Mangroves are ecosystems located in the transition zone between land and sea that serve as a potential source of biotechnological resources. Brazil's extensive coast contains one of the largest mangrove forests in the world (encompassing an area of 25,000km2 along all the coast). Endophytic bacteria were isolated from the following three plant species: Rhizophora mangle, Laguncularia racemosa and Avicennia nitida. A large number of these isolates, 115 in total, were evaluated for their ability to fix nitrogen and solubilize phosphorous. Bacteria that tested positive for both of these tests were examined further to determine their level of indole acetic acid production. Two strains with high indole acetic acid production were selected for use as inoculants for reforestation trees, and then the growth of the plants was evaluated under field conditions. The bacterium Pseudomonas fluorescens (strain MCR1.10) had a low phosphorus solubilization index, while this index was higher in the other strain used, Enterobacter sp. (strain MCR1.48). We used the reforestation tree Acacia polyphylla. The results indicate that inoculation with the MCR1.48 endophyte increases Acacia polyphylla shoot dry mass, demonstrating that this strain effectively promotes the plant's growth and fitness, which can be used in the seedling production of this tree. Therefore, we successfully screened the biotechnological potential of endophyte isolates from mangrove, with a focus on plant growth promotion, and selected a strain able to provide limited nutrients and hormones for in plant growth.
Mangroves are an important ecosystem in tropical biomes that occupy several million hectares of coastal area worldwide.1 Brazil possesses one of the largest mangrove forests, covering an area of 25.000km2 all along the coast. This ecosystem is located in the transition zone between land and sea2 and is characterized by periodic flooding, resulting in a unique environment with few plant species. Brazilian mangroves primarily comprise the following three tree species: Rhizophora mangle, Laguncularia racemosa and Avicennia sp.3 Furthermore, the mangroves harbor a diverse group of microorganisms.4,5 Several studies have examined the microbial community of mangroves by using metagenomic approaches to access the microorganisms involved in carbon,6 nitrogen7 and sulfer8 metabolism. Despite the high microbial diversity of mangroves, estimates suggest that less than 5% of species in this environment have been described.5
Moreover, the high diversity of culturable bacteria3 and culturable endophytic fungi9 within the Brazilian mangroves has not yet been explored. Few studies focus on the biotechnological potential of culturable mangrove isolates. Castro10 screened for enzymes for use in industrial processes, such as amylase, esterase, lipase, protease and endoglucanase. This large amount of microbial diversity can be exploited to improve crop science since the microorganisms produce phytohormones, such as indole acetic acid (IAA), enzymes, and antimicrobial molecules, and solubilize phosphate in the host plant.11,12 In addition, these organisms can fix nitrogen13 and increase drought resistance.14 More recently, the high tolerance of these microorganism to heavy metal was described15,16 in addition to characteristics that are important to the promotion of plant growth.
Bacteria that exhibit these features can be used to promote the growth of different plant species such as corn, soybeans, and sugarcane as well as arboreal species.17 These beneficial characteristics of the plant–microbe interactions can be used in other plants. Cross-colonization is common in nature in which the same bacterium can colonize different host plants. One example of cross-colonization is Pantoea agglomerans isolated from Eucalyptus grandis, which is able to colonize and promote plant growth in sugarcane.12 However, there are few studies evaluating the effects of bacterial inoculation in trees.18 The tree species Acacia polyphylla, of the Leguminosae family, commonly known as “monjoleiro” in Brazil, is widely used for the reforestation of degraded areas due to its ability to fix nitrogen19 and improve degraded soils, thus decreasing costs and benefitting the environment.20 Therefore, the aim of this study is to identify and analyze the biotechnological potential of endophytic bacteria isolated from a Brazilian mangrove environment and select strains able to promote the growth of A. polyphylla.
