar concentration (B2) and incubation temperature (C2) were also significant. The R2 value of the regression (0.9512) suggests that 95.12% of the variability in the response could be explained by this model. The p-value of “lack-of-fit tests” (0.0644) was not significant. These results clearly indicate that the model is reliable.
Comparison of repeated-batch ethanol production by the ALM-immobilized cells of thermotolerant yeast S. cerevisiae DBKKUY-53 and other immobilized cell systems.
| Strain | C-source | Carrier | P (g/L)a | Qp (g/Lh)a | Yp/s (g/g)a | References |
|---|---|---|---|---|---|---|
| S. cerevisiae | Glucose and sucrose | Sorghum bagasse | 96 (13 batches) 100 (21 batches) | NA | 0.48 | Yu et al.35 |
| S. cerevisiae NP 01 | Sweet sorghum juice | Fresh sorghum stalk | 99.28 (8 batches) | 1.36 | 0.47 | Ariyajaroenwong et al.34 |
| S. cerevisiae M30 | Palm sugar | Thin-shell silk cocoon | 81.9 (5 batches) | 1.71 | 0.45 | Rattanapan et al.1 |
| S. cerevisiae IR2 | Sugar beet juice | Loofah sponge | 76 (3 batches) | NA | 0.39 | Ogbonna et al.28 |
| S. cerevisiae M30 | Cane molasses | Alginate-loofah-matrix (ALM) | 81.4 (3 batches) | NA | 0.43 | Phisalaphong et al.15 |
| S. cerevisiae | Glucose Sucrose | Chitosan-cover calcium alginate | 30.7 (8 batches) 31.8 (8 batches) | NA NA | 0.31 0.34 | Duarte et al.36 |
| S. cerevisiae DBKKU Y-53 | Sweet sorghum juice | Alginate-loofah-matrix (ALM) | 82.4 (6 batches) | 1.37 | 0.42 | This study |
S0, initial sugar concentration; P, ethanol concentration; Qp, ethanol productivity; Yp/s, ethanol yield; NA, not available.
The 3-D response surface plots showing the effects of various parameters on ethanol productivity are illustrated in Fig. 1B. The results indicate that ethanol productivity increases when the inoculum size is increased. On the other hand, the ethanol productivity decreases when the initial sugar concentrations and the incubation temperatures are increased.
Repeated experiments were performed to verify the predicted optimum values. The final optimum values of the parameters from the repeated experiments were as follows: an inoculum size of 11.05%, an initial sugar concentration of 277.06g/L, and an incubation temperature of 33.55°C. A maximum ethanol concentration and a volumetric ethanol productivity of 97.54g/L and 1.36g/Lh, respectively, were obtained from the ALM-immobilized cell system under these optimum conditions (Fig. 2). The actual ethanol concentration obtained in this study is reliably close to the predicted value (97.76g/L), whereas that of a volumetric ethanol productivity is slightly different from the predicted value (1.76g/Lh). It should be noted from the current study that high levels of sugars and total soluble solid remained in the fermentation broth. This might be due to the negative effects of high concentrations of sugar and ethanol on growth and metabolic processes in yeast cells.15 By the way, the results of this study demonstrate that response surface methodology based on the CCD model is a powerful tool to determine the optimum values of the individual variables and the maximum response values as also described by Dong et al.33
Verification experiments of ethanol production from sweet sorghum juice using the ALM-immobilized cells of S. cerevisiae DBKKUY-53. The fermentation conditions used in this experiment were based on the CCD results, as follows: an inoculum size of 11.05%, an initial sugar concentration of 277.06g/L and an incubation temperature of 33.55°C. ●: ethanol, ■: total soluble solids, ▴: total residual sugar, ♦: pH.
