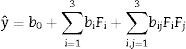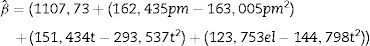A new strain of Thermomyces lanuginosus was isolated from the Atlantic Forest biome, and its β-xylosidases optimization in response to agro-industrial residues was performed. Using statistical approach as a strategy for optimization, the induction of β-xylosidases activity was evaluated in residual corn straw, and improved so that the optimum condition achieved high β-xylosidases activities 1003U/mL. According our known, this study is the first to show so high levels of β-xylosidases activities induction. In addition, the application of an experimental design with this microorganism to induce β-xylosidases has not been reported until the present work. The optimal conditions for the crude enzyme extract were pH 5.5 and 60°C showing better thermostability at 55°C. The saccharification ability of β-xylosidase in the presence of hemicellulose obtained from corn straw raw and xylan from beechwood substrates showed a xylo-oligosaccharide to xylose conversion yield of 80 and 50%, respectively, at 50°C. Our data strongly indicated that the β-xylosidases activities was not subjected to the effects of potential enzyme inhibitors often produced during fermentation process. These data suggest the application of this enzyme studied for saccharification of hemicellulose, an abundant residue in the American continents, thus providing an interesting alternative for future tests for energy production.
Thermomyces lanuginosus, previously known as Humicola lanuginosa, is a thermophilic fungus widely distributed and frequently isolated from organic waste.1 Several strains of Themomyces lanuginosus have been shown to be potential producers of enzymes with different applications.2–4 In general, microbial enzymes have received attention due to being precursors to clean technology for the production of industrial and commercially important compounds using residual biomass as substrates.
The biomass present in agro-industrial residues is composed of hemicellulose, and the enzymatic depolymerization of its structure is commercially interesting due to its soft condition and no formation of toxic compounds during the degradation of the products. The complete deconstruction of the hemicellulose structure requires the synergistic action of several enzymes, including xylanases (EC 3.2.1.8) and β-xylosidases (EC 3.2.1.37). β-Xylosidase cleaves β-1,4-xylo-oligosaccharides released by xylanases producing xylose, a monosaccharide that can be used by different microorganisms in the process of saccharification and production compounds of the biotechnological application.1,5
Currently, market analyses indicate that global maize production is abundant, and the US is the largest producer of the crop followed by Argentina and Brazil totaling approximately 53.2 million tons per harvest. Corn is mainly used for animal feed production but is also used to a lesser extent for the production of oils, flours and breakfast cereals. Thus, the residual biomass of the crop, such as straw and corncobs, should be considered for the production of energy and other products of biotechnological interest.1,2
Brazilian indices related to corn crops show that only 5% of production is intended for human consumption. The residues from the processing of this crop make up 58% of the produced biomass, and this portion is still neglected and could alternatively contribute as a source of renewable energy sources to generate wealth and to minimize the accumulation of residues that can be harmful to the environment.1,2
In the present report, an isolate from the Brazilian Atlantic Forest biome was optimized for the production total of β-xylosidases. The optimized enzyme crude extract was used for testing the saccharification of xylan from beechwood and hemicellulose derived from corn straw emphasizing the potential biotechnological role for the enzyme.
Materials and methodsIsolation, identification and maintenance of cultureThe fungus cataloged under the ID of PC-7S-1-T was isolated from a soil sample obtained from the Atlantic Forest biome of the Beautiful View Refuge (latitude 24°55′16″ S and longitude 53°54′35″ W) Foz do Iguaçu, Paraná, Brazil (collection authorized by Itaipu Bi-national). The isolate was subjected to morphological identification followed by molecular identification by nucleotide sequence analysis of the ITS region of DNA corresponding to the rRNA of the microorganism. This analysis was performed at the Laboratory of Molecular Biochemistry at the State University of West Paraná (Brazil) by extracting the total DNA of the fungus followed by replication of the target amplicon using specific primers. The amplicons obtained were sequenced by the sequencing service provided by HELIXXA (Sequencing Service, Brazil), and the sequences obtained were analyzed using sequence alignment tools (Blast-n from the National Center for Biotechnology Information; NCBI).
The generated sequence was deposited in GenBank under the access number KJ934703.1. In both analyses used to identify the fungus, the isolate was classified as belonging to the T. lanuginosus species. The microorganism was grown at 42°C for 7 days in tubes containing solid PDA medium (20% potato infusion (w/v), 1.5% dextrose (w/v) and 1.5% agar (w/v)) and was subsequently stored under refrigeration at 4°C with periodic maintenance every 30 days.
Preparation of agro-industrial residues and plant biomassThe following carbon sources tested for use in the experiments of fungal growth and induction of β-xylosidases enzyme activities were selected according to availability: corn straw, orange peels, banana peels and passion fruit peels. The plant biomass residues were dried at 70°C for 24h followed by trituration (SL30 slicer Willey) using a 20 mesh sieve, and the residues were then stored in glass vials at room temperature.
Cultivation conditions of T. lanuginosusNewly grown fungal conidia were used to prepare a suspension (1×105conidiamL−1) in sterile water, which was then inoculated into 25mL of mineral medium (distilled water, 0.3g of NaNO3, 0.1g of K2HPO4, 0.05g of MgSO4·7H2O, 0.05g of KCl, 0.001g of FeSO4·7H2O, and 0.1g of yeast extract; pH 6.0) supplemented with 1% (w/v) different selected carbon sources. The submerged fermentation (SMF) cultivations were performed two different ways as follows: stationary liquid and agitated liquid (150rpm) at 42°C for 7 days in biological duplicates. The carbon source most effective in inducing the highest enzyme activity was used for further optimization experiments.
The enzyme production was monitored, by collecting the mycelial cells of T. lanuginosus by vacuum filtration of the culture to sterile Whatman paper. The obtained mycelial cells were frozen, and they were then macerated with 1g of glass beads, resuspended in 5mL of ice-cold distilled water and centrifuged at 8000×g and 4°C for 5min. The supernatant was collected and used in the analytical tests.
Determination of fungal biomassThe samples used for the determination of biomass were filtered through 5μm filter paper (Whatman®), dried for 48h at 105°C and weighed on an analytical balance. The assay for measure the fungal biomass was conducted using a control for determination of biomass. In the control flask was added the residue in the absence of inoculum. The biomass was quantified by the difference of weight obtained in the test experiments containing residue and fungus, and, the control containing only the residue.
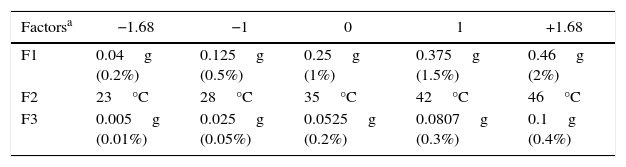
The optimal parameters determined for growth of the fungus under different conditions were applied to the experimental design based on the following three factors: concentration of the residue as a carbon source (corn straw), temperature and concentration of the nitrogen source (yeast extract) during 5 days of agitation at 150rpm in liquid culture. The 23 experimental matrix consisted of three central points and six axial points where the two factors combined with three levels totaling 17 tests. We selected the combination of 0.25g of corn straw with 0.0525g of yeast extract as a central point as this was defined as the optimal condition for β-xylosidases activities in preliminary tests.
The values were then added to the central point of the design, and the influence of temperatures between 23 and 46°C was also included in the tests (Table 1). Statistical analysis was performed to obtain the linear effects, and the model was adjusted according to Eq. (1) to describe the response surface.
