In this study, the cry1Ab gene of previously characterized and Lepidoptera-, Diptera-, and Coleoptera-active Bacillus thuringiensis SY49-1 strain was cloned, expressed and individually tested on Ephestia kuehniella (Lepidoptera: Pyralidae) and Plodia interpunctella (Lepidoptera: Pyralidae) larvae. pET-cry1Ab plasmids were constructed by ligating the cry1Ab into pET28a (+) expression vector. Constructed plasmids were transferred to an Escherichia coli BL21 (DE3) strain rendered competent with CaCl2. Isopropyl β-d-1-thiogalactopyranoside was used to induce the expression of cry1Ab in E. coli BL21(DE3), and consequently, ∼130kDa of Cry1Ab was obtained. Bioassay results indicated that recombinant Cry1Ab at a dose of 1000μgg−1 caused 40% and 64% mortality on P. interpunctella and E. kuehniella larvae, respectively. However, the mortality rates of Bt SY49-1 strains’ spore–crystal mixture at the same dose were observed to be 70% on P. interpunctella and 90% on E. kuehniella larvae. The results indicated that cry1Ab may be considered as a good candidate in transgenic crop production and as an alternative biocontrol agent in controlling stored product moths.
Bacillus thuringiensis (Bt) is a Gram-positive aerobic or facultative aerobic spore-forming entomopathogenic bacterium that can easily be isolated from a variety of environmental sources.1 It has specific toxicity against target insects and is safe to non-target organisms. Cry1 toxins are the most common crystal proteins characterized so far in Bt strains and have specific insecticidal activity against lepidopteran insects.2 They form typical bipyramidal parasporal inclusions with 130kDa molecular weight.3 Biotechnological developments in agriculture have caused scientists to seek new solutions to insect pest problems. Transgenic technology, involving a wide range of pesticidal genes from Bt, dominates the scenario of agricultural biotechnology. The improvement of broader spectrum biopesticides using novel Bt strains against target insects is an important aspect for improving their persistence on plants. The pyralid moths Ephestia kuehniella (Lepidoptera: Pyralidae) and Plodia interpunctella (Lepidoptera: Pyralidae) are global pests, particularly of stored grains, legumes, dried fruits, nuts, dates, and cocoa beans, and they are the most commonly reported pests of stored grains. Larvae can cause extensive damage to crops and a variety of processed foods. The presence of live insects and insect parts can result in the depreciation of the grain when sold. Chemical insecticides are commonly used in controlling these important pest insects worldwide. However, due to the risks of chemical pest control to the environment and human health, the cloning and expression of cry genes with activity to lepidopteran pests is an important issue concerning species-specific control. In the current study, we amplified and cloned the cry1Ab gene of a novel Bt SY49-1 strain.4 The strain had insecticidal activity to a variety of lepidopteran, dipteran and coleopteran pests, and we desired to improve the efficiency through transforming the cry1Ab gene into E. coli BL21 (DE3). Expression of the cry1Ab gene and its insecticidal activity against the serious stored product pests E. kuehniella and P. interpunctella were investigated. The results will reveal useful information for stored product pest control by providing Cry proteins from novel strains.
Materials and methodsStrains and plasmidsThe Bt SY49-1 was isolated from a soil sample collected from Adana, Turkey in 2008 using sodium acetate enriched medium.5 The strains B. thuringiensis SY49-1, Escherichia coli DH5α (kindly supplied by Middle East Technical University, METU, Molecular Biology Laboratory), cloning vector pGEMT-Easy, expression vector pET28a (+) and E. coli BL21(DE3) were used in experimental procedures. Bt and E. coli were cultured in Luria Bertani (LB, 10g/L Triptone, 5g/L yeast extract, 5g/L NaCl) medium at 30°C and 37°C, respectively.
Electron microscopyFor electron microscopy, a Bt SY49-1 spore–crystal mixture was suspended in dH2O on a microscope slide and fixed after air drying at room temperature. The sample was sputter-coated with 10nm Au/Pd using a SC7620 Mini-sputter coater and viewed using a scanning electron microscope (LEO440) at 20kV beam current.