Materials and methodsEndophyte isolation sitesMangrove forest samples were previously collected from São Paulo state, Brazil, as described by Castro.10 The following three locations were assessed: (A) the Bertioga location, which was contaminated by oil spills; (B) the uncontaminated Bertioga location, with anthropogenic impacts; and (C) the uncontaminated Cananéia location, with low anthropogenic impacts. The following three mangrove species were assessed: (1) R. mangle, (2) L. racemosa and (3) Avicennia sp. The oil spill in Bertioga occurred approximately 20 years ago, and the anthropogenic impacts (domestic and industrial sewer) are still occurring in Bertioga at both locations sampled.6,10
From the whole mangrove bacterial collection, we randomly selected 115 isolates that were endophytically isolated from the branches of mangrove plants belonging to the culture collection of the Laboratory of Bacterial Genetics Microorganism, School of Agriculture Luiz de Queiroz (Esalq).3,10
Selection of endophytes: nitrogen fixationWe started our screening by evaluating the ability of the randomly selected 115 strains to fix atmospheric nitrogen. Qualitative assays were performed using the process of Liba.21 The strains were inoculated in tubes containing 10mL semi-solid NFb medium (5gL−1 malic acid, 0.5gL−1 K2HPO4, 0.2gL−1 MgSO4.7H2O, 0.1gL−1 NaCl, 0.01gL−1 CaCl2·2H2O, and 4mL 1.64% Fe-EDTA), 2mL 0.5% bromothymol blue, 2mL micronutrients (0.2gL−1 Na2MoO4·2H2O, 0.235gL−1 MnSO4·H2O, 0.28gL−1 H3BO3, and 0.008gL−1 CuSO4·5H2O), and 1.75gL−1 agar. Bacterial growth was evaluated after 72h of incubation at 28°C in the dark. The formation of a growth disc in the culture medium indicated atmospheric nitrogen fixation by the bacterial strains. This procedure was repeated five times for confirmation.
Selection of endophyte phosphate solubilizationStrains that could solubilize inorganic phosphate were identified by a quantitative test. This test involved observing the presence of a halo after bacterial cultivation on medium supplemented with Ca3(PO4)2 after seven days of incubation at 28°C. The results were quantified by estimating the halo size (cm) and dividing it by the colony size (cm) to generate a solubilization index (SI).22
Selection of endophytes that produce IAAThe strains that tested positive for phosphate solubilization and nitrogen fixation were tested for their ability to produce IAA. The quantitative IAA production was evaluated using the Patten and Glick23 method with modifications. The bacterial strains were inoculated in 10% Tryptone Soy broth medium (Difco, Livonia, USA) supplemented with l-tryptophan (5mM) and incubated at 28°C for 48h in the dark. Triplicate cultures were centrifuged (5min, 10,000×g, at room temperature), and 1.5mL Salkowski reagent24 was added to 1.5mL of the supernatant. This mixture was incubated for 20min in the dark at room temperature and analyzed using a spectrophotometer (520nm; Ultrospec 3000, Amersham-Pharmacia Biotech). The absorbance values obtained were interpolated in a standard curve to determine the IAA concentration.
Identification of strains by partial sequencing of the 16S rDNAThe identification of 38 bacterial strains able to fix nitrogen, solubilize phosphorus and produce IAA was performed by partial sequencing of 16S rDNA. The amplification of 16S rDNA was performed directly from bacterial colonies grown on solid TSA medium (10%) (Difco, Livonia, USA) using the primers R1387 (5′-CGGTGTGTACAAGGCCCGGGAACG-3′) and PO27F (GAGAGTTTGATCCTGGCTCAG-5′–3′).25
The 16S rDNA gene PCR products (approximately 1500bp) were purified by the polyethylene glycol method of Lis26 and sequenced at the Institute of the Human Genome (USP, São Paulo, Brazil). The sequences were evaluated with BLASTn27 against the database of the GenBank Development National Center for Biotechnology Information website.
Sequences were deposited in GenBank under the following access numbers: KF356429–KF356431, KF356438, KF356439, KF356444, KF356453, KF356454, KF356457, KF356459, KF356462, KF356465 and KM438481–KM438506.