Levels of factors used in CCRD.
| Factorsa | −1.68 | −1 | 0 | 1 | +1.68 |
|---|---|---|---|---|---|
| F1 | 0.04g (0.2%) | 0.125g (0.5%) | 0.25g (1%) | 0.375g (1.5%) | 0.46g (2%) |
| F2 | 23°C | 28°C | 35°C | 42°C | 46°C |
| F3 | 0.005g (0.01%) | 0.025g (0.05%) | 0.0525g (0.2%) | 0.0807g (0.3%) | 0.1g (0.4%) |
It was used Derringer's6 desirability function a criterion decision-making method proposed by Derringer and Suich in 1980. This is the most current and frequently used technology for response optimization. The procedure involves the transformation of individual response to a desirability function (di), defined as a dimensionless partial desirability function that varies from 0 (considered a completely undesirable response) to 1 (considered a fully desired response).
β-Xylosidases activities and protein determinationThe β-xylosidase activity was determined using 0.5mL of p-nitrophenyl-β-d-xylo-pyranoside (pNPX) at a concentration of 10mM in 50mM sodium phosphate buffer (pH 6.0) with 0.05mL of the crude enzyme extract (with or without dilution), which was incubated for a period of 10–60min at 50°C in a water bath. The reaction was stopped with a saturated solution of sodium tetraborate. The samples were analyzed by spectrophotometer reading at λ 410nm using p-nitrophenol (0–0.6μmol/mL) for the standard curve. One unit of enzyme activity was defined as the amount of enzyme capable of releasing one μmol of p-nitrophenol per minute of reaction. The protein concentration was determined by the Bradford method using bovine serum albumin as the standard.
The enzymatic activity was expressed in U/mL of the homogenate or in specific enzymatic activity (U/mg) that correspond to the enzymatic activity obtained by mg of the total protein produced in the cell.
Influence of the test conditions on the production of β-xylosidaseAfter growth of T. lanuginosus cells in different test conditions, it was possible to define parameters to be considered in the experimental design as well as the carbon source, type of crop and the optimal production time for β-xylosidases activities.
Influence of pH and temperature on intracellular β-xylosidases activitiesThe intracellular β-xylosidase activity was analyzed for variations in different pH conditions (McIlvaine buffer pH 5–7.5). To analyze the effect of temperature, the optimum pH was maintained, and the crude extract containing the enzyme was incubated at temperatures ranging from 50 to 70°C in increments of 5°. The conditions for optimum pH and temperature were used for analysis of thermostability in the 3 optimal temperature conditions displayed.
Pretreatment for hemicellulose extraction from corn strawSamples of corn straw were placed in sealed tubes containing 0.35g of fresh corn straw with 10mL of deionized water, and the samples were placed in a block digester for 1h at 200°C. The samples were then immediately placed in an ice bath. After separation of the solid from the liquid phase, the liquid portion containing the hemicellulose was precipitated with absolute ethanol (three-fold the volume) for 48h. The obtained precipitate was recovered by filtration and then dried at 37°C for a period of 3 days.
Enzymatic hydrolysis of xylose and effect on β-xylosidases activitiesThe β-xylosidases from T. lanuginosus was evaluated for its ability to hydrolyze hemicellulose from corn straw and xylan from beechwood (both 1%, w/v) in treatments with and without pre-hydrolysis with crude extract containing high extracellular xylanase activity of the same T. lanuginosus strain subjected to the same conditions of growth and cultivation. The optimum condition indicated by the CCRD experiments was defined as the standard to grow T. lanuginosus and to obtain optimal β-xylosidases activities. In this situation, extracellular β-xylosidases activities were not present. Intracellular β-xylosidases and extracellular xylanase were incubated at two different temperatures (37 and 50°C) in the presence of 1% hemicellulose from corn straw (w/v) and 1% xylan from beechwood (w/v). During all experiments, the reducing sugars were measured by the DNS standard dosage method after the β-xylosidase activity was measured. As controlling of the hydrolysis experiment, two different assays were conducted under the follow conditions: 1 – incubation of DNS and substrates (hemicellulose from corn straw/xylan) without enzyme, and 2 – incubation of DNS containing the crude extract of enzymes without addition of hemicellulose from corn straw or xylan. Reducing sugars present in both the xylan from beechwood and hemicellulose from corn straw were measured before starting the hydrolysis, and the values obtained were used to present normalized results. Samples obtained after the enzymatic saccharification were subjected to thin layer chromatography to evaluate the conversion of the xylo-oligosaccharides to xylose by T. lanuginosus β-xylosidase. In parallel, the residual xylose was determined in the hemicellulose samples at 12h using the Megazyme® kit. The influence of xylose on β-xylosidases activities was also investigated by incubating the crude intracellular extract containing β-xylosidases with xylose at the levels detected in the saccharification process.
TLC silica product of the hydrolysisAnalysis of hydrolysis products formed from the combined action of the enzymes, namely xylanase (extracellular) and β-xylosidases (intracellular crude extract), of T. lanuginosus was performed by ascending thin layer chromatography on 10cm×10cm silica (Alufolien DC-Kieselgel 60 without fluorescent indicator, Merck®). The hydrolysis products were collected at both temperatures and at different times. The products were then boiled for 10min and frozen. The xylose standard (Sigma®) (2mg/mL; 5μL) and xylobiose/xylotriose standard (Megazime®) (3mg/mL; 4μL) (Sigma®) and each sample (4μL) were added to the silica. The plate was resolved twice with a mixture of butanol, pyridine and distilled water (7:3:1, v/v/v). Disclosure of the hydrolysis products was performed using a solution of 0.3% orcinol (w/v) sulfuric acid in methanol (1:9). Subsequently, the plate was placed in an oven at 100°C until the xylo-oligosaccharide degradation products were generated.
