The evolution of microorganisms resistant to many medicines has become a major challenge for the scientific community around the world. Motivated by the gravity of such a situation, the World Health Organization released a report in 2014 with the aim of providing updated information on this critical scenario. Among the most worrying microorganisms, species from the genus Candida have exhibited a high rate of resistance to antifungal drugs. Therefore, the objective of this review is to show that the use of natural products (extracts or isolated biomolecules), along with conventional antifungal therapy, can be a very promising strategy to overcome microbial multiresistance. Some promising alternatives are essential oils of Melaleuca alternifolia (mainly composed of terpinen-4-ol, a type of monoterpene), lactoferrin (a peptide isolated from milk) and chitosan (a copolymer from chitin). Such products have great potential to increase antifungal therapy efficacy, mitigate side effects and provide a wide range of action in antifungal therapy.
Fungal infections are responsible for many deaths around the world, in addition to contributing to significant expenses in public health.1 Among the possible etiologic agents, Candida spp. are noted due to their relevance and prevalence. According to McCarty and Pappas (2015), 15–20 microorganisms from the genus Candida are described as certain to cause infection.2 However, four species cause 96% of the infections: Candida albicans is the main etiologic agent, responsible for 42.5% of infections, followed by Candida tropicalis (27.3%), Candida parapsilosis (21.9%) and Candida glabrata (4.4%).3
Infections originating from C. albicans have been observed since the time of Hippocrates, who described Candida diseases in debilitated patients in 337 B.C.4 Now, Candida spp. are responsible for the majority of blood infections in intensive therapy units around the world, impacting hospitalization times and costs.5 In 2005, for example, the Centers for Disease Control and Prevention (CDC) showed that for each diagnosis of Candida spp. infection, the period of hospitalization is lengthened from 3 to 13 days. Financially speaking, this additional period leads to expenses from $6000 to $29,000. Furthermore, each year, these values are estimated to reach approximately $8 billion.6,7
Advances in medicine have enabled people who are immune suppressed (such as those who have received organ transplants or are HIV positive) to live longer lives.8 The greatest vulnerability of such individuals is to metabolically flexible microorganisms of the genus Candida, which have increased the rate of morbidity and mortality to 35% in this group of people.9 In addition, parallel to the increasing number of cases of fungal infection caused by Candida spp., the resistance of Candida spp. to antimicrobial drugs has progressively increased. The latter fact has sparked the attention of the World Health Organization (WHO) due to its relevance and led to the release of the report Antimicrobial Resistance: Global Report Surveillance.10
The development of resistance in Candida spp., from therapy with amphotericin B, against drugs from the azole group, echinocandin and fluconazole was among the key information provided in this report, along with reports of cases of multiresistance from different regions of the world such as Asia and the Middle East and some areas of South America.10 These data are alarming because such resistance has developed against antifungal drugs widely used to combat fungal diseases worldwide. Amphotericin B, for example, is a classical antifungal drug, and in many countries, it is the only medicine available for the treatment of fungal infections.
The azoles have been administered in formulations that combine different groups of chemotherapeutic drugs, attempting to overcome the increase in microbial resistance. This fact impacts both the cost and the success of treatments against that type of infection. Finally, the development of resistance to fluconazole—the most commonly used antifungal—drastically varies according to the Candida spp. and from country to country. For example, the intensity of the multiresistance in Candida spp. to fluconazole is higher in Denmark (33%) and lower in the Korean Republic (0.9%).10 Therefore, the aim of this paper is to discuss the most promising substances available for candidiasis treatment. In addition, this work reinforces the importance of using natural products along with those medicines traditionally employed in the treatment of such infections.