Growth promotion of the seedlings of the reforestation angiosperm tree A. polyphyllaWe selected two strains that presented positive results for all of the tests performed, including phosphorus solubilization, nitrogen fixation and IAA production. Both strains produced the highest amount of IAA of the bacteria studied. However, one strain had a high phosphorous solubilization index value while the other had a low value for this index. The selected bacteria were grown in liquid TSB culture medium and incubated for 24h at 28°C at 150rpm. The optical density (600nm) was adjusted to 108 cells mL−1. The plant growth assays were performed in the reforestation Bioflora Company located in Piracicaba, São Paulo, Brazil. The experiment was conducted in a plant nursery whose internal temperatures ranged from 20 to 30°C during this period. The treatments were as follows: I, seedlings of A. polyphylla uninoculated and unfertilized; II, seedlings uninoculated and fertilized (using Forth Solúveis Inicial; Forth Aqua Micros and Forth Aqua Calcio, Tiete, Brazil); III, seedlings inoculated with Pseudomonas fluorescens (strain MCR1.10); IV, seedlings inoculated with Enterobacter sp. (strain MCR1.48); V, seedlings inoculated with P. fluorescens (strain MCR1.10) and Enterobacter sp. (strain MCR1.48) consortium. The treatments were performed in a completely randomized design with 25 repetitions (1 plant per pot). Treatments inoculated with bacteria (III, IV and V) were not fertilized. Each inoculated sample received a 1.0mL suspension of 108 cellsmL−1, and the un-inoculated controls were treated with 1.0mL of sterile water only. The inoculation with bacteria was performed by adding the bacterial suspension to the substrate. After inoculation, the seedlings were maintained in the plant nursery for 60 days. After this period, the seedlings were collected and washed in water. Then, the root systems were separated from the shoot, and the dry mass of roots and shoots from the seedlings were measured to evaluate plant growth promotion.