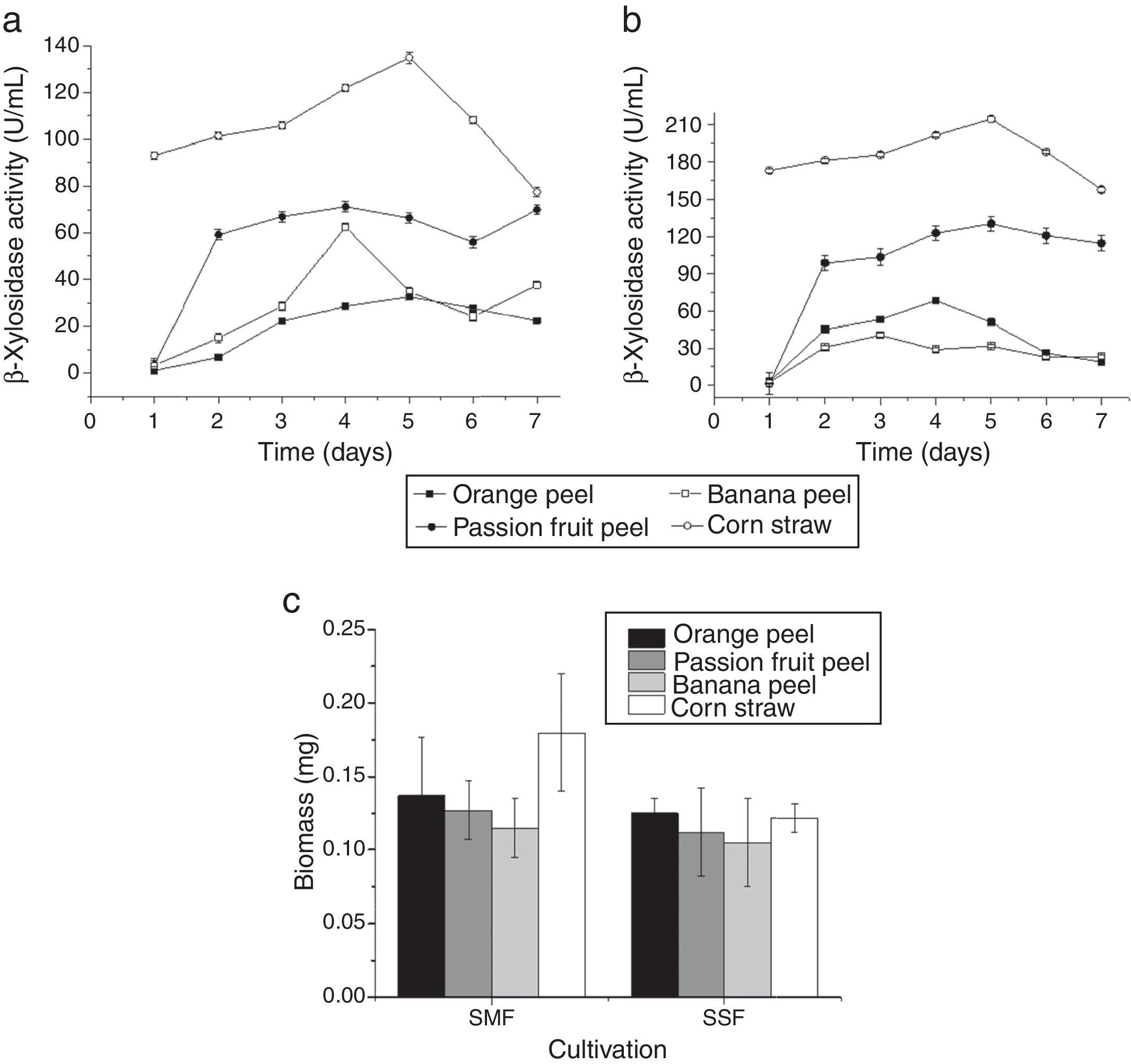
ResultsDetermination of optimal conditions for β-xylosidases productionThe T. lanuginosus fungus was grown under different conditions (type of residue, cultivation and incubation time) to determine the optimal conditions for the production of β-xylosidases with high activity. The plant biomass residue that led to higher enzymatic activity of T. lanuginosus was 1% corn straw (w/v) as compared to the other residues, namely 1% orange peel (w/v), 1% passion fruit peel (w/v) and 1% banana peel (w/v). With both steady cultivation and agitated cultivation, corn straw was the highest inducer of intracellular β-xylosidases leading to a maximum production of 214U/mL after 5 days of cultivation under shaking (Fig. 1A and B) The agitated cultivation using corn straw was also the most effective for the production of mycelial biomass (Fig. 1C).
(A) β-xylosidases activities in the crude extract of Thermomyces lanuginosus after 7 days of cultivation in mineral medium supplemented with 1% of various agro-industrial residues as a carbon source at 42°C and at stationary conditions. (B) Production of the intracellular β-xylosidases enzyme of T. lanuginosus during 7 days in culture containing mineral medium supplemented with 1% of agro-industrial residues as a carbon source at 42°C and agitation at 150rpm. (C) Evaluation of fungal growth on different carbon sources and with different cultivation conditions.
The parameters for enzyme production with the optimal conditions were applied to the CCRD experimental design as a way to further improve the yields of enzymatic activity. The experimental design was based on three different factors as follows: concentration of residue as a carbon source (corn straw), temperature and concentration of yeast extract (nitrogen source) during 5 days in SMF culture under agitation at 150rpm. The 23 experimental matrix consisted of three central points and six axial points where the two factors combined with three levels totaling 17 tests. We selected the combination of 0.25g of corn straw with 0.0525g of yeast extract as a central point as this was the optimum condition found for the activity of intracellular β-xylosidases in preliminary tests (Fig. 1). Therefore, these values were added to the central point of the design. The effect of temperature was also combined in the tests using temperatures between 23 and 46°C due to the thermophilic condition shown by the isolated strain (Table 1).
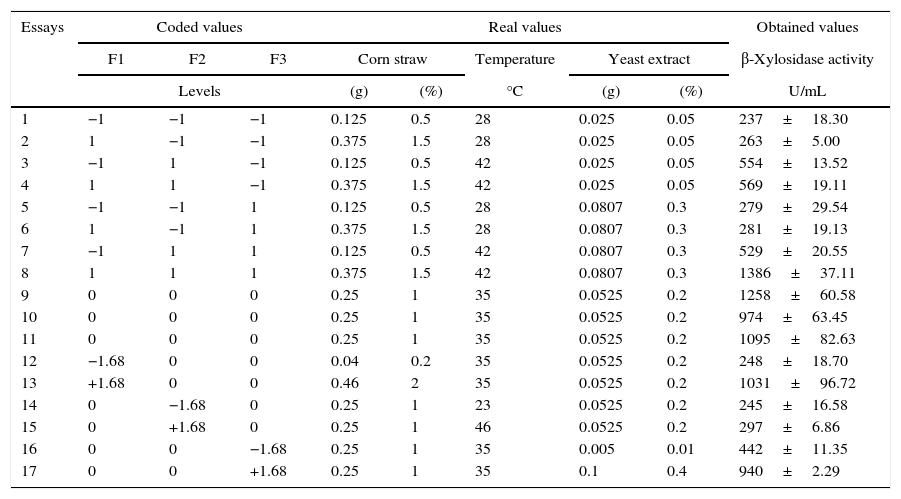
Table 2 shows the coded and real values of the experimental design performed with three center points and six axial points totaling 17 tests. The values of β-xylosidases activities are presented individually according to each assay including the corresponding standard error.
Matrix of design (CCRD) with the coded, real given values and values obtained in the experiments (residue concentration, temperature and concentration of the yeast extract).
| Essays | Coded values | Real values | Obtained values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | Corn straw | Temperature | Yeast extract | β-Xylosidase activity | |||
| Levels | (g) | (%) | °C | (g) | (%) | U/mL | |||
| 1 | −1 | −1 | −1 | 0.125 | 0.5 | 28 | 0.025 | 0.05 | 237±18.30 |
| 2 | 1 | −1 | −1 | 0.375 | 1.5 | 28 | 0.025 | 0.05 | 263±5.00 |
| 3 | −1 | 1 | −1 | 0.125 | 0.5 | 42 | 0.025 | 0.05 | 554±13.52 |
| 4 | 1 | 1 | −1 | 0.375 | 1.5 | 42 | 0.025 | 0.05 | 569±19.11 |
| 5 | −1 | −1 | 1 | 0.125 | 0.5 | 28 | 0.0807 | 0.3 | 279±29.54 |
| 6 | 1 | −1 | 1 | 0.375 | 1.5 | 28 | 0.0807 | 0.3 | 281±19.13 |
| 7 | −1 | 1 | 1 | 0.125 | 0.5 | 42 | 0.0807 | 0.3 | 529±20.55 |
| 8 | 1 | 1 | 1 | 0.375 | 1.5 | 42 | 0.0807 | 0.3 | 1386±37.11 |
| 9 | 0 | 0 | 0 | 0.25 | 1 | 35 | 0.0525 | 0.2 | 1258±60.58 |
| 10 | 0 | 0 | 0 | 0.25 | 1 | 35 | 0.0525 | 0.2 | 974±63.45 |
| 11 | 0 | 0 | 0 | 0.25 | 1 | 35 | 0.0525 | 0.2 | 1095±82.63 |
| 12 | −1.68 | 0 | 0 | 0.04 | 0.2 | 35 | 0.0525 | 0.2 | 248±18.70 |
| 13 | +1.68 | 0 | 0 | 0.46 | 2 | 35 | 0.0525 | 0.2 | 1031±96.72 |
| 14 | 0 | −1.68 | 0 | 0.25 | 1 | 23 | 0.0525 | 0.2 | 245±16.58 |
| 15 | 0 | +1.68 | 0 | 0.25 | 1 | 46 | 0.0525 | 0.2 | 297±6.86 |
| 16 | 0 | 0 | −1.68 | 0.25 | 1 | 35 | 0.005 | 0.01 | 442±11.35 |
| 17 | 0 | 0 | +1.68 | 0.25 | 1 | 35 | 0.1 | 0.4 | 940±2.29 |
The estimated effects of the variables evaluated were positive in this analysis as they demonstrated that the temperature, corn straw and yeast extract led to an increase in enzyme activity of 302, 324 and 247U/mL, respectively. Analyses of variables generated a mathematical model of the second order (Eq. (2)) where pm is corn straw, t is temperature and el is yeast extract.
