The aim of this study was to isolate and identify Candida species from the oral cavity of denture wearers with denture-related stomatitis who were attended at the University Federal of Pará (Belém City, Pará State, Brazil). A total of 36 denture wearers with denture-related stomatitis were included, and type I (50%), type II (33%) and type III (17%) stomatitis were observed. Candida spp. were isolated from 89% of the cases and included five different Candida species. C. albicans was the most frequently recovered species (78% of the cases), followed by C. famata and C. tropicalis. We observed a significant association between Candida species isolation and unsatisfactory denture condition (p=0.0017). Our results demonstrated the highly frequency of Candida species isolation in denture wearers with denture-related stomatitis and showed the relationship between these species and poor denture maintenance.
Approximately 200 Candida species are known, and 10% can cause infections in humans. C. albicans is the most frequently described species in cases of hospital infections, followed by C. parapsilosis, C. tropicalis and C. glabrata.1 The frequency at which these species are observed has significant implications for human infections. These species are commensal organisms that constitute part of the normal oral microbiota, and they are present in 30–60% of healthy individuals and 60–100% of patients with dentures.2 The long-term use of dentures is the most important risk factor for Candida species colonization of the mucosa surface and may be sufficient for the development of oral candidiasis.3 Oral candidiasis is associated with mucosal trauma caused by poor denture fit, the increasing age of the denture wearers, the increased age of the dentures, fungal infections (primarily C. albicans) and poor dental hygiene.4–6 In this context, the adherence of Candida to the surface of denture materials, such as polymethyl methacrylate, facilitates colonization by Candida.7,8 The mechanisms by which C. albicans adhere to polymeric surfaces (e.g., dentures) primarily include biofilm formation and morphological switching, which facilitate the colonization of these materials by the fungus. Colonization is the main risk factor for the development of denture-related stomatitis (DRS), which is the most common clinical manifestation of Candida infection in denture wearers.9
Infection by Candida spp. is frequently observed in patients with dentures and may lead to secondary oral lesions, such as lichen planus, leukoplakia and carcinoma.10Candida species colonization11,12 and infections9 in the oral cavity of denture wearers have been reported worldwide, and C. albicans is particularly prevalent.13 Moreover, the isolation of Candida species other than C. albicans has been increasing, which is likely because of the misuse of antifungals.14 In Brazil, few studies have demonstrated the profile of Candida species related to colonization of the denture surface or oral mucosa and the incidence of these lesions in denture wearers.15 Therefore, we focused this study on the isolating and identifying Candida species from the surface of dentures and the oral mucosa of denture wearers with DRS.
Materials and methodsEthical aspectsThe study was approved by the Ethics Committee of Evandro Chagas Institute (CEP/IEC 032/10) and conducted between March and October 2012. All patients were informed about the study and provided written informed consent.
PopulationThirty-six (n=36) patients fitted with acrylic-based dentures who presented with denture-related stomatitis were included. The patients were fitted with complete dentures (n=32) or partially removable dentures (n=4). All of the participants were attended at the dental school clinic at the University Federal of Pará (Belém, Pará, Brazil). Analyses were performed of the patient demographic data, which included age, gender, hygiene habits (poor or not), mouthwash use, present denture condition (satisfactory or unsatisfactory) and qualitative characteristic (new or old dentures). The presence of DRS was assessed according to a modified version of Newton's classification.16 The severity of the palatal inflammation was classified as (1) no stomatitis, which included no evidence of palatal inflammation or slight color change of the palate mucosa; (2) stomatitis type I, which included petechiae dispersed throughout all or any part of the palatal mucosa in contact with the denture; (3) type II, which included macular erythema without hyperplasia; and (4) type III, which included diffuse or generalized erythema with papillary hyperplasia.
The patient exclusion criteria included the presence of diabetes or autoimmune disease and the use of corticosteroids.
Isolation and identificationAfter an examination of the oral cavity, denture and mucosal specimens were harvested by scraping sterile swabs across the inner surface of the denture (basis of prosthesis, BP) and the oral mucosa (palatal mucosa, PM) in contact with the denture. Subsequently, the specimens were cultured in Sabouraud dextrose agar (Difco, Laboratories, Detroit, MI, USA), incubated at 35°C and observed daily for 7 days. When the growth of yeast colonies was observed, the Gram stain method was used to verify the absence of bacterial contamination. The yeasts were identified via carbohydrate assimilation profiles using the Vitek 2 System (BioMerieux I’Etoile, France) according to the manufacturer's instructions.