Statistical analysisData were subjected to analysis of variance, and means were compared by the Scott Knott test for IAA production and phosphorus solubilization index (<0.05) and the Tukey Test for plant experiments (<0.05). Statistical analyses were performed with R software (version 3.0.2).
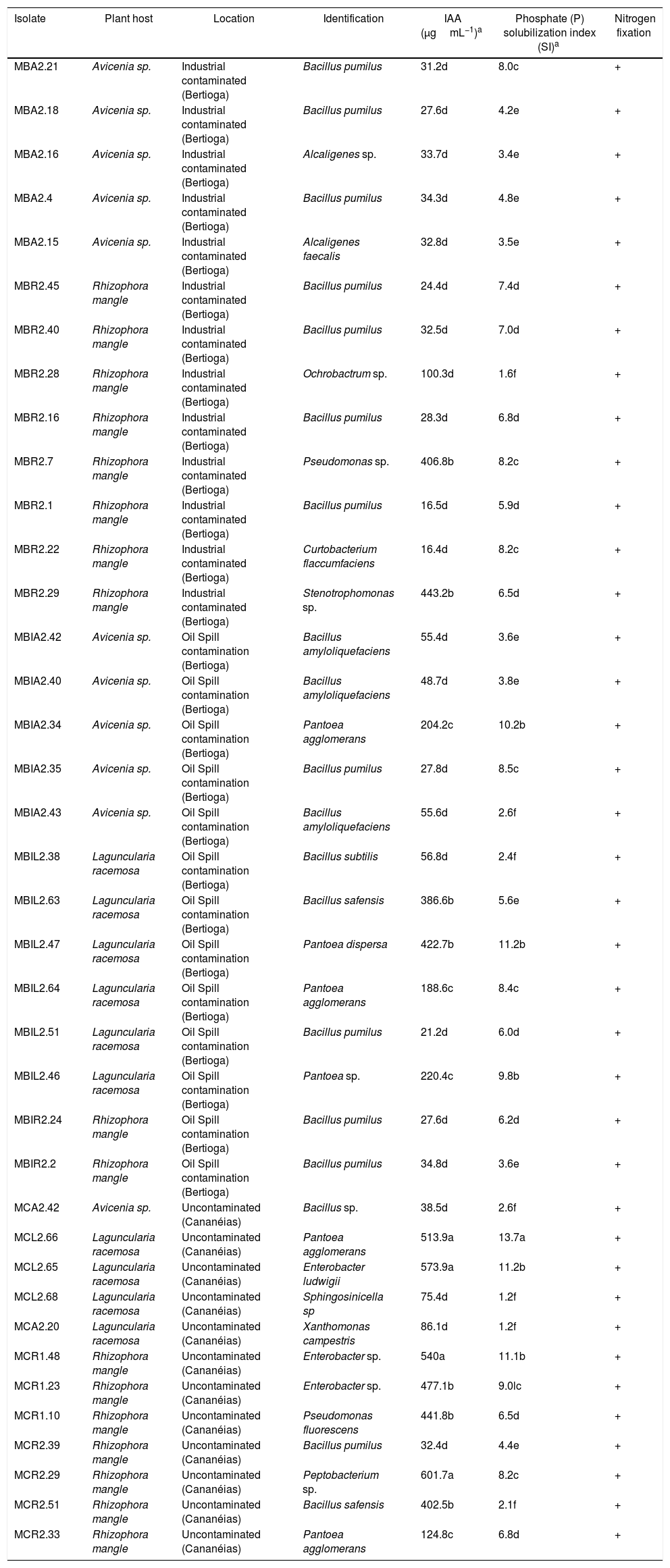
ResultsSelection of endophytes: nitrogen fixation and Phosphate solubilizationIn the nitrogen fixation test, 33% of the 115 strains examined (38) were able to grow in the medium free of nitrogen with a typical sub-surface growth, indicating their ability to fix nitrogen. This microaerophilic growth behavior resulted in a change of color of the culture medium from blue to green to yellow. This color change was caused by changes in the medium pH probably due to acidic molecules released by the isolate tested.
All 115 strains examined produced a halo during the phosphate solubilization test, indicating that they were all able to solubilize inorganic phosphate. The highest rates were observed in the genera Pantoea (MCL2.66), and the lowest rates were observed in the genera Sphingosinicella (MCL2.68), Xanthomonas (MCA2.20), Ochrobactrum (MBR2.28) and Bacillus (MBIA2.43, MCR2.51, MBIL2.38 and MCA2.42) with indices between 1.2 and 2.6.
Quantitative and qualitative assay of IAA productionThe IAA production assay was performed in 38 strains that were able to solubilize phosphate and fix nitrogen. All of the strains tested produced IAA ranging from 16.4 to 601.7μgmL−1. Pantoea (MCL2.66 and MBIL2.47), Enterobacter (MCL2.65, MCR1.48 and MCR1.23), Pectobacterium (MCR2.29), Bacillus (MCR2.51 and MBI2.63), Pseudomonas (MBR2.7, MCR1.10) and Stenotrophomonas (MBR2.29) genera showed the highest yields (Table 1). The lowest production was approximately 16μgmL−1 and was produced by strains MBR2.1 and MBR2.22, corresponding to the genera Bacillus and Curtobacterium, respectively (Table 1).
Identification and evaluation of in vitro tests for plant growth promoting bacteria isolated from branches of mangrove plants.