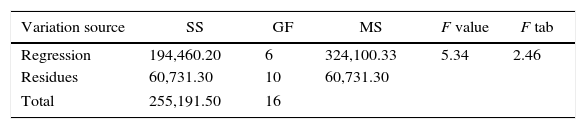
The summary of the analysis of variance (ANOVA) of the experimental design is presented in terms significant to 10% of probability (p<0.10) in Table 3. The coefficient of determination (R2) was 0.86, and the F-test showed that the model is appropriate to predict the results via response surface, thus confirming the mathematical model presented in Eq. (2). Fig. 2 shows the behavior of the design data with the three variables tested, thus indicating the preferred optimization.
Summary of ANOVA of the 2nd order mathematical model for the production of β-xylosidase.
| Variation source | SS | GF | MS | F value | F tab |
|---|---|---|---|---|---|
| Regression | 194,460.20 | 6 | 324,100.33 | 5.34 | 2.46 |
| Residues | 60,731.30 | 10 | 60,731.30 | ||
| Total | 255,191.50 | 16 | |||
SS, sum of the squares; GF, degrees of freedom; MS, mean squares; F value, calculated F value; F tab, tabulated F value; R2=0.86; p-value<0.10.
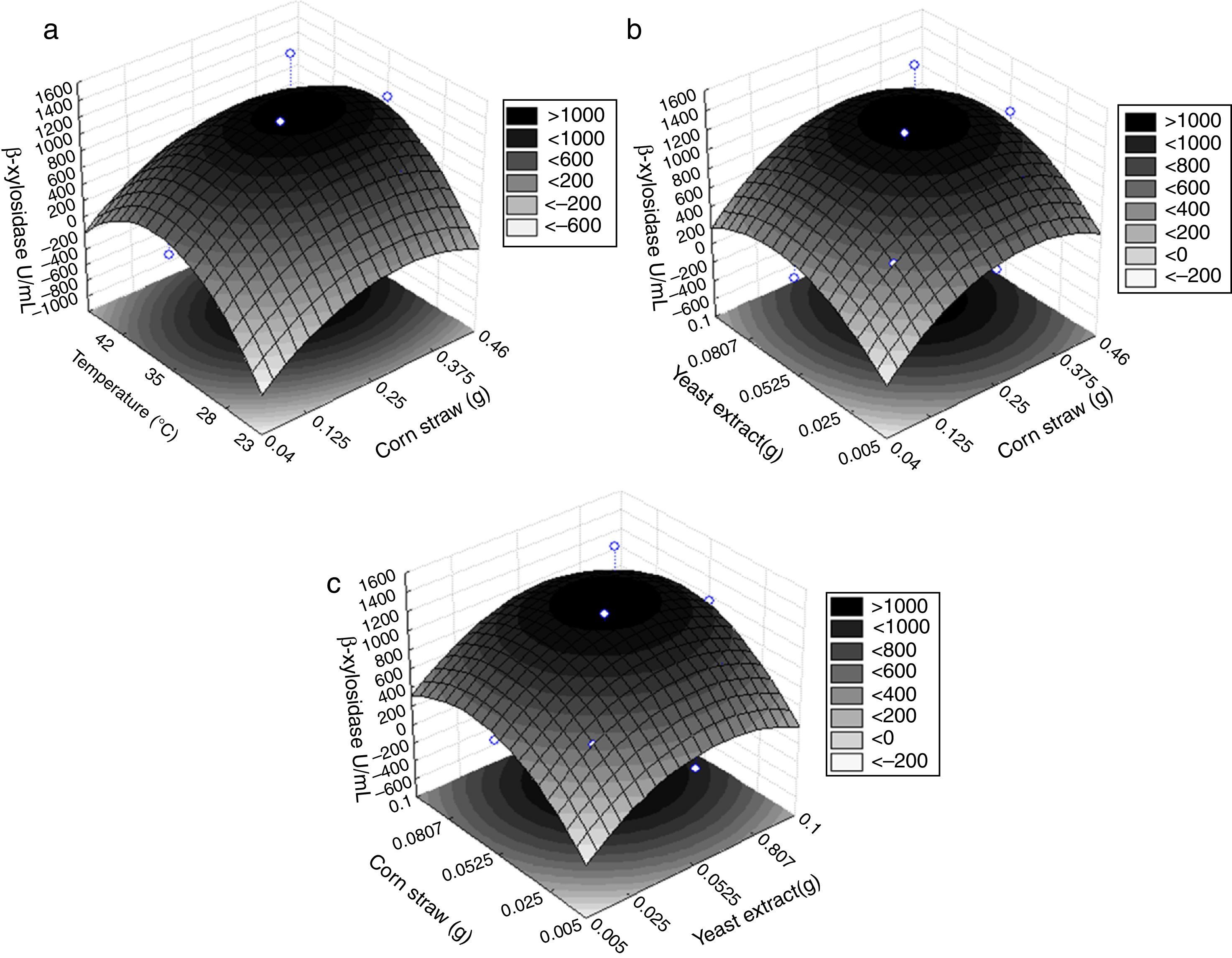
Response surfaces generated by analysis of variance for the production of β-xylosidases in terms of (A) temperature and residual corn straw, (B) yeast extract and corn straw and (C) yeast extract and corn straw. The darkest portion of the graph shows higher levels of enzyme activity.
The surface responses represent the mathematical model that enables the verification of the combination of three factors analyzed in the experiment, the influence of each one and the maximum enzyme activity of β-xylosidase. Conditions of increased production of β-xylosidase were obtained at concentrations that were close to the central point condition for the three factors studied. However, contour curves were present indicating slightly lower values of the center point for all three variables, thus leading to the same responses.
