A bioassay-guided fractionation of two samples of Brazilian red propolis (from Igarassu, PE, Brazil, hereinafter propolis 1 and 2) was conducted in order to determine the components responsible for its antimicrobial activity, especially against Candida spp. Samples of both the crude powdered resin and the crude ethanolic extract of propolis from both locations inhibited the growth of all 12 tested Candida strains, with a minimum inhibitory concentration of 256μg/mL. The hexane, acetate and methanol fractions of propolis 1 also inhibited all strains with minimum inhibitory concentration values ranging from 128 to 512μg/mL for the six bacteria tested and from 32 to 1024μg/mL for the yeasts. Similarly, hexane and acetate fractions of propolis sample 2 inhibited all microorganisms tested, with minimum inhibitory concentration values of 512μg/mL for bacteria and 32μg/mL for yeasts. The extracts were analyzed by HPLC and their phenolic profile allowed us to identify and quantitate one phenolic acid and seven flavonoids in the crude ethanolic extract. Formononetin and pinocembrin were the major constituents amongst the identified compounds. Formononetin was detected in all extracts and fractions tested, except for the methanolic fraction of sample 2. The isolated isoflavone formononetin inhibited the growth of all the microorganisms tested, with a minimum inhibitory concentration of 200μg/mL for the six bacteria strains tested and 25μg/mL for the six yeasts. Formononetin also exhibited fungicidal activity against five of the six yeasts tested. Taken together our results demonstrate that the isoflavone formononetin is implicated in the reported antimicrobial activity of red propolis.
Propolis is a resinous material collected by melliferous bees from various plant exudates, such as secretions of trees, leaves and flowers. This resin is used by bees in the protection of the hive against bacterial and fungal infections.1
The chemical composition of propolis samples is complex and varies according to its source. Amongst the compounds reported to occur in these samples, phenolic acids and flavonoids are particularly important since many of propolis’ alleged biological activities are attributed to these substances.2
Brazilian propolis has been classified into 12 different chemotypes, according to its chemical profile as determined by UV/VIS spectrophotometry, thin layer and high performance liquid chromatography, as well as its antimicrobial and antioxidant activities.3 Red propolis constitutes a new, separate chemotype occurring at mangrove regions of northeastern Brazilian states and is characterized by its intense red color. This chemotype is the only propolis type whose botanical origin can be traced to a plant species (Dalbergia ecastaphyllum) from the Leguminosae family and one of its characteristics is its high isoflavones content.4 Red propolis has attracted considerable attention for its biological properties5 which include antimicrobial,6 antioxidant,7 leishmanicidal,8 and antileukemic6 activities. To our knowledge there is no chemical or biological characterization reported for the propolis sample from Igarassu (Pernambuco, Brazil).
Considering that fractionation is the first step toward identification of new bioactive natural products,9 the present work aimed at performing a bioassay-guided fractionation of red propolis samples from Igarassu (Pernambuco, Brazil) in order to determine the main constituents associated with its antimicrobial activity, especially against Candida sp.
Material and methodsRed propolis samples and chemical standardsRed propolis samples were obtained from collecting traps placed at the hives of Apis mellifera bees. Collection took place between April 2011 and May 2012 at two apiaries (7°50′37.05″S and 34°53′00.66″W elev. 7m; 7°49′57.90″S and 34°56′02.38″W elev. 64m), located in the municipality of Igarassu, Pernambuco state, Brazil. The samples were labeled as Sample 1 (apiary 1) and Sample 2 (apiary 2). After collection, samples were cleaned by removing any foreign material and then powdered in a shaker until a thin red powder was obtained. The sample was weighed and stored at −18°C and will be referred to from now on as crude powdered propolis. The crude powdered propolis was submitted to chromatographic analysis, tested for antimicrobial activity and was used for the preparation of the crude ethanol extract and its fractions. Solvents used were HPLC-grade unless otherwise specified. The chemical standards ferulic acid, rutin, daidzein, quercetin, luteolin, formononetin, pinocembrin, and biochanin A (≥98–99% purity) were acquired from Sigma–Aldrich (São Paulo, Brasil).