| Isolate | Plant host | Location | Identification | IAA (μgmL−1)a | Phosphate (P) solubilization index (SI)a | Nitrogen fixation |
|---|---|---|---|---|---|---|
| MBA2.21 | Avicenia sp. | Industrial contaminated (Bertioga) | Bacillus pumilus | 31.2d | 8.0c | + |
| MBA2.18 | Avicenia sp. | Industrial contaminated (Bertioga) | Bacillus pumilus | 27.6d | 4.2e | + |
| MBA2.16 | Avicenia sp. | Industrial contaminated (Bertioga) | Alcaligenes sp. | 33.7d | 3.4e | + |
| MBA2.4 | Avicenia sp. | Industrial contaminated (Bertioga) | Bacillus pumilus | 34.3d | 4.8e | + |
| MBA2.15 | Avicenia sp. | Industrial contaminated (Bertioga) | Alcaligenes faecalis | 32.8d | 3.5e | + |
| MBR2.45 | Rhizophora mangle | Industrial contaminated (Bertioga) | Bacillus pumilus | 24.4d | 7.4d | + |
| MBR2.40 | Rhizophora mangle | Industrial contaminated (Bertioga) | Bacillus pumilus | 32.5d | 7.0d | + |
| MBR2.28 | Rhizophora mangle | Industrial contaminated (Bertioga) | Ochrobactrum sp. | 100.3d | 1.6f | + |
| MBR2.16 | Rhizophora mangle | Industrial contaminated (Bertioga) | Bacillus pumilus | 28.3d | 6.8d | + |
| MBR2.7 | Rhizophora mangle | Industrial contaminated (Bertioga) | Pseudomonas sp. | 406.8b | 8.2c | + |
| MBR2.1 | Rhizophora mangle | Industrial contaminated (Bertioga) | Bacillus pumilus | 16.5d | 5.9d | + |
| MBR2.22 | Rhizophora mangle | Industrial contaminated (Bertioga) | Curtobacterium flaccumfaciens | 16.4d | 8.2c | + |
| MBR2.29 | Rhizophora mangle | Industrial contaminated (Bertioga) | Stenotrophomonas sp. | 443.2b | 6.5d | + |
| MBIA2.42 | Avicenia sp. | Oil Spill contamination (Bertioga) | Bacillus amyloliquefaciens | 55.4d | 3.6e | + |
| MBIA2.40 | Avicenia sp. | Oil Spill contamination (Bertioga) | Bacillus amyloliquefaciens | 48.7d | 3.8e | + |
| MBIA2.34 | Avicenia sp. | Oil Spill contamination (Bertioga) | Pantoea agglomerans | 204.2c | 10.2b | + |
| MBIA2.35 | Avicenia sp. | Oil Spill contamination (Bertioga) | Bacillus pumilus | 27.8d | 8.5c | + |
| MBIA2.43 | Avicenia sp. | Oil Spill contamination (Bertioga) | Bacillus amyloliquefaciens | 55.6d | 2.6f | + |
| MBIL2.38 | Laguncularia racemosa | Oil Spill contamination (Bertioga) | Bacillus subtilis | 56.8d | 2.4f | + |
| MBIL2.63 | Laguncularia racemosa | Oil Spill contamination (Bertioga) | Bacillus safensis | 386.6b | 5.6e | + |
| MBIL2.47 | Laguncularia racemosa | Oil Spill contamination (Bertioga) | Pantoea dispersa | 422.7b | 11.2b | + |
| MBIL2.64 | Laguncularia racemosa | Oil Spill contamination (Bertioga) | Pantoea agglomerans | 188.6c | 8.4c | + |
| MBIL2.51 | Laguncularia racemosa | Oil Spill contamination (Bertioga) | Bacillus pumilus | 21.2d | 6.0d | + |
| MBIL2.46 | Laguncularia racemosa | Oil Spill contamination (Bertioga) | Pantoea sp. | 220.4c | 9.8b | + |
| MBIR2.24 | Rhizophora mangle | Oil Spill contamination (Bertioga) | Bacillus pumilus | 27.6d | 6.2d | + |
| MBIR2.2 | Rhizophora mangle | Oil Spill contamination (Bertioga) | Bacillus pumilus | 34.8d | 3.6e | + |
| MCA2.42 | Avicenia sp. | Uncontaminated (Cananéias) | Bacillus sp. | 38.5d | 2.6f | + |