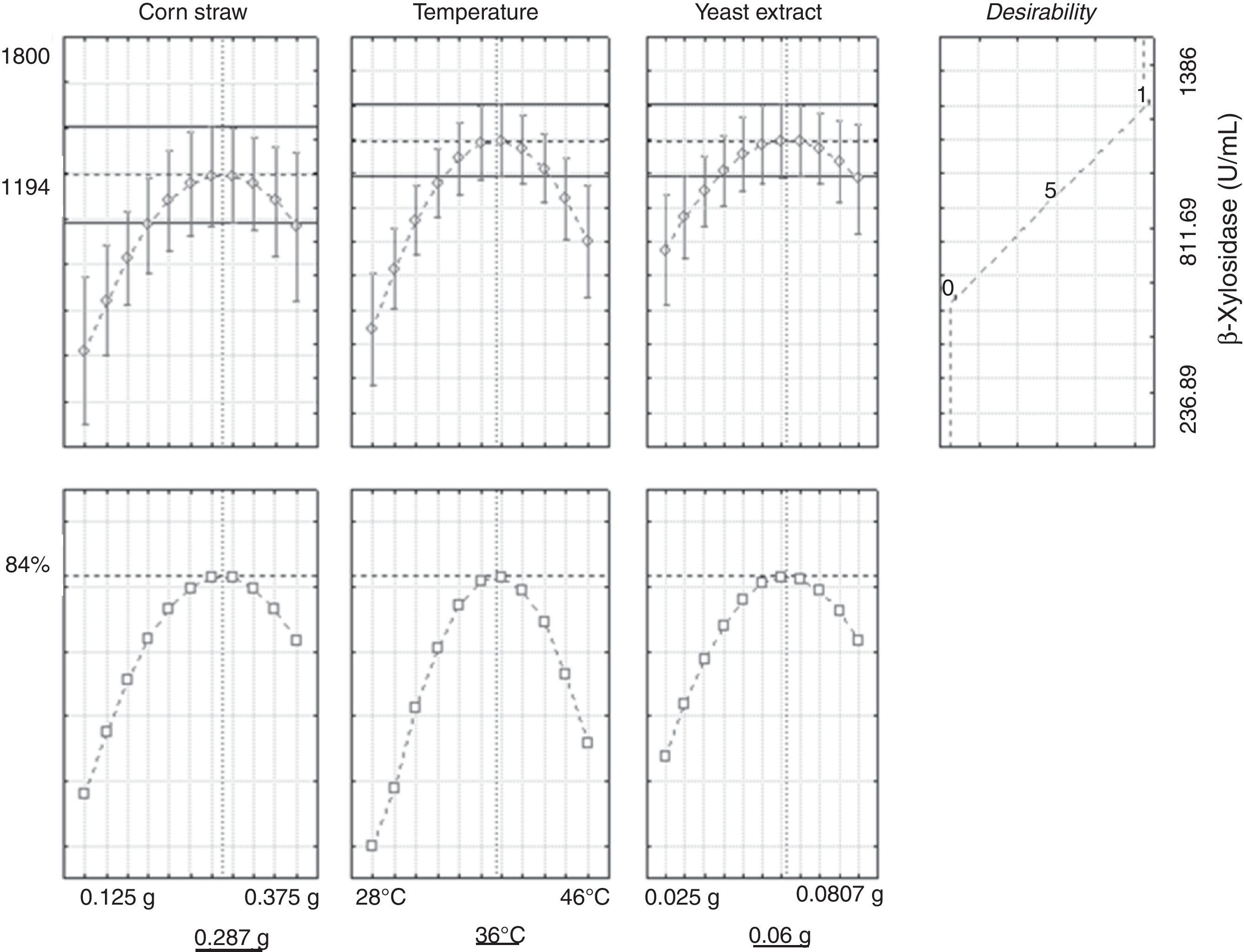
Optimization of the conditions that maximize enzymatic production of β-xylosidase requires analysis of individual behavioral responses in acceptable regions. The desirability conditions (Fig. 3) showed that the combination of factors that achieved the highest production of β-xylosidase must contain 0.287g of corn straw with 0.06g of yeast extract at a temperature of 36°C. The profile of the predicted values and desirability of the model showed that it is possible to obtain a maximum of 1194U/mL of β-xylosidase enzyme with an expected yield of 84%.
Validation of optimized experimental conditionsThe conditions determined for desirability of the experiment were validated after performing 6 repetitions with duplicate biological and enzymatic dosages for the extracts obtained from mycelia. The cultivations were performed in liquid medium with shaking (150rpm) for 5 days at 36°C as indicated by the desirability settings (Fig. 3). The intracellular crude enzymatic extract and dosages of β-xylosidase activity followed the protocol in “Materials and methods” section. The average enzymatic activity obtained in the tests was 1003U/mL, and the specific activity was 1683U/mg. Initially, the experiments without application of the experimental design had a maximum β-xylosidase activity of 214U/mL. However, optimization achieved almost 500% more than the initial values with a 4.7-fold increase in β-xylosidase activity.
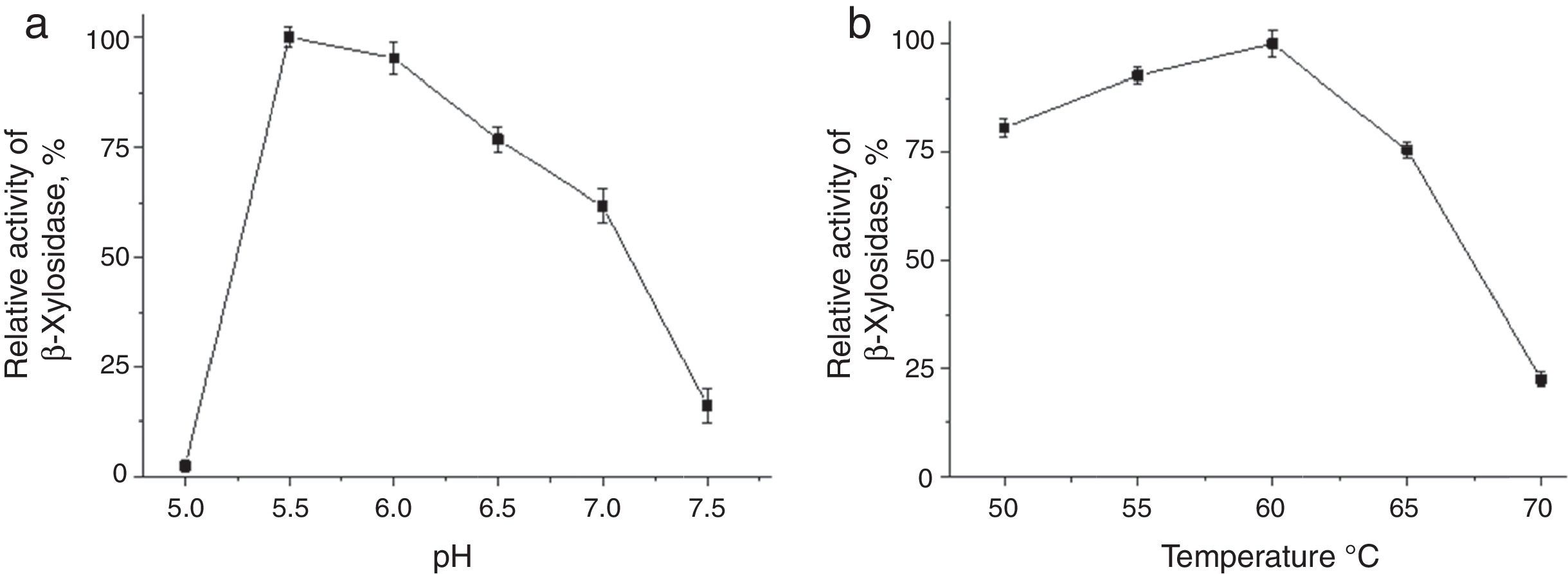
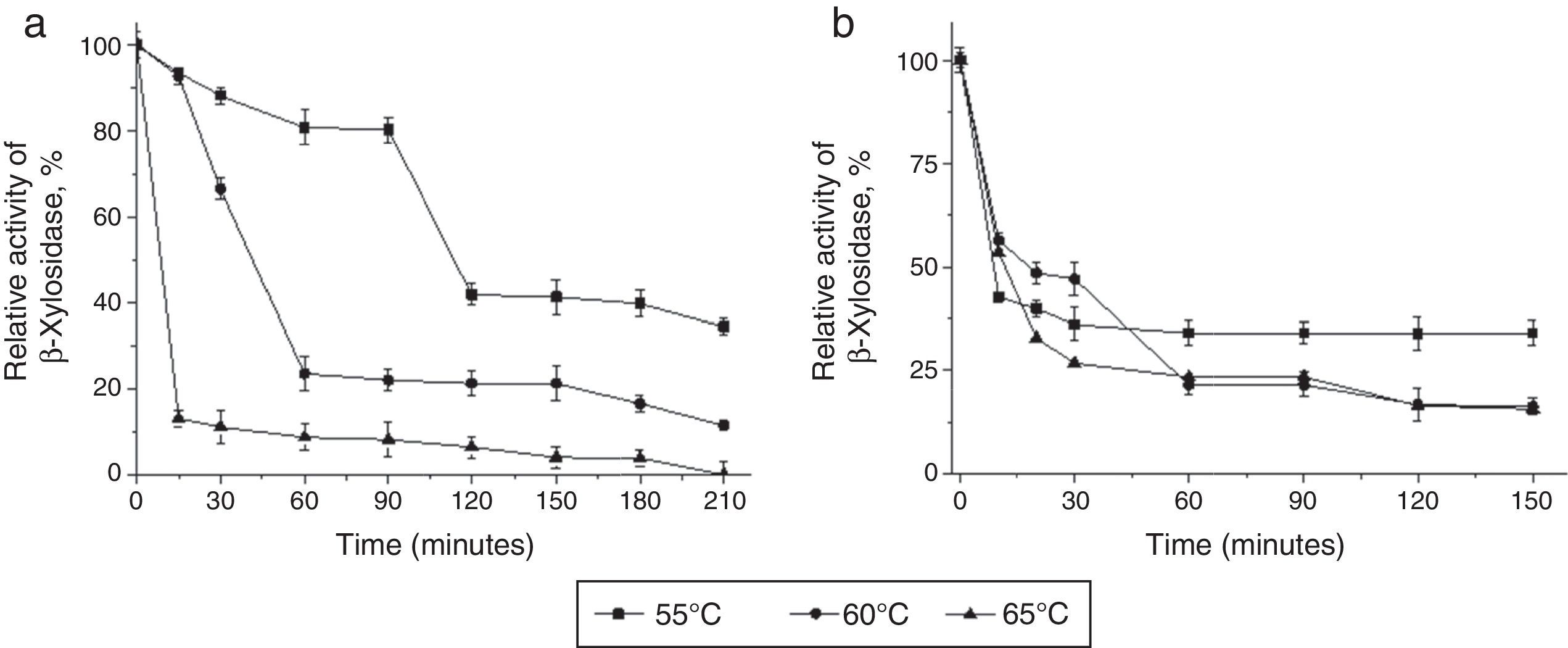
The β-xylosidase activity in the crude extract was subjected to a partial characterizationThe β-xylosidase activity in the crude extract was subjected to a partial characterization. The test conditions at different pH values (McIlvaine buffer) indicated that the optimal pH value was 5.5 (Fig. 4A) at 60°C (Fig. 4B). In this study, the intracellular β-xylosidase of T. lanuginosus was more thermostable at 55°C and pH 7 (Fig. 5A) retaining 80% activity for 90min. However, the thermostability of β-xylosidase was low at the optimum pH as there was a significant decrease in enzyme activity after 30min of incubation, but the activity remained stable thereafter at the three tested temperatures (Fig. 5B). In the same extract were quantified the β-glucosidase activity at 4.9U/mL, α-l-arabinofuranosidase 2.6U/mL and Xylanase 35.59U/mL.