Preparation of red propolis extractsThe crude powdered propolis (100g) was extracted with 500mL of ethanol 96%. Extraction was carried out at room temperature (24°C) in an ultrasonic bath for 30min. The solution was then filtered through a cotton plug and the resulting crude ethanol extract left overnight in the freezer (−18°C) in order to precipitate the waxes. The extract was then placed in a separating funnel for better separation from the waxes and the supernatant was evaporated to dryness in a rotary evaporator at 40°C. This crude ethanolic extract was submitted to chromatographic analysis, tested for antimicrobial activity and used for preparation of the fractions.
Fractionation of the crude ethanol extract and isolation of formononetinThe crude ethanol extract (50g) was suspended in a mixture of 100mL HPLC-grade methanol and 100mL water (1:1, v/v) in a separating funnel and fractioned by liquid-liquid extraction with hexane (700mL, 7 extractions for sample 1 and 400mL, 4 extractions for sample 2) and ethyl acetate (500mL, 5 extractions for sample 1 and for sample 2). The resulting fractions were evaporated to dryness to afford the hexane (27.86g), acetate (8.34g) and methanol (2.13g) fractions for sample 1 and hexane (27.89g), acetate (9.14g) and methanol fractions (1.70g) for sample 2. All fractions were submitted to chromatographic analysis and tested for antimicrobial activity. Since the acetate fraction was the most flavonoid-rich fraction obtained (see Fig. 2 and Table 1), it was also used for further purification. An acetate fraction of sample 1 (8.34g) was dissolved in a mixture of CHCl3:MeOH (1:1, v/v). This sample was submitted to column chromatography with Sephadex® LH-20 and eluted isocratically with CHCl3:MeOH (1:1, v/v), yielding 48 fractions. These fractions were monitored by thin layer chromatography using silica gel plates, a solvent system of CHCl3:MeOH (9:1, v/v) and a visualization reagent PEG-NP (for phenolics). Fractions were grouped according to their TLC profile into four groups (F7-8, F9-14, F15-29 and F30-48).
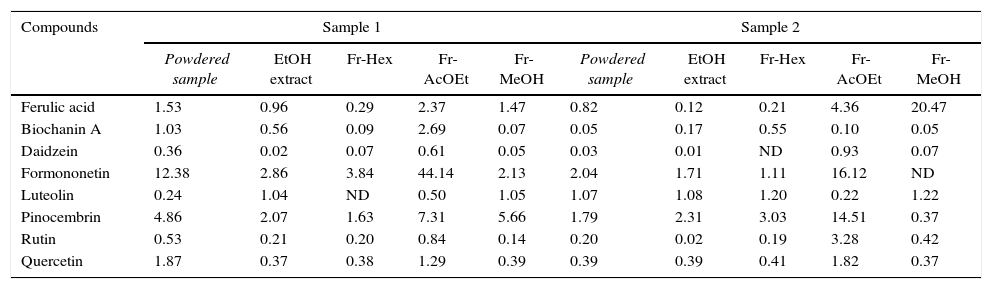
Content (μg/mg) of phenolic compounds found in the analyzed samples as determined by HPLC.