Natural productsNatural products have been widely used in society since 3500 B.C.11 Medicinal methods range from folk medicine to validated treatments.12 The doctor Paracelsus is considered the founder of plant-based medicine due to his great contributions in the field.13 His findings, along with those of other researchers, led to a consolidation of knowledge in the field of natural products and created other domains such as pharmacognosy. Among the medicinal properties of natural products, one can find anti-inflammatory, antispasmodic, anticancer, antimutagenic, antibacterial, antifungal, antiviral and vermicidal properties.14
Essential oils compose the most utilized group of substances. Isolated from plants, essential oils are compounds largely described in the scientific literature as versatile in the treatment of many infirmities.15 Their active ingredients are usually various terpenes, which are secondary metabolites excreted by vegetables to expel predators or harmful agents.16 One example of a bioactive essential oil is the one obtained from Melaleuca alternifolia leaves; the activity of this oil is attributed to terpinen-4-ol, a monoterpene.17
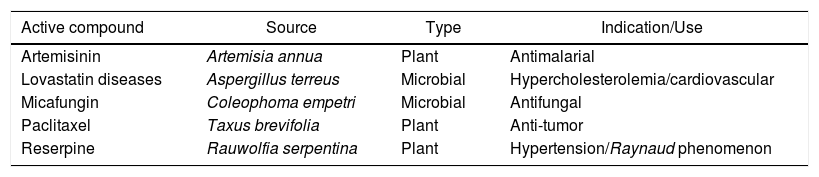
The isolation of bioactive molecules is also performed on natural sources other than vegetable cells. Some metabolites produced by microorganisms are also important sources of new bioactive substances.18 In Table 1, therapeutic agents obtained through bioprospection are presented; these agents are responsible for significant impact on the medicinal treatments that have been offered in recent years.
Conventional medicines originally obtained from natural sources.
| Active compound | Source | Type | Indication/Use |
|---|---|---|---|
| Artemisinin | Artemisia annua | Plant | Antimalarial |
| Lovastatin diseases | Aspergillus terreus | Microbial | Hypercholesterolemia/cardiovascular |
| Micafungin | Coleophoma empetri | Microbial | Antifungal |
| Paclitaxel | Taxus brevifolia | Plant | Anti-tumor |
| Reserpine | Rauwolfia serpentina | Plant | Hypertension/Raynaud phenomenon |
In addition to the compounds listed in Table 1, lactoferrin (a peptide isolated from milk) and chitosan (a copolymer derived from chitin) also show distinct biological activity, including antioxidant, anti-allergy and antimicrobial actions.19 The association of such natural products with other antimicrobial drugs has been recently investigated to identify a possible synergistic effect. This can provide alternative treatments against multiresistant microorganisms, thereby reducing the amount of drugs used in treatments, which can impact drug-resistance phenomena.
However, as noted by Harvey et al. (2015), due to the environmental legislation that protects biodiversity, bioprospection, among other pursuits, has suffered a considerable slowdown in the last 20 years.20 The reduction in the rate of drug discovery and new medicines provided by the pharmaceutical industry reflects such constraints.
Bioprospection is a feasible way to discover new biomolecules. One example of such a discovery is teixobactin, which was isolated from soil samples by Ling et al. (2015).21 This molecule proved to be very promising as a new antibiotic class because its administration in mice was able to successfully eliminate infections caused by Staphylococcus aureus super bacteria strains. Therefore, after nearly 30 years without a new drug discovery, this promising molecule—found through bioprospection—may revolutionize the current antibacterial treatments.21
Finding bioactive compounds is not an easy task. Therefore, natural products have been used along with synthetic drugs to overcome, mainly, resistance to antimicrobial drugs or to alleviate the side effects associated with specific therapies.
CandidiasisMicroorganisms from the genus Candida are described as yeasts and include more than 160 species.22 In terms of morphology, the genus is characterized as being thick, curd-like (cheesy), and yellowish-white. Candida albicans is found extensively in humans, and these yeasts are easily isolated from the skin and gastrointestinal tract.23 Therefore, this type of microorganism lives commensally, co-inhabiting and existing in equilibrium with human microbiota.24 Nonetheless, under specific conditions, these microorganisms are favored, which usually enables the activation of the virulence factor, making such organisms pathogenic and harmful to the health of the host.25,26 Thus, C. albicans is known as an opportunistic microorganism.27
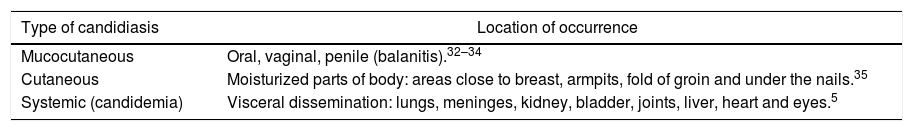
People with immunodeficiency compose the group with the highest tendency to develop candidiasis. Among the most vulnerable people are elderly people and babies; patients with cancer, AIDS or with transplants; people whose treatment uses catheters (in parenteral nutrition or in hemodialysis); and people submitted to therapies based on antimicrobial drugs of large spectrum/corticoids.28–30 The type and the intensity of the infection (Table 2) are directly related to the immunological response of the patient.31
Types of infection described for candidiasis and its respective places of occurrence.