Yeasts identified as C. dubliniensis were subjected to molecular confirmation because of the close phenotypical relationship of this species with C. albicans. Briefly, genomic DNA was extracted as previously described,17 and when necessary, molecular identification was performed as described by Mannarelli and Kurtzman18 (C. dubliniensis/forward: CDU2 – 5′-AGT TAC TCT TTC GGG GGT GGC CT-3′; C. dubliniensis/reverse: NL4CAL – 5′-AAG ATC ATT ATG CCA ACA TCC TAG GTA AA-3′) and by Luo and Mitchell19 (C. albicans/forward: CALB1 – 5′-TTT ATC AAC TTG TCA CAC CAG A-3′; C. albicans/reverse: CALB2 – 5′-ATC CCG CCT TAC CAC TAC CG-3′). The mix was prepared to a final volume of 25μL as follows: 10× MgCl2 (2μL), 10mM dNTP (1μL), 10× PCR buffer (2.5μL), Q solution (2μL), Taq DNA polymerase (1U; Invitrogen Life Technologies, Carlsbad, Calif.) and genomic DNA template (2μL). Amplification was performed in a thermal cycler (TX96 plus, Amplitherm, Axigen) as follows: for C. dubliniensis: 98°C for 3min; followed by 35 cycles of 95°C for 1min, 52°C for 1.5min, and 72°C for 10min; and then 72°C for 10min; and C. albicans: 96°C for 5min; followed by 40 cycles of 94°C for 30s, 58°C for 30s, and 72°C for 30s; and then 72°C for 15min. The PCR products were submitted to horizontal electrophoresis. The amplified fragments were 175bp and 273bp for C. dubliniensis and C. albicans, respectively.
Statistical analysisStatistical inferences of the descriptive results were performed based on non-parametric tests, such as the G adherence independence test, using BioEstat version 5.3 (Instituto Maumirauá, Belém, Pará, Brazil). Statistical significance was considered at p≤0.05.
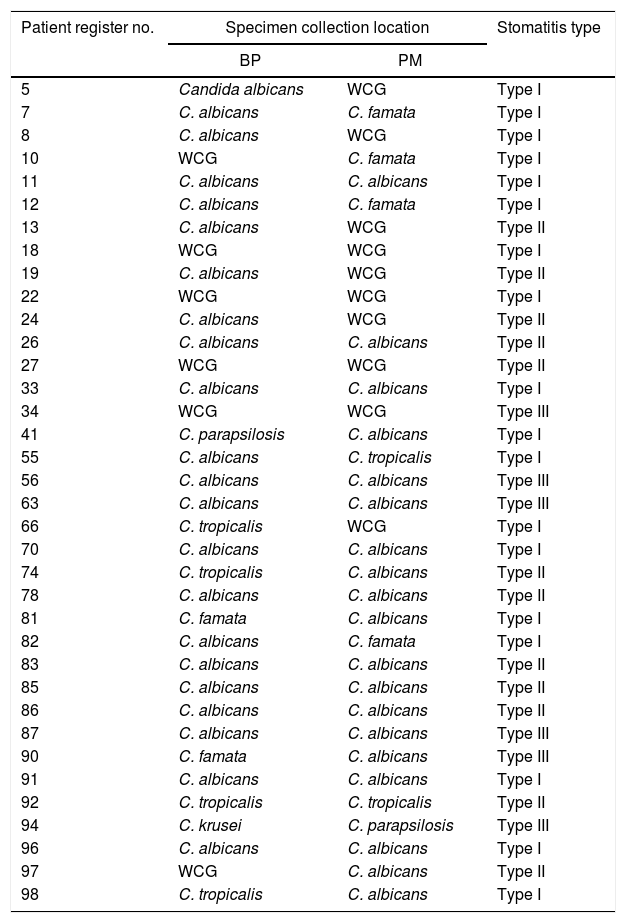
ResultsThirty-six (n=36) denture wearers with DRS were included in this study. The patients ranged in age from 40 to 83 years (mean age=62 years) and included 12 males (33%) and 24 females (67%). According to Newton's classification, the DRS cases were distributed as follows: Type I (50%), Type II (33%), and Type III (17%) among the cases. Candida species were isolated from the BP only (17%), FM only (5%) and BP and FM simultaneously (67%). In four cases (11%), Candida species were not isolated. Based on biochemical or biochemical and molecular identification, we observed five different Candida species. C. albicans was the most frequently observed species (78% of the cases), and it was observed alone or in simultaneous isolations with C. famata, C. tropicalis or C. parapsilosis. The restricted isolation of Candida species other than C. albicans was observed in 11% of the cases. All of these results are summarized in Table 1.