Insect culturesThe larvae of E. kuehniella were reared on a diet containing a mixture of wheat flour, wheat bran and glycerol, and the Indian meal moth P. interpunctella larvae were obtained from naturally infested dried apricot in Kayseri province. The larvae of P. interpunctella were maintained continuously on a diet containing 10% glycerol, 50% dried apricot and 40% wheat flour–wheat bran mixture. Throughout the experiments, insect cultures were maintained at constant temperature (27±1°C), photoperiod (14L:10D) and relative humidity (60±5%).6,7
Amplification of cry1Ab gene from Bt SY49-1Total DNA of Bt SY49-1 was extracted according to the method of Bravo et al.8 and used as template for polymerase chain reactions (PCR). The cry1Ab gene was amplified using the primer pairs F-5′-GGA TCC ATG GAT AAC AAT CCG AAC ATC-‘3; R- 5′-GTC GAC TTA TTC CTC CAT AAG AAG TAA-3′.9BamHI and SalI restriction enzyme recognition sequences were added to the 5′ end of the forward and reverse primers, respectively. PCR mixes contained the reagents at a final concentration of 2.3mM MgCl2, 1× Taq buffer, 0.2mM dNTP mix, 0.3pmol of each primer, 0.5 U MaxTaq DNA polymerase (Vivantis, PL2201), and 30–100ng template DNA. The PCR amplification was performed under the following conditions: Initial denaturation at 95°C for 5min, followed by 30 cycles at 94°C for 1min, 52°C for 1min, 72°C for 3.5min, and a final extension step at 72°C for 10min.9 Adenine was added to the 3′ end of the PCR products after amplification for the TA cloning process.
Transfer of cry1Ab gene into pGEM-T Easy cloning vectorTo construct the pGEM-cry1Ab, PCR products (∼3.5kb) were purified and ligated into pGEM-T Easy vector. The ligation procedure was conducted according to the manufacturer's protocol (Promega, vector system I), and then ligate was transformed into CaCl2-rendered competent E. coli DH5α.
Expression of cry1Ab gene in E. coliBamHI and SalI-treated cry1Ab PCR amplicon and pET28a (+) vector were ligated according to the manufacturer's protocol (pET manual system). The pET-cry1Ab was transferred into E. coli BL21(DE3) cells rendered competent with CaCl2. The positive clones were selected using the PCR method and incubated at 37°C until reaching OD600=0.5–1 in 100mL of LB medium containing 30μg/mL kanamycin. The 50mL of culture was induced by 1mM IPTG for 4h and followed by centrifuging at 4°C and 5000rpm for 5min. The pellet was solubilized in 12mL of 20mM Tris–HCl (pH 7.5) and centrifuged as described above. The remaining pellet was solubilized in 5mL of 20mM Tris–Cl (pH 7.5) containing 50μL lysozyme (10mg/mL) and incubated for 15min at 30°C. The cells were then sonicated for 1min to release proteins from lysate. Subsequently, it was centrifuged at 4°C and 14,000rpm for 10min, and the pellet was resuspended in 2mL of 20mM Tris–HCl (pH 7.5) for SDS-PAGE analysis. The same procedure was applied for isolating the proteins from E. coli BL21(DE3) and Bt SY49-1. Total protein quantitation was determined according to the Bradford10 method.
Cry1Ab protein quantificationThe SDS-PAGE image of the approximately 130kDa Cry1Ab band was used for determining the quantities. Cry protein concentrations were calculated by the following formula: Cry protein concentration (μg/mL)=(μg/mL total protein)×(proportion of Cry protein to total protein).11 The proportion of Cry protein to total protein was determined using the Biorad Chemi Doc MP Imaging System Image Lab version 5.1 (Biorad).
Insecticidal activity of Cry1AbLyophilized samples of Bt SY49-1, E. coli carrying pET-cry1Ab, and plasmid-free E. coli BL21(DE3) were applied to 10 third-instar larvae of P. interpunctella and E. kuehniella at doses of 10, 25, 50, 100, 250, 500 and 1000μgg−1 supplied in the diet. Bioassay experiments on P. interpunctella and E. kuehniella were performed according to the method of Obeidat et al.12 Experiments were carried out as three replicates. The data from the experiments were subjected to analysis of variance (ANOVA), and means were separated at the 5% significance level by using the Tukey HSD post hoc test. LC50 were estimated by probit analysis.13
ResultsAmplification of cry1Ab geneThe total DNA of the Bt SY49-1 strain was screened by the PCR method using the cry1Ab-specific primer pairs for amplifying the full-length gene region (Fig. 1).
Spore–crystal mixture and electron microscopyBt SY49-1 was incubated in T3 sporulation medium at 30°C for 4 days to obtain a spore–crystal mixture. Investigations of the mixture indicated that bipyramidal crystals were compatible with the presence of cry1 gene products. Spherical, cubic and irregularly shaped crystals were also observed via electron micrograph (Fig. 2).