| Type of candidiasis | Location of occurrence |
|---|---|
| Mucocutaneous | Oral, vaginal, penile (balanitis).32–34 |
| Cutaneous | Moisturized parts of body: areas close to breast, armpits, fold of groin and under the nails.35 |
| Systemic (candidemia) | Visceral dissemination: lungs, meninges, kidney, bladder, joints, liver, heart and eyes.5 |
When Candida spp. take advantage of a host's immunosensitivity, the major mechanisms of virulence and the invasive capacity of the Candida spp. are mainly related to (i) the ability to form biofilm reinforced by a mechanism of quorum sensing and (ii) phenotypic switching, that is, when yeasts change to the filamentous form, which is a determinant of tissue invasion.36,37
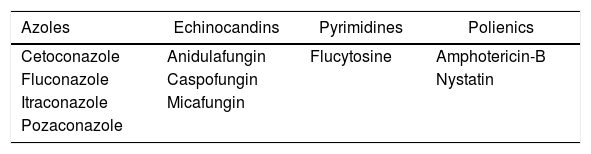
Many classes of drugs are available for the treatment of this type of infection, as shown in Table 3. However, considering that fungi, as well as human cells, are eukaryotic, patients may suffer from several side effects that arise from such drugs. Nephrotoxicity (especially in amphotericin administration), nausea/vomiting and stomachache are among the main effects.
In addition to side effects, another major challenge in the campaign against Candida spp. infections is the refractoriness to those medicines that are traditionally used.38 The latter problem arose from the indiscriminate use of antifungal drugs or long antifungal therapies, favoring the natural selection of Candida spp.-resistant strains. Some examples of resistant strains are C. albicans, C. glabatra, C. krusei and C. auris.39Candida lusitaniae has also been reported to develop resistance during therapy with Amphotericin-B.40
Therefore, different studies have indicated that the association of traditional medicines with alternative treatments (herein, natural products or biomolecules) as a promising option for therapeutic success against Candida spp. infection.41 Among the alternatives, the most prominent are lactoferrin, chitosan and Malaleuca alternifolia extracts. Promising biomolecules in the treatment of candidiasis
LactoferrinAntimicrobial peptides (AMPs) have been described in the scientific literature and correspond to a group of approximately 1200 molecules.42,43 They can be isolated from different sources such as bacteria, insects, plants and vertebrates.44 Lactoferrin (LF) is an example of an AMP.
LF was initially identified in cow milk in 1939.45 In human milk, LF was not isolated until 1960.46,47 Then, this peptide began being recognized as one of the essential components in the defense system for combatting infections caused by different etiologic agents—bacteria, fungi, parasites and viruses.48 In addition to being in milk, LF is also widely found on dermal mucosa, in neutrophils (where responses to inflammatory stimuli are released) and in external secretions—tears, saliva, vaginal mucus and seminal plasma.49,50 In body fluids, the concentration of LF depends on where the LF is found: in the colostrum, the concentration is from 5 to 7mgL−1, whereas in tears, the concentration is approximately 2mgL−1.51,52 In blood, depending on the inflammatory process, the concentration of LF also varies from 10−3 to 200μgmL−1.53 In contrast, according to Cohen et al. (1987), the concentration of LF in vaginal mucus varies as a function of the menstrual cycle: before menstruation, the concentration is 3.8–11.4μgmg−1, whereas after menstruation, this value increases to 62.9–218μgmg−1.54 Cohen et al. (1987) also showed that women submitted to continuous contraceptive-based treatment have drastically affected LF concentrations in the vaginal mucus, with concentrations remaining stable during the entire menstrual cycle at approximately 19.8μgmg−1.54
Structurally, LF is one glycoprotein bound to an iron nucleus, with a reddish-pink color, made up of 692 amino acids and with a molecular weight of 80kDa. Fifty-five percent of these amino acids are similar to the ones found in transferrin (the protein responsible for iron transport in plasma). Lactoferrin exists in two different forms: halo-lactoferrin (rich in iron) and apo-lactoferrin (free of iron). It is also described as being able to bind to other metals (aluminum, copper, gallium, manganese and zinc) better than iron.55