Ethanol production from SSJ by repeated batch fermentation for six cycles using the ALM-immobilized cells of the thermotolerant S. cerevisiae strain DBKKUY-53 was evaluated. The time profile of repeated batch ethanol production by the ALM-immobilized cells is shown in Fig. 3, and the main fermentation parameters are summarized in Table 3. Overall, the almost constant ethanol concentrations and volumetric ethanol productivities, ranging from 80.63 to 84.75g/L and from 1.34 to 1.41g/Lh, respectively, suggest that the ALM-immobilized cells are stable throughout the entire operation. Although a longer period of fermentation may be needed to determine the long-term stability of the ALM-immobilized cell system, repeated fermentation had to be ceased after six cycles due to a limited amount of SSJ. A previous study by Ogbonna et al.19 reported that cells immobilized on loofah sponges were stable after more than 35 cycles of repeated batch fermentation and more than 500h of continuous ethanol production using either sucrose or molasses as raw materials.
Fig. 4 shows the yeast cells adsorbed on the inner and outer surfaces and in the micro-porous structure of the ALM. The number of yeast cells at the end of the repeated batch fermentation (360h) increased as compared to the initial time of fermentation.
SEM image of the ALM-immobilized cells in loofah sponges with a dimension of 20mm×20mm. (A) Pure loofah sponge; (B) alginate-loofah sponge (without yeast cells); (C) yeast cells incorporated on the outer surface of the ALM; (D) yeast cells incorporated on the inner surface of the ALM; (E) ALM-immobilized cells at 0h, and (F) ALM-immobilized cells at 360h after fermentation. The white and black arrowheads indicate the yeast cells and loofah sponge, respectively.
A comparative analysis between repeated batch ethanol fermentation by ALM-immobilized cells of the thermotolerant yeast S. cerevisiae DBKKUY-53 and other immobilized cell systems reported in the literature was illustrated (Table 4). The ethanol concentration, volumetric ethanol productivity and ethanol yield produced by the ALM-immobilized cells of S. cerevisiae DBKKUY-53 were comparable to those reported by Rattanapan et al.,1 Phisalaphong et al.15 and Ogbonna et al.28 However, the ethanol concentration, volumetric ethanol productivity and ethanol yield obtained in this study were lower than those reported by Ariyajaroenwong et al.34 and Yu et al.35 This might be due to the differences in yeast strains and carriers used for cell immobilization. On the other hand, lower levels of ethanol concentrations and ethanol yields using immobilized cells system have also been reported. For example, Duarte et al.36 reported the ethanol concentration of 30.7g/L with the ethanol yield of 0.31g/g from glucose and 31.8g/L with the ethanol yield of 0.34g/g from sucrose using S. cerevisiae immobilized in chitosan-covered calcium alginate. In this system, chitosan acted as a barrier to substrate and products, resulting in the low substrate consumption and ethanol production rate.
DiscussionCell-immobilization has been proposed as one of the fermentation approaches to improve the ethanol production efficiency since it provides several advantages, such as prolonged activity and stability of the cells, the ability to recycle the biocatalysts, increased tolerance to a high substrate concentration, and easy product recovery.1,13–17 In this study, S. cerevisiae DBKKUY-53 was successfully immobilized in the ALM, one of the most promising natural carriers for cell immobilization, and its ethanol fermentation activities using SSJ as a raw material were determined. Although the ethanol production efficiencies in terms of ethanol concentrations and volumetric ethanol productivities between the ALM-immobilized cells and the free cells were not significantly different, the ethanol concentrations and volumetric ethanol productivities produced by the ALM-immobilized cells were slightly higher than those of the free cells. The residual sugar concentrations in the fermentation broth of the immobilized cell cultures were also less than those in the broth of the free cell cultures (Table 1). Considering the cell number in this study, the ALM-immobilized cells exhibited slightly lower cell number than the free cells. These findings demonstrated that the immobilized cell cultures exhibited higher ethanol fermentation activity than the free cell cultures. One possibility is that the matrix of carriers may protect yeast cells from stress conditions such as high temperature or high ethanol concentration during fermentation, allowing them to perform better fermentation activity than the free cells.15 With respect to the ethanol yield in the current study, the ALM-immobilized cells exhibited slightly lower ethanol yield than the free cells. This might be due to the fact that the immobilized cell cultures utilized sugars not only for ethanol production but also for synthesis of other metabolizes such as saturated fatty acids, glycerol or trehalose, which are involved in the ethanol tolerance of yeast cells under stress conditions.35 To clarify this hypothesis, further investigation is needed.