| Compounds | Sample 1 | Sample 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Powdered sample | EtOH extract | Fr-Hex | Fr-AcOEt | Fr-MeOH | Powdered sample | EtOH extract | Fr-Hex | Fr-AcOEt | Fr-MeOH | |
| Ferulic acid | 1.53 | 0.96 | 0.29 | 2.37 | 1.47 | 0.82 | 0.12 | 0.21 | 4.36 | 20.47 |
| Biochanin A | 1.03 | 0.56 | 0.09 | 2.69 | 0.07 | 0.05 | 0.17 | 0.55 | 0.10 | 0.05 |
| Daidzein | 0.36 | 0.02 | 0.07 | 0.61 | 0.05 | 0.03 | 0.01 | ND | 0.93 | 0.07 |
| Formononetin | 12.38 | 2.86 | 3.84 | 44.14 | 2.13 | 2.04 | 1.71 | 1.11 | 16.12 | ND |
| Luteolin | 0.24 | 1.04 | ND | 0.50 | 1.05 | 1.07 | 1.08 | 1.20 | 0.22 | 1.22 |
| Pinocembrin | 4.86 | 2.07 | 1.63 | 7.31 | 5.66 | 1.79 | 2.31 | 3.03 | 14.51 | 0.37 |
| Rutin | 0.53 | 0.21 | 0.20 | 0.84 | 0.14 | 0.20 | 0.02 | 0.19 | 3.28 | 0.42 |
| Quercetin | 1.87 | 0.37 | 0.38 | 1.29 | 0.39 | 0.39 | 0.39 | 0.41 | 1.82 | 0.37 |
Fr-Hex, hexane fraction; Fr-AcEOt, acetate fraction; Fr-MeOH, methanol fraction. ND, not detected.
HPLC analysis was performed using a Shimadzu liquid chromatograph composed of a LC-10AD vp pump, FCV-10AL vp solvent mixer, DGU-14A degasser unit, CTO-10AS column oven, SIL-10AD vp autosampler, SP-10AV vp UV/Vis detector, and a SCL-10A vp system controller. Samples (20μL) of the crude powdered propolis, ethanol extract, hexane fraction, acetate fraction and methanol fraction at a concentration of 5mg/mL (dissolved in MeOH:H2O 8:2, v/v) or formononetin at 100μg/mL were injected into the chromatograph. Separation was achieved using a Luna C-18 column (250mm×4.6mm, 5μm) from Phenomenex (Torrance, USA) and a C-18 guard column (Security guard®, Phenomenex, Torrance, USA). The mobile phase consisted of ultrapure water (A) and methanol (B) delivered at a flow rate of 0.8mL/min. A gradient of 45–65% B from 0 to 90min, 65–75% B from 90 to 120min, 75–95% B from 120 to 170min, and 95–45% B from 170 to 185min was used. Detection was done at 254nm and identification of compounds was done by comparing with the retention time of standards at two different mobile phase compositions. Calibration curves for each of the standards were obtained by dissolving an appropriate amount of each compound in HPLC-grade MeOH to produce a 1mg/mL stock solution and then diluting these stock solutions with a mixture of MeOH:H2O (80:20 v/v) to produce the final standard concentrations (0.5–100μg/mL). The concentration range for each standard was chosen so as to bracket the peak area of each compound detected in the samples in preliminary chromatographic runs. The calibration curves were used if the correlation coefficient was 0.99 or higher.