(A) Effect of pH on the activity of intracellular β-xylosidase of T. lanuginosus incubated in McIlvaine buffer (pH 5.0–7.5) followed by determination of β-xylosidase using ρNPX as a substrate. (B) Effect of temperature on the activity of intracellular β-xylosidase of T. lanuginosus incubated in McIlvaine buffer (pH 5.5) during 10min at temperatures of 50–70°C with subsequent standard determination of β-xylosidase.
Thermostability profile of the intracellular T. lanuginosus β-xylosidase activity. (A) Thermostability displayed in the top three temperatures of 55°C, 60°C and 65°C during 210min of incubation time. (B) The enzyme stability at pH 5.5 at temperatures of 55°C, 60°C and 65°C during 150min.
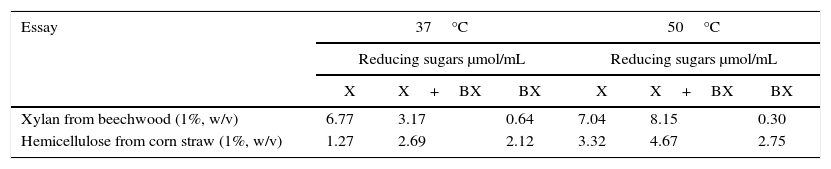
The intracellular T. lanuginosus β-xylosidase was subjected to a test for enzymatic saccharification at two temperatures of 37 and 50°C with and without pre-hydrolysis with the filtrate containing high extracellular xylanase activity and total absence of β-xylosidase activity obtained from T. lanuginosus. Each crude enzyme (2U/mL), including intracellular β-xylosidase and extracellular xylanase, was incubated with the following two different substrates: 1% xylan from beechwood (w/v) and 1% hemicellulose from corn straw (w/v). In the hydrolysis performed at two temperatures in the presence of xylan from beechwood, the enzymes had similar behavior when they were tested individually or in combination (Table 4). However, in the presence of hemicellulose from corn straw and in assays containing xylanase previously added, the β-xylosidases of T. lanuginosus was more efficient in promoting saccharification. These results suggest a combined action of both enzymes, and indicate an important role for the deconstruction structure of hemicellulose from vegetable biomasses derived from residues (Table 4).
Production of reducing sugars in the enzymatic hydrolysis by action of xylanase (X) or β-xylosidase (BX) tested individually or in combination (X+BX).