Regarding the antimicrobial activity of LF, the following mechanisms are described in the literature: (i) microbial growth is inhibited by iron deprivation because LF takes iron away from the pathogen or the media where the microorganism is present56; (ii) the protein presents different enzymatic activities in its purified sub-fractions, which include ATPase, DNase, RNase, phosphatase and hydrolysis of malto-oligosaccharide57; (iii) LF increases cell wall permeability, leading to the leakage of cytoplasmatic content and microorganism death because lactoferrin's N-terminus region has a strong positive charge that easily interacts with lipopolysaccharides, which have a negative charge58; and (iv) in contrast, as suggested by Ulvatne et al. (2001), LF is not able to induce cellular lyses in unscathed bacteria but leads to vesicle formation through a plasmatic membrane depolarization mechanism.59 Considering the facts mentioned previously, lactoferrin has been recently explored in several scientific investigations with different aims, especially in combination with antifungal drugs.60 In the latter case, a synergistic effect is the focus with regard to fighting against resistant and nonresistant microorganism strains in the genus Candida.61
Lactoferrin: antifungal drugs with a synergistic effectCandidemia in newborns is a critical problem that has continuously sparked the attention of the scientific community. Venkatesh and Rong (2008) evaluated the combined use of human recombinant lactoferrin (talactoferrin, TLF) with fluconazole and Amphotericin-B against three strains of Candida spp.; two strains were isolated from newborns diagnosed with septicemia caused by C. albicans. The results showed that either the interaction of TLF with fluconazole and/or TLF with Amphotericin-B presented a synergistic effect.62
Thereafter, Venkatesh et al. (2009) studied the prevention of biofilm formation (one of the virulence mechanisms observed in Candida) by the association of TLF with antifungal drugs; C. albicans ATCC MYA 4441 was the strain used in this study. A synergistic effect was observed in the mixture containing both TLF and Amphotericin-B and TLF and fluconazole. This study is especially important because Candida biofilm formation is extremely critical in intensive therapy units around the world, leading to high rates of mortality and morbidity from infections of the bloodstream related to catheters. Other studies have focused on the interaction of lactoferrin with fluconazole, showing that LF probably interacts with the microorganism's cell wall, raising its permeability and thereby increasing the rate of drug penetration into the cell.63
In an in vivo study, Tanida et al. (2001) carried out assays with rodents to investigate the synergistic effect of LF and other elements. However, in that study, the LF that was used was synthesized in the laboratory (known as peptide 2). Another similar synthetic molecule (peptide 2′) was also tested. Mice were inoculated with 5.0×108 cells of Candida (considered a lethal dose). Furthermore, the mice that did not receive treatment or were treated with peptide 2′ died after 8 and 7 days, respectively. On the other hand, mice submitted to intravenous administration of peptide 2 (5 and 100μgday−1) over 5 days lived longer (8.4±2.9 and 22.4±3.6 days, respectively). Additionally, in studies carried out by the same authors, a mouse that received 1.0×109Candida cells (above the lethal dose) had its life extended for 8.2±2.4 days (treated with 10μgday−1 of peptide 2) compared with the control (lived for a period of 3.2±1.3 days). When peptide 2 was administered (10μgday−1) along with Amphotericin-B (0.1μgday−1) and granulocyte macrophage colony-stimulating factor (GM-CSF) (0.1μgday−1), 60% of the mice lived for more than 22 days, even when receiving Candida doses higher than the lethal amount. When peptide 2 was administered along with GM-CSF, the phagocytic activity was raised 1.5 times due to an immune system stimulus.64
A few years later, Okamoto et al. (2004) confirmed the results of Tanida et al. (2011). In this case, the same methodology was adopted. However, another group of mice was also inoculated with 1.0×108Aspergillus fumigatus cells. For both microorganisms, the combination of lactoferrin, Amphotericin-B and GM-CSF increased the survival time of the rodents for 35 days.65
Melaleuca alternifoliaMelaleuca alternifolia belongs to the Myrtaceae family.66 This plant is described as a scrubland species and can be found primarily in South America, western India and Australia. In the latter country, the plant is known as tea tree and has been used in folk medicine learned from aboriginal people. Some biological activities of M. alternifolia include antibacterial, antiviral and antifungal action.67