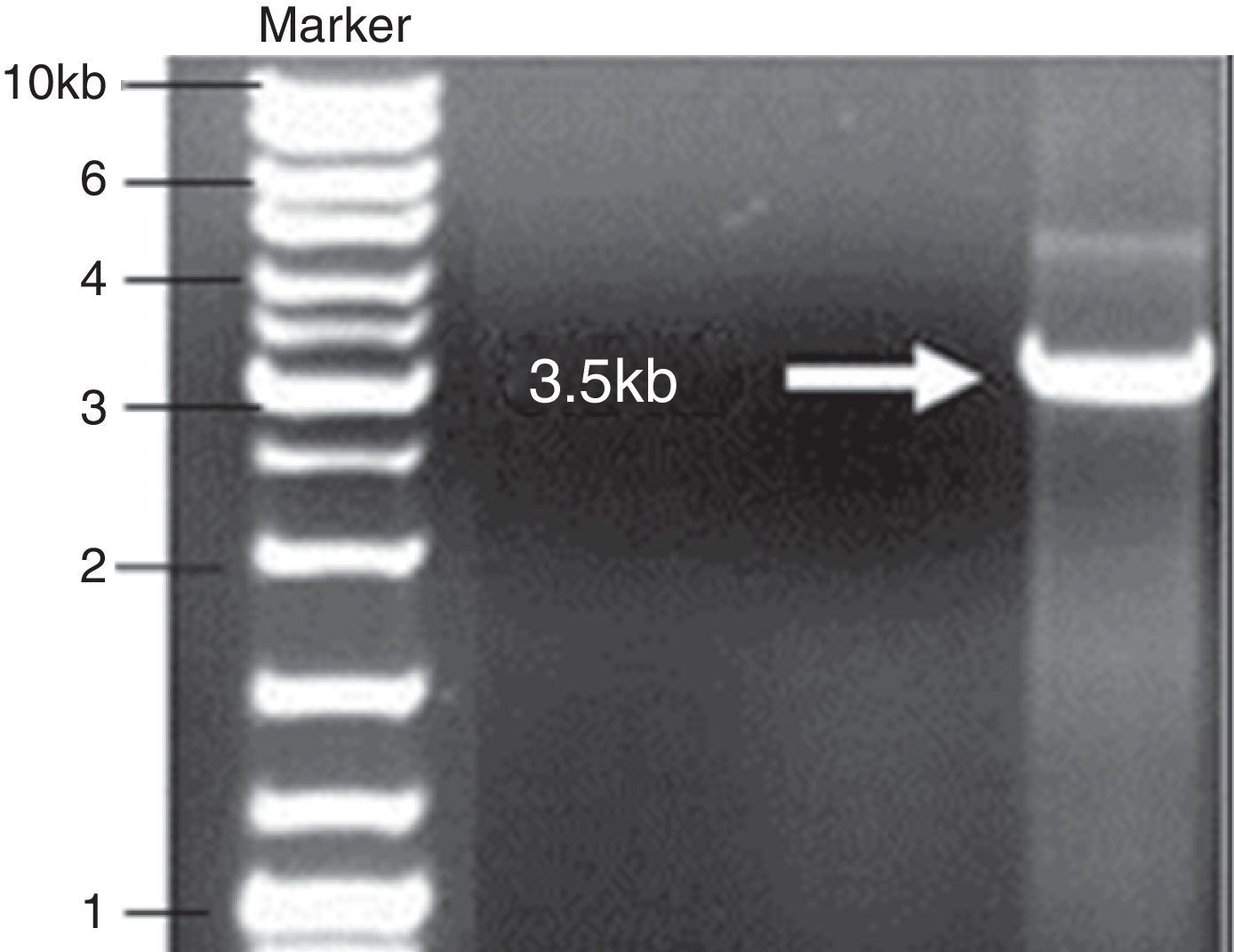
Cry1Ab gene TA cloningPCR product corresponding to the open reading frame of cry1Ab gene (∼3.5kb) was amplified and inserted into the pGEM-T Easy vector system to preserve the gene for further use. The resulting combination was transferred into E. coli DH5α. Subsequently, pGEM-cry1Ab from a positive clone of E. coli DH5α was digested with EcoRI to validate the ligation (Fig. 3).
Expression of cry1Ab in E. coli BL21(DE3)The cry1Ab gene excised with BamHI and SalI was inserted into expression vector pET28a (+) for obtaining pET-cry1Ab. The resulting pET-cry1Ab was transformed into the E. coli BL21(DE3) strain, and positive clones were validated by colony PCR (Fig. 4). A positive clone, E. coli BL21 (DE3) pET-cry1Ab, was cultured in kanamycin containing LB medium until OD600=0.5–1 was achieved and subsequently induced with IPTG. The expression of recombinant products was analyzed by SDS-PAGE, verifying the presence of a 130kDa protein band (Fig. 5).
SDS-PAGE analysis of cry1Ab gene expressed in E. coli BL21(DE3). M; Marker (Fermentas SM0661), Lane 1: IPTG-induced E. coli BL21(DE3) pET-cry1Ab; lane 2: E. coli BL21(DE3) pET-cry1Ab; lane 3: IPTG-induced E. coli BL21(DE3) pET-cry1Ab; lane 4: E. coli BL21(DE3) pET-cry1Ab; lane 5: E. coli BL21(DE3); lane 6: IPTG-induced E. coli BL21(DE3) IPTG; lane 7: B. thuringiensis SY49-1.