Presence of Candida species in the oral specimens from 36 denture wearers with denture-related stomatitis.
| Patient register no. | Specimen collection location | Stomatitis type | |
|---|---|---|---|
| BP | PM | ||
| 5 | Candida albicans | WCG | Type I |
| 7 | C. albicans | C. famata | Type I |
| 8 | C. albicans | WCG | Type I |
| 10 | WCG | C. famata | Type I |
| 11 | C. albicans | C. albicans | Type I |
| 12 | C. albicans | C. famata | Type I |
| 13 | C. albicans | WCG | Type II |
| 18 | WCG | WCG | Type I |
| 19 | C. albicans | WCG | Type II |
| 22 | WCG | WCG | Type I |
| 24 | C. albicans | WCG | Type II |
| 26 | C. albicans | C. albicans | Type II |
| 27 | WCG | WCG | Type II |
| 33 | C. albicans | C. albicans | Type I |
| 34 | WCG | WCG | Type III |
| 41 | C. parapsilosis | C. albicans | Type I |
| 55 | C. albicans | C. tropicalis | Type I |
| 56 | C. albicans | C. albicans | Type III |
| 63 | C. albicans | C. albicans | Type III |
| 66 | C. tropicalis | WCG | Type I |
| 70 | C. albicans | C. albicans | Type I |
| 74 | C. tropicalis | C. albicans | Type II |
| 78 | C. albicans | C. albicans | Type II |
| 81 | C. famata | C. albicans | Type I |
| 82 | C. albicans | C. famata | Type I |
| 83 | C. albicans | C. albicans | Type II |
| 85 | C. albicans | C. albicans | Type II |
| 86 | C. albicans | C. albicans | Type II |
| 87 | C. albicans | C. albicans | Type III |
| 90 | C. famata | C. albicans | Type III |
| 91 | C. albicans | C. albicans | Type I |
| 92 | C. tropicalis | C. tropicalis | Type II |
| 94 | C. krusei | C. parapsilosis | Type III |
| 96 | C. albicans | C. albicans | Type I |
| 97 | WCG | C. albicans | Type II |
| 98 | C. tropicalis | C. albicans | Type I |
BP, basis of prosthesis; PM, palatal mucosa; WCG, without Candida growth.
According to the demographics data, an unsatisfactory denture condition was an influencing factor for the isolation of Candida species (p=0.0017); however, gender (p=0.7015), mouthwash use (p=0.6514), hygiene habits (p=0.3897), continuous denture use (p=0.4011) and old dentures (p=0.2502) were not related to Candida species isolation.
DiscussionDRS exhibits a multifactorial etiology and is associated with denture use, indicating that disease presentation can be affected by both endogenous and exogenous factors.7,9 A critical risk factor, however, is the colonization of the oral mucosa by Candida species.20 In the present study, Candida spp. were isolated in 89% of the cases of denture wearers with DRS (according to Newton's classification), thereby reinforcing the relationship between Candida spp. and DRS. In Brazilian denture wearers, DRS was previously observed as the major lesion in the oral cavity,21 which is consistent with our results. In the present study, the factor that influenced whether Candida was isolated was an unsatisfactory denture condition. The frequent maintenance of dentures allows for improvements to certain functional characteristics, such as the RVD (rest vertical dimension) and VDO (vertical dimension of occlusion) of the patient, the adhesion of the denture to the mucosa and the diminution of the resin roughness. Improving these factors may decrease microorganism contamination of the surface denture, which will influence the oral health of denture wearers. In this context, Pereira-Cenci et al.22 and Park et al.23 observed that the colonization of dentures by Candida species is caused by surface roughness, which facilitates the adhesion of the yeast to resin dentures. Because the adherence of C. albicans to acrylic surface resins is related to the surface porosity and roughness, denture surfaces can be considered an infection source, and these findings are consistent with the significant colonization observed in denture wearers presenting with DRS because all patients were fitted with acrylic resin dentures. Moreover, the results support the relationship between unsatisfactory denture condition and Candida species isolation.
Others factors have been frequently reported as influential in the development of DRS. For example, Bulad et al.4 mentioned that the continuous use of dentures facilitated denture stomatitis because extended mucosal exposure to dental plaques may intensify certain injuries. Additionally, Darwazeh et al.24 observed a strong association between poor dental hygiene and Candida colonization and suggested that plaque accumulation on the denture surface may create an ideal environment for yeast. However, an association between poor hygiene, continuous denture use or old dentures and the isolation of Candida species was not observed in this study.
Among the colonized denture wearers in our study, C. albicans was the major isolated species, followed by C. famata and C. tropicalis, which were the most frequent non-C. albicans species. These results corroborated the findings of other studies,11,14,21,25–27 including other Brazilian studies.16,28–30 Additionally, Sant’Ana et al.31 reported that the presence of two or more species in the same patient can predispose the patient to recurrent stomatitis, and double isolations were also observed in denture wearers with DRS in the present study. Moreover, C. famata was observed in the PM of denture wearers who exhibited DRS and found in association with C. albicans. These results demonstrated the potential for emergent pathogens in cases of DRS.
We observed a significant frequency of DRS in Brazilian patients colonized by Candida species, and the infection was primarily caused by C. albicans. The results indicate that in the observed patients, DRS developed along a pathway that was dependent on the unsatisfactory state of the prosthesis and the colonization of the oral cavity by Candida species.
Conflicts of interestThe authors declare no conflicts of interest.
FundingThe preparation of this manuscript was supported by the Post-graduate Program in Biology of Infectious and Parasitic Agents, Federal University of Pará (Edital PAPQ/2013-UFPA).