| MCL2.66 | Laguncularia racemosa | Uncontaminated (Cananéias) | Pantoea agglomerans | 513.9a | 13.7a | + |
| MCL2.65 | Laguncularia racemosa | Uncontaminated (Cananéias) | Enterobacter ludwigii | 573.9a | 11.2b | + |
| MCL2.68 | Laguncularia racemosa | Uncontaminated (Cananéias) | Sphingosinicella sp | 75.4d | 1.2f | + |
| MCA2.20 | Laguncularia racemosa | Uncontaminated (Cananéias) | Xanthomonas campestris | 86.1d | 1.2f | + |
| MCR1.48 | Rhizophora mangle | Uncontaminated (Cananéias) | Enterobacter sp. | 540a | 11.1b | + |
| MCR1.23 | Rhizophora mangle | Uncontaminated (Cananéias) | Enterobacter sp. | 477.1b | 9.0lc | + |
| MCR1.10 | Rhizophora mangle | Uncontaminated (Cananéias) | Pseudomonas fluorescens | 441.8b | 6.5d | + |
| MCR2.39 | Rhizophora mangle | Uncontaminated (Cananéias) | Bacillus pumilus | 32.4d | 4.4e | + |
| MCR2.29 | Rhizophora mangle | Uncontaminated (Cananéias) | Peptobacterium sp. | 601.7a | 8.2c | + |
| MCR2.51 | Rhizophora mangle | Uncontaminated (Cananéias) | Bacillus safensis | 402.5b | 2.1f | + |
| MCR2.33 | Rhizophora mangle | Uncontaminated (Cananéias) | Pantoea agglomerans | 124.8c | 6.8d | + |
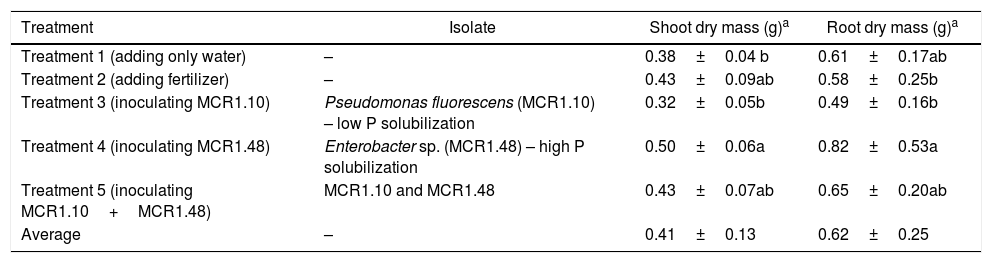
Two high IAA producing strains were selected. One strain had the highest phosphorous solubilization index, strain MCR1.48 (Enterobacter sp.), while strain MCR1.10 (P. fluorescens) had one of the lowest phosphorous solubilization indices. Both stains (with high and low phosphorous solubilization) were inoculated in A. polyphylla plants. The seedlings tested displayed different results when compared to the control (Table 2, Fig. 1).
The effect of soil inoculation of endophytic mangrove bacteria on A. polyphylla after 60 days following inoculation.
| Treatment | Isolate | Shoot dry mass (g)a | Root dry mass (g)a |
|---|---|---|---|
| Treatment 1 (adding only water) | – | 0.38±0.04 b | 0.61±0.17ab |
| Treatment 2 (adding fertilizer) | – | 0.43±0.09ab | 0.58±0.25b |
| Treatment 3 (inoculating MCR1.10) | Pseudomonas fluorescens (MCR1.10) – low P solubilization | 0.32±0.05b | 0.49±0.16b |
| Treatment 4 (inoculating MCR1.48) | Enterobacter sp. (MCR1.48) – high P solubilization | 0.50±0.06a | 0.82±0.53a |
| Treatment 5 (inoculating MCR1.10+MCR1.48) | MCR1.10 and MCR1.48 | 0.43±0.07ab | 0.65±0.20ab |
| Average | – | 0.41±0.13 | 0.62±0.25 |
The treatment with MCR1.10 (the low phosphorous solubilization strain) as well as the consortium treatment (V) was statistically similar to the control (not inoculated and not fertilized), indicating a lack of ability to promote plant growth. In contrast, seedlings inoculated with MCR1.48 (high phosphorous solubilization strain) caused an increase in dry root and shoot biomass. Some of the treated seedlings had an increase in the number of root hairs in their adventitious roots along with root page thickening when compared with the control (Table 2, Fig. 1). The seedlings with MCR1.48 (high phosphorous solubilization strain) inoculum and associated with fertilization were statistically similar.