The protein concentration was calculated according to the following formula: Cry1Ab concentration (μg/mL)=(μg/mL total protein)×(% proportion of Cry1Ab to total protein). Here, the total protein was 7.63μg/mL, the proportion of Cry1Ab to total protein was 2.58μg/mL, and the proportion of Cry1Ab to total protein was 33.82%.
Toxicity of pET-cry1Ab, Bt SY49-1 and plasmid-free E. coli BL21(DE3)The LC50 values of recombinant Cry1Ab on third-instar larvae of E. kuehniella and P. interpunctella were found to be 685.67 and 1320.84μgg−1, respectively. The toxicity of the source strain SY49-1 was higher compared with recombinant protein (LC50=365.17μgg−1 for E. kuehniella and 582.179μgg−1 for P. interpunctella). Plasmid-free E. coli BL21(DE3) did not exert significant activity on both pest larvae. The mortality rates are supplied in Figs. 6 and 7. (Fig. 6; (A) F=7.710; df=7; P≤0.0001; (B) F=22.136; df=7; P≤0.0001; (C) F=0.635; df=7; P=0.721; Fig. 7; (A) F=4.082; df=4; P≤0.037; (B) F=8.799; df=4; P≤0.003; (C) F=0.474; df=4; P=0.754).
Bt SY49-1 is a novel strain, and the toxicity of its wettable spore–crystal powder was previously determined against insect pests from different orders.4E. kuehniella and P. interpunctella are two troublesome pests posing serious problems in stored products worldwide. In the present study, the cry1Ab gene (∼3.5kb) of a previously characterized Bt SY49-1 strain was used thorough cloning and expression in E. coli BL21(DE3). It is well known that cry1A genes have specific insecticidal activity against lepidopteran pests.14,15Bt SY49-1 produces a 130–140kDa Cry1 band corresponding to the bipyramidal crystal structure (Fig. 2). Cry1Ab (∼130kDa) was overexpressed in IPTG-induced E. coli BL21 (DE3), and its bioactivity was tested on E. kuehniella and P. interpunctella. The results indicated that recombinant Cry1Ab exhibited considerable mortality on pest larvae. However, increasing doses of Cry1Ab from 50 to 500μgg−1 had little additional toxicity on the larvae when compared with the spore–crystal mixture of source organism. Similar trends were reported with the recombinant Cry3Aa on Hypothenemus hampei.16 Zhang et al.17 also reported that the activity of recombinant cry8Ab1 has lower toxicity compared with the source organism, suggesting that this phenomenon may originate from less Cry8Ab1 expression in the host organism and more than one type of differently sized crystals being expressed in wild-type Bt. Comparatively lower toxicity was also reported by some other researchers on E. kuehniella using Cry1Aa, Cry1Ac, and Cry2Aa18 and on Chironomus tepperi using Cry11A and Cry4B.19 On the other hand, Park et al.20 reported similar toxicity to the parent strain against Culex mosquitoes with Cry11B from B. thuringiensis subsp., jegathesan. Similarly, Bt SY49-1 harbors cry1, cry2, cry4, cry5 and cry9 genes, and its higher activity may possibly result from the combined activity of these gene-corresponding products. Therefore, this study was intended to determine the effectiveness of Cry1Ab, independent of other genes encoding insecticidal proteins.
In the present study, the individual toxicity of Cry1Ab (expressed in E. coli) was evaluated on E. kuehniella and P. interpunctella to avoid potential synergistic interactions between spores and crystals.21 As far as we know, this is the first report evaluating the toxicity of E. coli BL21(DE3) pET-cry1Ab on E. kuehniella and P. interpunctella. Significant differences were not observed with respect to toxicity between E. coli BL21 (DE3) and watery control. The individual toxicity of recombinant Cry1Ab on the third-instar larvae of these two stored product pests was precisely estimated.
In conclusion, the cry1Ab gene of the Bt SY49-1 strain was successfully cloned, expressed and tested on E. kuehniella and P. interpuctella larvae. The results indicated that cry1Ab may be considered as a good source in transgenic crop production and as an alternative biocontrol agent in controlling stored product moths.
Ethical statementThe authors declare that the experiments complied with the current laws of the country in which they were performed.
Conflict of interestThe authors declare that they have no conflict of interest.
The authors thank Dr. Mikail Akbulut and Zehra Büşra Atciyurt for their valuable assistance. Special thanks to METU Molecular Biology Laboratory for supplying E. coli DH5α. This project was funded by the Erciyes University Scientific Project Unit under the codes of FBD11-3634 and ÖNAP-3638 and also funded by Bilim, Sanayi ve Teknoloji Bakanlığı Türkiye (TGSD-0802).