Antimicrobial activityDetermination of the antimicrobial activity of the crude powdered propolis, ethanol extract, fractions and formononetin was carried out by determining the minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC). The following bacteria species were tested: Staphylococcus aureus ATCC 13150, Staphylococcus aureus ATCC 25923, Staphylococcus epidermides ATCC 12228, Pseudomonas aeruginosa ATCC 9027, Pseudomonas aeruginosa ATCC P-12, Pseudomonas aeruginosa ATCC P-03; for antifungal tests: Candida albicans ATCC 76645, Candida albicans LM P-20, Candida tropicalis ATCC 13803, Candida tropicalis LM 6, Cryptococcus neoformans ICB 59, Cryptococcus neoformans LM 2601. The culture medium for tests was RPMI 1640 with l-glutamine and no bicarbonate (Sigma–Aldrich®) for antifungal activity assays and nutrient broth (Difco Laboratories/USA/FRANCE) for the antibacterial activity tests. The inoculum was prepared from colonies taken from recently-grown cultures in appropriate media incubated at 35–37°C for 24–48h for bacteria and 24–72h for yeast. The colonies were suspended in sterile 0.9% NaCl. The inoculum suspensions were shaken for 2min and the inoculum density was adjusted to the turbidity of a 0.5 McFarland standard with sterile saline (equivalent to 1–5×106cfu/mL).10–13
Determination of the minimum inhibitory concentration (MIC)The determination of the minimum inhibitory concentration (MIC) was done in duplicate by the broth microdilution method using U-shaped round-bottomed 96 well microplates. In each well, 100μL of the doubly concentrated medium was added and on the first row of each plate, 100μL of the tested substances were added to give final concentrations of 2048μg/mL for all samples except for isolated formononetin which was added to give a final concentration of 400μg/mL. Then, by serial dilution the test substances and formononetin were diluted up to 32μg/mL or 6.25μg/mL respectively. After the dilution step, 10μL of the microorganism inoculum was added in each well. For each microorganism, wells containing the following controls were also added: chloramphenicol 100μg/mL (on the plates used in the antibacterial tests), nystatin 100UI/mL (on the plates used in the antifungal tests) and 1% DMSO (on all plates). Plates were incubated at 35–37°C for 24–48h for bacteria and 24–72h for yeast. After the incubation period, 20μL of resazurin sodium salt solution at 0.01% (w/v) was added to the plates used in the antibacterial tests and 20μL of 0.5% triphenyl tetrazolium chloride (TTC) was added to the plates used for antifungal activity as indicators of cell viability. The MIC was defined as the lowest concentration capable of inhibiting the bacterial and fungal growth when compared with the control wells. Stock solutions of test substances were diluted in DMSO (final concentration of DMSO in each well was no higher than 0.1%).
Determination of the minimum bactericidal (MBC) and minimum fungicidal concentrations (MFC)A modification of the microdilution method was used to estimate MBC and MFC values. Aliquots of 10μL from wells that did not present growth during the experiments for MIC determination (taken from those wells corresponding to 1×, 2× and 4× the MIC value calculated for each tested substances) were transferred to sterile microtitration plates (DISPOPETRI, Brazil) containing 100μL/well of incubation medium for determination of MBC and MFC. Plates were incubated at 35–37°C for 24–48h for bacteria and 24–72h for yeast. The MBC and MFC were considered as the lowest concentration of tested substances able to induce inactivation of the inoculum for bactericidal and fungicidal tests respectively.14,15 All antimicrobial tests were performed in duplicate and the results were expressed as the geometric mean of the MIC, MBC, or MFC.11,16,17 For interpretation of the results the guidelines adopted by Sartoratto et al.18 were followed: high activity for values of MIC between 50 and 500μg/mL, average activity for MIC values between 600 and 1500μg/mL and low activity for MIC values above 1600μg/mL.
ResultsHPLC profile of the samples and formononetin isolationThe chromatograms of samples are shown in Fig. 2. The chromatograms of the crude ethanolic extract of both propolis samples were remarkable similar considering the geographic separation of the apiaries from which the samples were obtained. The acetate fraction concentrates most of the constituents found in the ethanolic extract, while the hexane and methanol fractions are less abundant in compounds. The chromatogram of the methanol fraction displayed a single major peak with a retention time of 10min was identified as ferulic acid.