Tea tree oil (TTO) can be obtained from the hydrodistillation of M. alternifolia leaves, and the chemical composition varies with the extraction method and crop region. Generally, this oil is a complex mixture of monoterpenes and terpenoids (50% oxygenated and 50% hydrocarbons) and approximately 100 other substances.68 Among these substances, terpinen-4-ol is the primary compound (≈30% TTO composition) and is indicated as the active ingredient responsible for the therapeutic properties of TTO. However, studies such the one performed by Miller (1984), where TTO's antimicrobial activity was higher than the activity of the isolated terpinen-4-ol, have shown the synergy among the compounds of the plant.69
According to Calo et al. (2015), the antimicrobial activity of the M. alternifolia terpenes is associated with hydrophobicity in which the terpenes interact with the pathogen's cellular membrane lipids, changing the membrane's permeability.70,71 Among the main results of membrane permeability are the (i) modification of proton-motive force, leading to a deficit in the production of cellular energy caused by the decrease in ATP generation and (ii) cellular lyses due to leakage or coagulation of the cytoplasm.72,73
Melaleuca alternifolia (tea tree oil): studies in the treatment of candidiasisMelaleuca alternifolia has been considered a promising alternative in the treatment of stomatitis caused by dentures, which is a recurrent problem in the elderly. de Campos Rasteiro et al. (2014) determined both the MIC and minimum biofilm eradication concentration (MBEC) of TTO in in vivo experiments. The results showed a 0.195% MIC and 12.5% MBEC. The latter concentration was used in topical application in animal experiments. Morphologic analysis through scanning electron microscopy (SEM) of mouse tongue tissue showed considerable wound area reduction, whereas colony-forming units were reduced by 5.33 log, which highlights the potential of M. alternifolia.74
Pachava et al. (2015) found colony-forming units of approximately 30, 43 and 19% of the control when discs impregnated with TTO were submerged in a C. albicans suspension. That result indicates that TTO could be used to coat dentures and reduce the wounds caused by Candida.75 Sharma and Hegde (2014) also investigated the impregnation of dentures with TTO and fluconazole. TTO's MIC was equal to 30% m/m, while fluconazole's MIC was 5% m/m. At the same concentrations, fluconazole lost its antifungal activity after 7 days, whereas TTO did not.76 Similar results were obtained by Dalwai et al. (2014), where both chlorhexidine gluconate and TTO retained their bioactivity after 14 days of incubation, whereas fluconazole became inactive.77 On the other hand, Van Vuuren et al. (2009) evaluated the association of TTO with amphotericin-B (AMP-B) against C. albicans (ATCC 10231), through MIC and fractionary inhibitory concentration index (FICI) calculation.78 The following antimicrobial agent ratio resulted in a synergistic interaction (FICI<1): 6:4 AMP-B:TTO (FICI 0.93) and 1:9 AMP-B:TTO (FICI 0.95).79 Similarly, Rosato et al. (2009) combined TTO and nystatin and used them to challenge C. albicans, C. krusei and C. tropicalis. This resulted in FICIs>0.5, also indicating a synergistic effect.80
Recently, in a study published by Di Vito et al. (2015), the combination of Amphotericin-B and TTO showed a synergistic effect against different species of Candida. That study focused on the development of a vaginal suppository for vulvovaginal candidiasis treatment. Candida strains were isolated from patients who had vaginitis. A synergistic effect was obtained for concentrations equal to 0.25μgmL−1 of AMP-B and 0.08μgmL−1 of TTO. In the same study, TTO was shown not to kill vaginal microbiota at concentrations below 2% v/v (Bifidobacterium animalis remained alive) and only concentrations above 4% v/v were bactericidal.81
Nonetheless, as described by Kon and Rai (2013), a large gap remains in the development of in vivo studies to evaluate the biological activity of M. alternifolia combined or not with antifungal drugs. Therefore, such studies should be encouraged to obtain more consistent results about the commercial use of TTO for the treatment of candidiasis.82
ChitosanChitin is defined as a natural polymer, and after cellulose, it is the most widespread and abundant polysaccharide found in nature. It has structural and protective functions and is found mainly in arthropods, the carapaces of shrimp, insects, and the cell walls of some fungi.