It should be noted that the utilization of sugars by the ALM-immobilized cells was not restricted with the carrier system, suggested that the diffusion of the substrates was not prevented by the carriers, which were highly porous and thus, facilitated the mass transfer of the system. The results of the current study were in good agreement with those reported by Phisalaphong et al.,15 Pacheco et al.37 and Le et al.38
There are several factors influencing the ethanol production efficiency6,7,14,18; however, in this study, only three major factors including the inoculum size, the initial sugar concentration and the incubation temperature were focused. As found in this study, increasing the inoculum size resulted in the increases of the ethanol concentration, which was similar with that reported by Nuanpeng et al.7 On the contrary, the ethanol concentration and ethanol productivity decrease when the initial sugar concentrations and the incubation temperatures are increased (Fig. 1). This might be due to the negative effects of high sugar concentrations and high incubation temperatures on growth, cell viability and metabolic processes in yeast cells. Bai et al.39 and Ozmichi and Kargi40 reported that high sugar concentrations caused negative effects on cell viability and morphology due to an increase in the osmotic pressure, leading to a reduction in the cell biomass and ethanol production efficiency. Likewise, it has also been reported that relatively high temperature conditions cause a modification of plasma membrane fluidity and a reduction in the effectiveness of the plasma membrane, resulting in the leakage of essential cofactors and coenzymes required for the activity of enzymes involved in glucose metabolism and ethanol production.41 Relatively high temperature conditions have also been reported to cause a denaturation of cellular proteins, leading to the reduction of cell growth and fermentation activity.42
It should be noted from the experimental using CCD model that the maximum ethanol concentration and volumetric ethanol productivity were derived from different fermentation conditions. In this study, a maximum ethanol concentration was achieved from run 14 with the conditions as follows; 13% inoculum size, 295g/L sugar concentration and 33°C, while a maximum volumetric ethanol productivity was achieved from run 19 with the conditions as follows; 15% inoculum size, 260g/L sugar concentration and 37.5°C. There is a correlation between the inoculum size, the initial sugar concentration and the incubation temperature on the volumetric ethanol productivity. It clearly indicated from this study that increasing the inoculum size and incubation temperatures resulted in the increases of the volumetric ethanol productivity. On the other hand, the volumetric ethanol productivity decreases when the initial sugar concentrations increased.
Based on the CCD model, the optimal conditions for ethanol production from SSJ using the ALM-immobilized cells were obtained in the current study. In order to verify the predicted optimal values of the individual variables from CCD results, repeated ethanol fermentation was performed (Fig. 2). Although the actual ethanol concentration (97.54g/L) obtained in this experiment was relatively close to the predicted value (97.76g/L), the actual volumetric ethanol productivity (1.36g/Lh) was slightly lower than that of the predicted value (1.76g/Lh). It was plausible that high sugar concentration (277.06g/L) may cause prolongation of complete sugar utilization, resulting in lower volumetric ethanol productivity.7,39,40
One of the advantages of the cell-immobilization system is the ability to recycle the biocatalysts. In this study, the ethanol production from SSJ by repeated batch fermentation using the ALM-immobilized cells of S. cerevisiae DBKKUY-53 was performed. As found in this study, the ALM-immobilized cells could be used for at least six successive cycles without any loss of ethanol production efficiency (Fig. 3). It has been reported that high concentrations of ethanol inhibit the growth and metabolic processes of yeast cells.15 The enhanced cell stability of the immobilized cells as observed in the present study suggests that the ALM carriers may protect the yeast cells from severe conditions during the fermentation process. The increased cell stability and cell productivity of the immobilized system demonstrated in this study are consistent with the reports by Rattanapan et al.,1 Phisalaphong et al.15 and Ariyajaroenwong et al.34 In addition, an increase in the number of yeast cells adsorbed on the inner and outer surfaces and in the micro-porous structure of the matrix based on SEM observation at the end of the repeated batch fermentation (360h) as compared with that at the initial time (0h) was observed (Fig. 4), suggesting that high ethanol and sugar concentrations in the fermentation broth had no effect on the yeast growth. We propose, from these findings, that the regeneration and protection of immobilized cells by the ALM are the main factors that work synergistically to prevent cell activity.