| Essay | 37°C | 50°C | ||||
|---|---|---|---|---|---|---|
| Reducing sugars μmol/mL | Reducing sugars μmol/mL | |||||
| X | X+BX | BX | X | X+BX | BX | |
| Xylan from beechwood (1%, w/v) | 6.77 | 3.17 | 0.64 | 7.04 | 8.15 | 0.30 |
| Hemicellulose from corn straw (1%, w/v) | 1.27 | 2.69 | 2.12 | 3.32 | 4.67 | 2.75 |
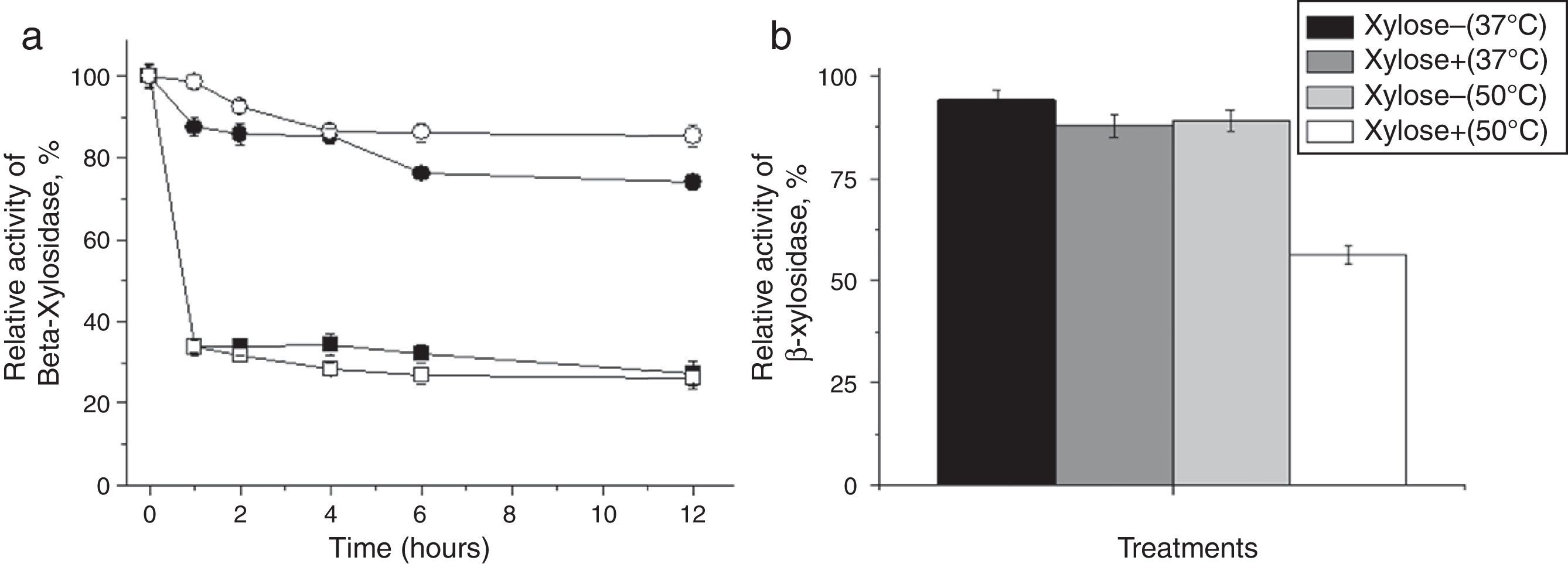
The yield of the reducing sugars generated from the individual action of β-xylosidase in the assay demonstrated the high conversion capacity of the enzyme because the hydrolysis at 50°C was 80% compared to saccharification performed only by xylanase in hemicellulose derived from corn straw (Table 4). A slightly lower conversion was found using xylan as a substrate at the same temperature where the efficiency of β-xylosidase was only 50% higher than that of the individual action of the xylanase enzyme. The enzymatic activity of β-xylosidase was monitored during the entire saccharification process and showed a constancy maintaining approximately 80% of its activity over time in the presence of the test hemicellulose substrate from corn straw (Fig. 6A).
Performance T. lanuginosus β-xylosidases activities during saccharification. Relative activity after hydrolysis by intracellular β-xylosidase of T. lanuginosus for 12h in xylan from beechwood (square) (1%, w/v) and hemicellulose from corn straw (circle) (1%, w/v) at 37°C (closed circle and closed square) and 50°C (open circle and open square). Relative activity exhibited by T. lanuginosus β-xylosidase in the absence and presence of 200mM xylose after 12h at 37°C and 50°C.
The yield of β-xylosidase conversion into xylo-oligosaccharides was represented by the amount of total reducing sugars which indicated the ability to generate 0.64 and 0.30μmol/mL from xylan and between 2.12 and 2.75μmol/mL from hemicellulose from corn straw in the two test conditions of 37 and 50°C, respectively. The xylose content was also measured using the d-xylose Assay Kit (Megazyme®) which indicated a production of 207μmol/mL of xylose at 37°C and 205μmol/mL of xylose at 50°C in 24h of testing. The amount of xylose produced is shown in Fig. 7.
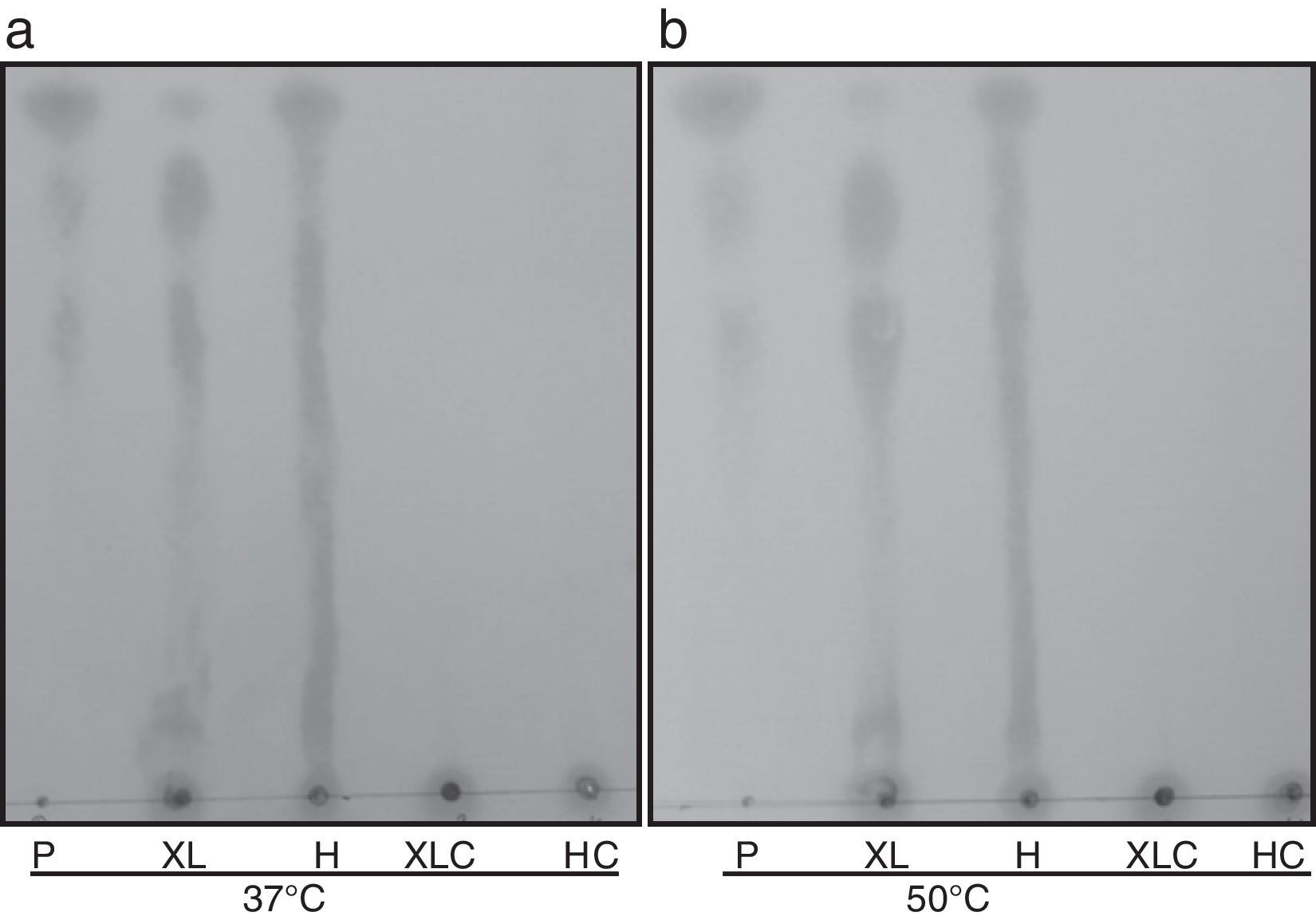
Analysis of thin layer chromatography. TLC of the hydrolysis of T. lanuginosus β-xylosidase products obtained after 12h of incubation with xylan from beechwood (1%, w/v) and hemicellulose from corn straw (1%, w/v) at 37°C (A) and 50°C (B). P, xylose, xylobiose and xylotriose; XL, xylan from beechwood (1%, w/v); H, hemicellulose from corn straw (1%, w/v); XLC, xylan from beechwood (1%, w/v) control; HC, hemicellulose from corn straw (1%, w/v) control.