DiscussionThe ability of endophytic bacteria to promote plant growth is attributed to direct mechanisms such as nitrogen fixation, plant hormone production (mainly IAA),28 phosphate solubilization, and siderophore production29 as well as the increased absorption of water and nutrients and the suppression of deleterious microorganisms by metabolite production.30
Phosphate solubilization by microorganisms has an important function in supplying phosphorus (P) to plants31 with the potential to be used as inoculants. Many authors32 report that the ability of microorganisms to solubilize phosphate correlates with the ability to produce organic acids and/or extracellular polysaccharide.33–35 In this study, all of the endophytes tested could solubilize P in vitro, although their solubilization indices varied.
In the IAA production assay, strains produced similar or higher amounts of IAA than previous reports indicated36,37 demonstrating their potential to improve plant growth. Some strains of the same species had different values, possibly due to the different amounts of tryptophan in the strains with each cell line having an optimal concentration for interfering with the synthesis of IAA.38,39
Several studies report that endophytic microorganisms are able to promote plant growth in different plants,17,40 such as corn (Zea Mays), peppermint (Mentha piperita),41Vitis vinifera L.42 and pineapple (Ananas comosus).43 However, there are few reports on the use of endophytic bacteria on growth promotion in woody plants. Previous studies were performed mainly on trees important in the paper and cellulose industry, such as pines and eucalyptus.18 Burns and Schwarz44 found that an unidentified bacterium induced 90% of root explant in Pinus elliottii when compared to the control.44 Other tree species studied included poplar (Populus), which was used as a model plant, and the bacterium Burkholderia multivorans, a nonpathogenic strain, reported to be able colonize the roots and significantly promote the growth of poplar seedlings.45 Moreover, Bashan46 reported that Azospirillum brasilense and Bacillus pumilus promoted the growth of two trees used in reforestation (Prosopis articulata and Parkinsonia microphylla), thus increasing plant development and survival.
Two high IAA producing strains, P. fluorescens and Enterobacter sp., were selected to inoculate plants and evaluate their growth in field conditions since both the Pseudomonas and Enterobacter genera have been previously reported to promote the growth of plants.47–49 The main difference between the strains selected is that Enterobacter sp. (MCR1.48) has a high P solubilization index, while P. fluorescens (MCR1.10) has one of the lowest P solubilization indices. We selected the commonly used reforestation tree A. polyphylla, which has few published studies involving inoculation by the bacteria of interest. The present work demonstrates that the inoculation of a high P solubilization strain MCR1.48 (Enterobacter sp.) increased the shoot dry mass of monjoleiro. This result indicates that phosphorous solubilization plays a key role in tree plant growth. Therefore, this study is the first known report of the growth promotion of A. polyphylla from the inoculation of an endophytic bacterium.
In reforestation, the rotation time is long, so the inoculation of microorganisms to accelerate the trees’ development should help them grow more quickly over the long term. We report an increase in the rate of seedling survival, early establishment in the field after planting along with improvement in quality and characteristics of the root system, i.e., all advantages desirable for better production. Therefore, we successfully screened the biotechnological potential of endophyte isolates from mangroves, focusing on plant growth promotion. We selected a strain able to provide limited nutrients by fixing nitrogen since not all forms of nitrogen in the soil are available to the plant; by solubilizing phosphorus, which is present in soil but is also not available to plants; and by producing plant hormones involved in plant growth such as auxin (IAA).
The use of an endophytic microorganism in the substrate as inoculum for the production of seedlings of A. polyphylla is a highly successful strategy that can be utilized in the reforestation nursery. The inoculant effect of the Enterobacter sp. endophytic bacteria in A. polyphylla seedlings affect the quality of seedling production, improving the development and possibly reducing the cost of chemical fertilization performed by the nursery company.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by a grant from FAPESP (Proc.2004/15414-6) to R.A. and by a fellowship from CNPq to M.N.D. (Proc 150228/2017-1). We thank Bioflora for providing the structure and staff to develop nursery experiments.