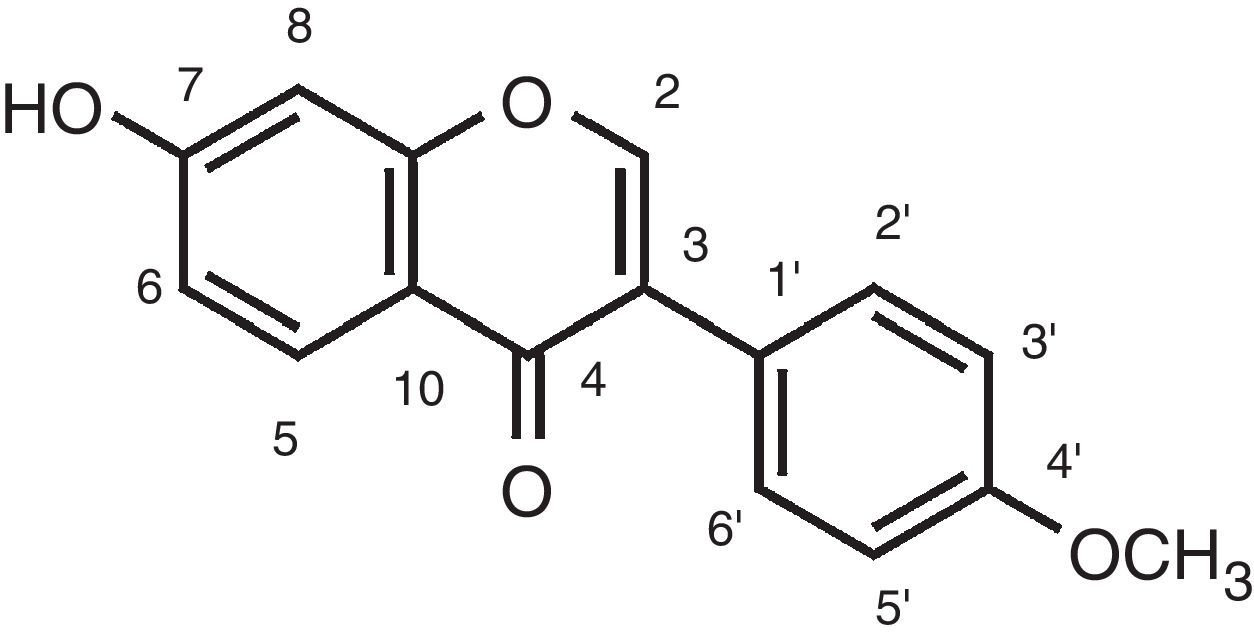
After chromatography with Sephadex® LH-20, fractions 9–14 produced a precipitate that was filtered, dried and submitted to 1H and 13C NMR spectroscopy for structure elucidation. The spectral data confirmed the identity of the substance as the isoflavone formononetin (12.3mg) (Fig. 1). 1H NMR data (DMSO-d6, 300MHz): 3.79 (3H, s, OCH3), 6.99 (2H, d, J=9.0Hz, H-3′, 5′), 6.87 (1H, d, J=2.2Hz, H-8), 6.95 (1H, dd, J=8.9, 2.2Hz, H-6), 7.51 (2H, d, J=8.7Hz, H-2′, 6′), 8.34 (1H, s, H-2), 7.98 (1H, d, J=9Hz, H-5).
The phenolic compounds identified and quantified in the samples are listed in Table 1. One phenolic acid and seven flavonoids were identified in the samples. Amongst the compounds identified, formononetin and pinocembrin were the most abundant compounds and their concentration in the samples varied from 220.72μg/mL in the acetate fraction of sample 1 to 5.56μg/mL in the hexane fraction of sample 2 for formononetin and from 72.54μg/mL in the acetate fraction of sample 2 to 1.84μg/mL in the methanol fraction of sample 2 for pinocembrin.
Antimicrobial activityMinimum inhibitory concentration (MIC)The results of MIC determination for the tested materials are presented in Table 2. The powdered red propolis of both samples 1 and 2, and the ethanol extract of both samples inhibited the growth of all twelve microorganisms tested (bacteria and yeasts) with a MIC of 256μg/mL, which according to Sartoratto et al.18 should be considered a high activity. The hexane and acetate fractions from both samples, together with the methanol fraction of sample 1 inhibited all microorganisms tested with MIC values varying from 32μg/mL to 1024μg/mL. These fractions had a very strong antifungal activity with all of them exhibiting a MIC value of 32μg/mL for the six yeasts tested, except for the methanol fraction of sample 1 which had a MIC of 128μg/mL against C. tropicalis ATCC 13803 and the acetate fraction of sample 1, with a MIC of 1024μg/mL against C. tropicalis LM 6. The methanol fraction of sample 2 did not inhibit the growth of any of the bacteria strains tested and was active against five out of six yeasts tested. The isoflavone formononetin, isolated from the acetate fraction was active against all microorganisms tested, with a MIC of 200μg/mL for the six bacteria and a MIC of 25μg/mL for the six yeast strains.