The cationic copolymer chitosan can be obtained from the deacetylation of chitin. In this process, chitin's acetyl groups (COCH3) are replaced by amine groups (–NH2). This process can be done through three different mechanisms: (i) the alkaline hydrolysis of the chitin at high temperatures, (ii) the employment of the enzyme chitinase, and (iii) through the direct action of microorganisms. Depending on the degree of acetylation, chitosan may have several byproducts of different composition, which will affect the molecule's solubility. For example, while chitin is insoluble in most solvents, chitosan is soluble in organic and inorganic acids. Structurally, chitosan is defined as a copolymer that is composed of d-glucosamine monomers and N-acetyl-d-glucosamine residues bound with glycoside β (1→4).83
Chitosan is functional, nontoxic, biocompatible, biodegradable, and bioactive, with chelating capacity, selective permeability and polyelectrolytes. All of these characteristics are dependent on chitosan's purity, viscosity, degree of acetylation and molecular weight. Another property of chitosan is its antimicrobial characteristics. The mechanisms of action seem to be through (i) chitosan's interaction with the phospholipids’ sialic acid (anionic group) because chitosan is positively charged, leading to cell agglutination and preventing cells from exchanging substances with external media, and (ii) the penetration of chitosan into the cell, where the chitosan blocks DNA and RNA transcription and inhibits cell growth. Generally, chitosan's amine groups are responsible for its antimicrobial activity.84
Chitosan: potential in candidiasis treatmentThe combination of chitosan with antifungal drugs has shown promising results. Soliman et al. (2015), for example, inoculated 0.1mL of C. albicans suspension into 80 mice with induced neutropenia. Twenty-four hours later, 20 animals were treated with saline solution (control group), 20 with chitosan extract (CE), 20 with Amphotericin-B (150mg kg−1) (AMB), and the last 20 with chitosan and amphotericin simultaneously (150mgkg−1) (CE+AMB) for 72h. When compared to the control group, the animals that received CE, AMB and CE+AMB showed a reduction in fungal density of 73%, 87% and 90%, respectively. In addition, oxidative stress markers, such as malondialdehyde (MDA), reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT) and nitric oxide (NO), were analyzed from the mouse lung tissue. The results showed that the groups that were treated with CE and CE+AMB showed a significant reduction in the levels of MDA, SOD, CAT and NO compared to the control group. On the other hand, the levels of GSH increased, which clearly indicates that chitosan can mitigate oxidative stress or lung wounds caused by systemic candidiasis.85
Alternatively, Senyiğit et al. (2014) evaluated the formulation of chitosan gel for vulvovaginal candidiasis treatment. Such a gel was produced with chitosan of different viscosities (20,000, 200,000 and 800,000mPas) and two antifungal drugs: miconazole nitrate and econazole nitrate. In vivo studies showed that chitosan of 200,000mPas led to the best results with both antifungal drugs. This conclusion was based on the following gel properties: mucosal adhesion, mechanical properties and adequate drug release.86
Chhonker et al. (2015) investigated the potential of chitosan, in combination with Amphotericin-B, as a treatment for fungal keratitis. The active compounds were used to coat 161.9–230.5-nm nanoparticles to extend the drug delivery time. The nanocarriers containing chitosan and amphotericin were found to be more active against C. albicans than the commercial formulation (Fungizone®). In addition, in vivo studies were carried out in rabbits for pharmacokinetic studies, where it was observed that the bioavailability of the medicine was increased 2.04 times, whereas the precorneal residence time was increased 3.36 times. Finally, the best results were achieved with chitosan of average molecular weight.87 Such results are relevant because fungal keratitis is an eyes disease that is responsible for blindness of many people around the world, and medicines currently available for its treatment have strong side effects.
Parker et al. (2016) investigated the role of chitosan in association with Amphotericin-B and polyethylene glycol in the local treatment of muscle wounds. Sponges were utilized as a means of delivering the formulation. The results of in vivo tests led to the conclusion that sponges coated with Amphotericin-B released the formulation (at concentrations above 0.25μgmL−1) for more than 72h.88
Final remarksThe prospection of biomolecules and natural products is clearly an important strategy for discovering and making available new drugs for the treatment of different diseases, as well as for use in increasing the activity of common drugs through synergism, which can be seen as a promising strategy to overcome the alarming current drug multiresistance scenario. In this context, M. alternifolia, lactoferrin and chitosan have been demonstrated to be viable options in the treatment of fungal infections, mainly against Candida spp., when used in association or not with antimicrobial dugs. This is especially important because Candida has led to a high mortality rate worldwide. Therefore, studies have indicated the feasibility of using synthetic and natural products in combination to improve their activity though synergism and overcome the problem of microbial multiresistance.
Conflicts of interestThe authors declare no conflicts of interest