S. cerevisiae DBKKUY-53 has been reported as one of the good candidates for ethanol production at high temperature conditions.7 Although, in this work, the predicted optimal temperature based on the CCD results for ethanol production from SSJ by the ALM-immobilized cells of this strain was 33.55°C, it has an ability to produce relatively high levels of ethanol concentrations and volumetric ethanol productivities at relatively high temperature of up to 40°C. Previous study by Nuanpeng et al.,7 demonstrated that the maximum ethanol concentrations and volumetric ethanol productivities produced under the optimal conditions using the free-cell system of S. cerevisiae DBKKUY-53 were 106.82g/L and 2.23g/Lh at 37°C, and 85.01g/L and 2.83g/Lh at 40°C, respectively, using SSJ containing sugar concentration of 250g/L as a raw material. These findings suggests that S. cerevisiae DBKKUY-53 has a high potential for high-temperature ethanol fermentation (HTEF), which provides several advantages, such as increasing the rate of fermentation, decreasing a risk of contamination, and reducing the cost of the cooling system and operating processes.43,44 It should be noted that the level of ethanol concentration and volumetric ethanol productivity obtained at 37°C using the free-cell system in this study (Table 1) was lower than that of Nuanpeng et al.7 This might be due to the differences in the fermentation conditions. In this study, the sugar concentration used in the ethanol fermentation experiment using the free-cell system was 200g/L, while that of Nuanpeng et al.7 was 250g/L. Furthermore, the condition for ethanol production using the free-cell system in this work was not yet optimized as compared with that of Nuanpeng et al.7
ConclusionALM-immobilized cells were successfully developed, and their ethanol fermentation activities using SSJ as a raw material were evaluated. The ethanol concentration and volumetric ethanol productivity produced by the free cells and the cells immobilized in different dimensions of loofah sponges were not significantly different. Based on the stability and mass transfer characteristics, the ALM with a dimension of 20×20×5mm3 was shown to be effective for cell immobilization. The optimum conditions for ethanol production by the ALM-immobilized cells were as follows: the inoculum size of 11.05%, the initial sugar concentration of 277.06g/L and the incubation temperature of 33.55°C. Under these optimum conditions, the maximum ethanol concentration and the volumetric ethanol productivity of 97.54g/L and 1.36g/Lh, respectively, were achieved. The ability of ALM-immobilized cells to be used in repeated batch fermentation for at least six successive cycles without any loss of ethanol production efficiency indicates that ALM-immobilized cells can be potentially used for industrial ethanol production. Further studies to improve ethanol productivity and yield such as testing ethanol production by ALM-immobilized cells during continuous fermentation, need to be performed.
Conflict of interestThe authors declare that they have no conflict of interest.
This research was financially supported by the Energy Policy and Planning Office, Ministry of Energy, Thailand, and by the Research Fund for Supporting a Lecturer to Admit a High-Potential Student to Study and Perform Research on His Expert Program Year 2009, by the Graduate School, Khon Kaen University, Thailand, by the Fermentation Research Center for Value Added Agricultural Products (FerVAAP), and by the Khon Kaen University. We would like to thank Associate Prof. Dr. Prasit Jaisil, Faculty of Agriculture, Khon Kaen University, Thailand for providing sweet sorghum juice.