Minimum inhibitory concentration – MIC (μg/mL) of propolis samples 1 and 2, its fractions and extract+(presence of microbial growth).
| Microorganisms | Sample 1 | Sample 2 | Formononetin | Chloramphenicol | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Powdered sample | Ethanol extract | aFr-Hex | aFr-AcOEt | aFr-MeOH | Powdered sample | Ethanol extract | aFr-Hex | aFr-AcOEt | aFr-MeOH | |||
| Gram-positive | ||||||||||||
| S. aureus ATCC 13150 | 256 | 256 | 512 | 512 | 1024 | 256 | 256 | 512 | 512 | + | 200 | 32 |
| S. aureus ATCC 25923 | 256 | 256 | 256 | 512 | 1024 | 256 | 256 | 512 | 512 | + | 200 | 32 |
| S. epidermides ATCC 12228 | 256 | 256 | 128 | 512 | 1024 | 256 | 256 | 512 | 512 | + | 200 | 64 |
| Gram-negative | ||||||||||||
| P. aeruginosa ATCC 9027 | 256 | 256 | 128 | 512 | 1024 | 256 | 256 | 512 | 512 | + | 200 | 128 |
| P. aeruginosa ATCC P-12 | 256 | 256 | 512 | 512 | 1024 | 256 | 256 | 512 | 512 | + | 200 | 128 |
| P. aeruginosa ATCC P-03 | 256 | 256 | 256 | 128 | 1024 | 256 | 256 | 512 | 512 | + | 200 | 128 |
| Yeasts | Nystatin | |||||||||||
| C. albicans ATCC 76645 | 256 | 256 | 32 | 32 | 32 | 256 | 256 | 32 | 32 | 32 | 25 | 32 |
| C. albicans LM P-20 | 256 | 256 | 32 | 32 | 32 | 256 | 256 | 32 | 32 | 32 | 25 | 32 |
| C. tropicalis ATCC 13803 | 256 | 256 | 32 | 32 | 128 | 256 | 256 | 32 | 32 | + | 25 | 64 |
| C. tropicalis LM 6 | 256 | 256 | 32 | NDb | 64 | 256 | 256 | 32 | 32 | 32 | 25 | 64 |
| C. neoformans ICB 59 | 256 | 256 | 32 | 32 | 32 | 256 | 256 | 32 | 32 | 32 | 25 | 32 |
| C. neoformans LM 2601 | 256 | 256 | 32 | 32 | 32 | 256 | 256 | 32 | 32 | 32 | 25 | 32 |
Only the acetate fraction of sample 2 presented bactericidal activity, with MBC of 1.024μg/mL against four of the six tested bacterial strains. It showed no bactericidal activity against Staphylococcus aureus ATCC 13150 and Staphylococcus aureus ATCC 25923. The results for MFC determination are presented in Table 3. The ethanol extract of sample 2 had fungicidal activity against all strains tested, with a MFC of 256μg/mL. Isolated formononetin exhibited fungicidal activity against five of a total of six strains tested with a MFC of 200μg/mL, and was inactive only against C. tropicalis LM 6. It is worth noting that from all samples tested, the acetate fraction of sample 1 was the one with the lowest MFC at 64μg/mL, and this was the fraction with the highest formononetin concentration, at 220.72μg/mL.