As a parameter determining the levels of xylose detected during saccharification of hemicellulose from corn straw with β-xylosidase of T. lanuginosus, an experiment was conducted to investigate the behavior of enzyme activity in the absence and presence of 200mM of xylose even without the presence of another substrate at both temperatures used in the hydrolysis (37 and 50°C) for 12h. This assay showed that the β-xylosidase activity at 37°C remained at approximately 85% of the original activity at 50°C and that the enzyme activity remained slightly more than 56% of the enzyme activity when compared with the total absence of xylose (Fig. 6B). Importantly, in the actual test conditions of saccharification, the hydrolysis products of β-xylosidase are gradually released into the reaction medium from the natural substrate, either xylan or hemicellulose from corn straw. However, when trying to evaluate the inhibition by the final hydrolysis product of β-xylosidase in the case of xylose alone, the compound is added in the presence of enzyme, which justifies a greater inhibition by product, thus overestimating what actually occurs in the process of saccharification (Fig. 6A).
DiscussionDespite of the fact the β-xylosidase activity in the crude extract was hight optimized (1003U/mL), other enzymes intracellular xylanase, β-glucosidase and α-L-arabinofuranosidase were obtained in low levels. This result suggests that the high level of β-xylosidase intracellular obtained in the crude extract is a consequence of the extracellular deconstruction of corn straw residue by other xylanolitic enzymes.
In general, the growth of fungi can be influenced by the rate of aeration in cultivation due to the higher availability of oxygen during growth, which can positively influence enzyme productivity.7 However, various microorganisms behave differently to growth conditions, so it is essential to evaluate the combination of different effects exerted on the enzymes of interest. Several carbon sources have been used to evaluate the induction of hemicellulase activity in other isolates of T. lanuginosus. Among these studies, there is an emphasis on the use of materials derived from corn processing as promising enzyme inducers.2,7,8
The corn cob has been reported as a complex residue in the induction of xylanase in T. lanuginosus DSM 5826 compared to xylan8 to induce low enzyme productivity. The effects of aeration on cultivating were also investigated the production of hemicellulose in T. lanuginosus SSBP cells grown in a bioreactor.3 These tests showed an overall induction of hemicellulase proportional to the increased agitation.2
Although some studies have focused on T. lanuginosus, the use of agro-industrial residues in fermentation processes with this filamentous fungus for enzyme production is still incipient. However, the corn cob,7,9 citrus pectin/beet pulp,4 wheat straw10 and wheat bran11 have been shown to have potential applicability for enzyme induction of the xylanolytic and hemicellulolytic complex. However, the application of an experimental design with this microorganism to induce β-xylosidases has not been reported until the present work.
Data generated using Statistica 7® software to analyze the combined factors indicated a maximum predicted activity of 1194U/mL, and the experimental conditions indicated a production of 1003U/mL. Thus, 84% of the predicted value was achieved with the application of the experimental model. According our known, this study is the first to show so high levels of β-xylosidase activity induction. In addition, the application of an experimental design with this microorganism to induce β-xylosidases has not been reported until the present work.
In this study, the need for experimental design to determine the optimal conditions for enzyme induction was demonstrated. One of the major advantages using this statistical approach to obtain high enzymatic activity is laboratory economics; in a recent work has reported5 the presence of beta-xylosidases in thermophilic fungi in smaller quantities than this study, the ability to obtain high enzyme levels is desirable.
The number of tests performed to achieve the optimal state is less with higher efficiency due to less time spent for the determination of further parameters and the reduced use of reagents needed in the cultures and enzyme dosages. The data indicated that straw corn residue (abundant in the American continents, largely unexplored and considered poor in nutrients) is an excellent inducer of intracellular β-xylosidase from T. lanuginosus in optimized conditions.
The Aspergillus fumigatus grown on corn cobs at temperatures of 30 and 42°C showed an optimum pH of 5.4 and an optimum temperature of 70°C.9Lactobacillus brevis NCDC01 grown in 1% wheat bran has an optimum pH and temperature of 6.0 and 40°C, respectively.12 In the bacterium Selenomonas ruminantium GA192, showed an optimum pH of 5.3 and temperature of 25°C for β-xylosidase.12,13Alicyclobacillus acidocaldarius in optimized conditions is assayed at pH 5.5 and at 50°C.14 All of these data suggest the versatility and heterogeneity of biochemical characteristics of β-xylosidases in pro- and eukaryotic microorganisms.
The synergistic ability to hydrolyze xylan (1%, w/v) and sugarcane bagasse (1%, w/v) with enzyme purified fungal xylanase and purified bacterial β-xylosidase to measure the contribution of β-xylosidase in the process of deconstructing hemicellulose, and they showed increased production of reducing sugars with the purified enzymes as compared to the amount produced with pre-hydrolysis by xylanase.15 In other study with recombinant proteins β-xylosidase and xylanase purified from Humicola insolens expressed in E. coli to hydrolyze xylan from birchwood and beechwood at 37°C, which resulted in saccharification increases of 1 and 1.29 times, respectively, indicating the synergistic action of these enzymes.16
Xylanase and β-xylosidase isolated from Neurospora crassa have also been used for the saccharification of xylan resulting in 18% hydrolysis with the individual action of xylanase, 68% with the combination of both and 48% with the individual action of β-xylosidase, thereby indicating that β-xylosidase is an important tool for depolymerization of xylo-oligosaccharides.17
In this study, the β-xylosidase activity was monitored throughout the test showing high performance with hemicellulose from corn straw, and it retained approximately 80% of the activity over time (Fig. 6A). Most of the tests showed stability that lasted for 1h after the start of the saccharification, which strongly suggested that the enzyme was not subjected to the effects of potential enzyme inhibitors often produced during this type of process.
In general, the data presented here strongly suggest that β-xylosidase from T. lanuginosus can be used for the saccharification of hemicellulose derived from corn straw, an abundant residue in the American continents, thus providing an alternative for future trials to produce energy that depends on the conversion of plant biomass. Successful strategies for the production of hemicellulases with biotech traits not only depend on the selection of new microorganisms but also on the improvement of experimental strategies that take maximum advantage of its potential.18,19
ConclusionsThis study demonstrates the potential of using corn straw as a substrate for achieving high levels of intracellular β-xylosidase (1003U/mL/1683U/mg) for T. lanuginosus. This abundant agro-industrial waste biomass is easily accessible and can lead to significant cost reductions for production of enzymes when used as a carbon source. Although it is widely used for feed, corn straw is underutilized in biotechnological processes, thus leading to its accumulation in the environment. In this study, we tested the optimal conditions for producing β-xylosidase using an optimized experimental design resulting in an increase of approximately 500% or 4.7 times in terms of enzyme yield. Our results suggest an application for the use of corn straw not only for enzyme production but also for processes involving the saccharification of hemicellulose. Additional studies are still needed and are being conducted for the application of corn straw in the production of biofuels.
Conflict of interestThe authors declare that they have no conflict of interest.
J.M. Corrêa was fellow of the Coordination of Improvement of Higher Education Personnel (CAPES, Brazil). R.C.G. Simão (process 630/2014) was partially supported by the Araucária Foundation. M.K. Kadowaki were supported by the Araucária Foundation (process 395/2013) and National Council for Scientific and Technological Development (CNPq 563260/2010-6/Brazil).