Minimum fungicidal concentration – MFC (μg/mL) of propolis samples 1 and 2, its fractions and extract+(presence of microbial growth).
| Microorganisms | Sample 1 | Sample 2 | Formononetin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Powdered sample | Ethanol extract | aFr-Hex | aFr-AcOEt | aFr-MeOH | Powdered sample | Ethanol extract | aFr-Hex | aFr-AcOEt | aFr-MeOH | ||
| Yeasts | |||||||||||
| C. albicans ATCC 76645 | + | + | 1.024 | 1.024 | + | + | 256 | 1.024 | 512 | + | 200 |
| C. albicans LM P-20 | + | + | + | 64 | + | + | 256 | 1.024 | 512 | + | 200 |
| C. tropicalis ATCC 13803 | + | + | 1.024 | 1.024 | 1.024 | + | 256 | 256 | 128 | + | 200 |
| C. tropicalis LM 6 | + | + | + | + | + | + | 256 | + | 512 | + | + |
| C. neoformans ICB 59 | + | + | 256 | + | + | + | 256 | 1.024 | 512 | + | 200 |
| C. neoformans LM 2601 | + | + | + | + | + | + | 256 | + | 512 | + | 200 |
The antimicrobial activity of red propolis has been described before7,19 but reports are scarce in comparison to other types of Brazilian propolis. The comparison of the antimicrobial activity of different propolis samples is difficult, especially when they are from different geographical origins, since their chemical composition and thus their antimicrobial activity can vary considerably under these circumstances.20
Junior et al.21 studied the antimicrobial activity of red propolis from Alagoas state (Brazil). The ethanol extract presented antimicrobial activity against Gram-positive (100% of tested strains) and Gram negative bacteria (62.5% of tested strains) as well as antifungal activity (100% of strains). In our study the powdered sample and the ethanol crude propolis extract of samples 1 and 2 from Igarassu (Pernambuco state, Brazil) presented activity against all the Gram-positive and Gram-negative bacteria, as well as against all yeast strains.
Cabral et al.7 studied the antibacterial activity of red propolis samples from the state of Alagoas (Brazil) and found that the antibacterial activity of the chloroform fraction and its subfractions were higher (MIC values ranging from 15.8 to 31.7μg/mL) than that of the crude ethanol extract (MIC values ranging from 62.5 to 125μg/mL). The authors thus suggested that the antimicrobial activity of red propolis is not due to a synergic effect of its components, but to individual constituents. In the same study, formononetin was the major compound identified in the ethanol extract and chloroform fraction. For the bacterial strains tested here, the acetate fraction showed a general trend to exhibit weaker antibacterial activity than the ethanol fraction, but the antifungal activity against Candida strains of the acetate fraction was considerably higher than the one exhibited by the ethanol extract. In addition, formononetin, the isoflavone isolated from the samples exhibited good antifungal activity against all strains tested (MIC=25μg/mL), and it presented also fungicidal activity against all strains (MFC=200μg/mL), except for C. tropicalis LM 6. However, the antifungal activity of the acetate fraction cannot be attributed solely to formononetin, since the concentration of the isoflavone in the acetate fraction of sample 1 was determined to be 2.7 times higher than that of sample 2, and yet the fungicidal activity of the acetate fraction of sample 2 was higher (see Table 3). We also did not observe a trend of higher antibacterial potency of the fractions compared to the crude ethanol extract as observed by Cabral et al.7 However, the MIC of the acetate fraction against all tested Candida strains was lower than the MIC of the crude ethanol fraction, demonstrating that formononetin is an important compound for the antifungal activity demonstrated by red propolis.
The botanical origin of propolis samples is difficult to ascertain on the basis of palynological analysis only, and a more definitive confirmation depends on analysis comparing the chemical profile of the samples with the chemical profile of resins and extracts from the plants found in close vicinity of the bee's hives. It should be stressed that red propolis has been suggested to be the only propolis type derived from a plant from the Leguminosae family (D. ecastaphyllum), rich in isoflavones such as genistein and formononetin.4
Although flavonoids exhibit pleiotropic activity affecting several different targets, and synergistic effects cannot be ruled out, our results suggest that the isoflavone formononetin is responsible at least partially for the antimicrobial activity of red propolis.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil) for financial support (PhD grant).