This study aimed to explore the effects of two siderophore-producing bacterial strains on iron absorption and plant growth of peanut in calcareous soil. Two siderophore-producing bacterial strains, namely, YZ29 and DZ13, isolated from the rhizosphere soil of peanut, were identified as Paenibacillus illinoisensis and Bacillus sp., respectively. In potted experiments, YZ29 and DZ13 enhanced root activity, chlorophyll and active iron content in leaves, total nitrogen, phosphorus and potassium accumulation of plants and increased the quality of peanut kernels and plant biomass over control. In the field trial, the inoculated treatments performed better than the controls, and the pod yields of the three treatments inoculated with YZ29, DZ13, and YZ29+DZ13 (1:1) increased by 37.05%, 13.80% and 13.57%, respectively, compared with the control. Based on terminal restriction fragment length polymorphism analysis, YZ29 and DZ13 improved the bacterial community richness and species diversity of soil surrounding the peanut roots. Therefore, YZ29 and DZ13 can be used as candidate bacterial strains to relieve chlorosis of peanut and promote peanut growth. The present study is the first to explore the effect of siderophores produced by P. illinoisensis on iron absorption.
Iron is an essential trace mineral for plants. Most of the iron in the soil exists as ferric iron (Fe3+), which cannot be absorbed by plants. The effective iron content in the soil is very low, especially in calcareous soil; thus, plant iron deficiency is a serious global problem.1 Peanut is one of the most important oil crops and cash crops in China. The northern region is one of the largest cultivated areas in China and is composed of sandy alkaline soil. Due to the sensitivity to iron, iron-deficiency chlorosis in peanut frequently occurs. This disease has become an important limiting factor for peanut yield and quality.2 Therefore, it is extremely urgent to find a safe, effective and economical approach to relieve iron-deficiency chlorosis in peanut.
A set of microbes has been discovered to be able to synthesize and secrete siderophores, a group of small molecular compounds (molecular weight: 1–2kDa) that chelate Fe3+ at high specificity to absorb iron in the surrounding environment.3 The Fe3+-siderophore can be recognized and absorbed by many types of plant and is vital for iron absorption, especially in calcareous soil. In the past years, the possible implication of siderophores produced by plant growth promoting rhizobacteria (PGPR) has been considered as a potential way to alleviate iron-deficiency chlorosis and improve plant growth. Many studies focus on the role of Pseudomonas in promoting plant iron intake. For example, Manwar et al. have discovered that the siderophore pyoverdine produced by Pseudomonas aeruginosa increases chlorophyll content, percentage germination, root ramification, nodulation, height and foliage of groundnut. Siderophores produced by Pseudomonas putida are able to enhance the chlorophyll content of peanut in calcareous soil.4 Siderophores produced by P. putida P3 have been found to enhance the chlorophyll content and the presence of 59Fe in the roots of peanut in nutrient solution.5 Furthermore, siderophores produced by some fungi have also drawn much attention. For instance, the siderophore mixture produced by Penicillium chrysogenum is able to increase the chlorophyll content of cucumber and maize under hydroponic conditions.6 Rhizoferrin, siderophores secreted by Rhizopus arrhizus, can induce normal growth in plants with iron deficiency chlorosis.7 However, reports on the effect of Paenibacillus sp. on iron absorption of plants, especially under calcareous soil conditions, are limited. In addition, all of the aforementioned experiments were conducted in the laboratory or iron-deficient indoor conditions, which were quite different from the outdoor natural conditions.
In this study, we hypothesized that siderophore-producing Paenibacillus sp. can promote the iron absorption of plant in calcareous soil, thus promoting plant growth. To confirm this hypothesis, potted assays and field trial were performed to investigate the effects of two siderophore-producing and spore-producing strains on iron absorption and peanut growth in calcareous soil in indoor and outdoor natural conditions. This study was expected to provide a basis for preventing iron-deficiency chlorosis of peanut using a biological method.
Materials and methodsMedium used in the assaysLB medium was used to activate and culture the isolates for the identification of strains. This medium contained 1% tryptone, 0.5% yeast extract, 1% NaCl and 1.5% agar.
Bean sprouts medium was used to prepare the inocula of the isolates and contained 1% bean sprouts, 3% saccharose and 0.8% (NH4)2SO4.
CAS (chrome azurol S) medium was used to evaluate the siderophore production of the isolates and was carried out by the Schwyn method.8
Used bacterial strains and strain identificationThe two strains, namely, YZ29 and DZ13, were cultivated on LB plates in a constant temperature incubator at 37°C for 24h. These strains were subjected to morphological observation, physiological and biochemical determination (such as fermentation of saccharides and hydrolysis of starch and gelatin)9 and analysis of 16S rRNA gene sequences.
The DNA of the two strains was extracted using a commercially available bacteria DNA extraction kit (Bacteria DNA Kit, Tiangen, Beijing, China). Genomic DNA samples were subjected to 16S rDNA amplification using fluorescent primers 27F and 1492R. Amplified DNA was purified using a commercially available kit (Common DNA Product Purification Kit, Tiangen, Beijing, China). PCR amplified fragments were then subjected to detection in Sangon Biotech (Shanghai) Company (Shanghai, China). A phylogenetic tree based on 16S rRNA gene sequences was constructed using Molecular Evolutionary Genetics Analysis (MEGA) 4.0 software (available at: http://www.megasoftware.net).10
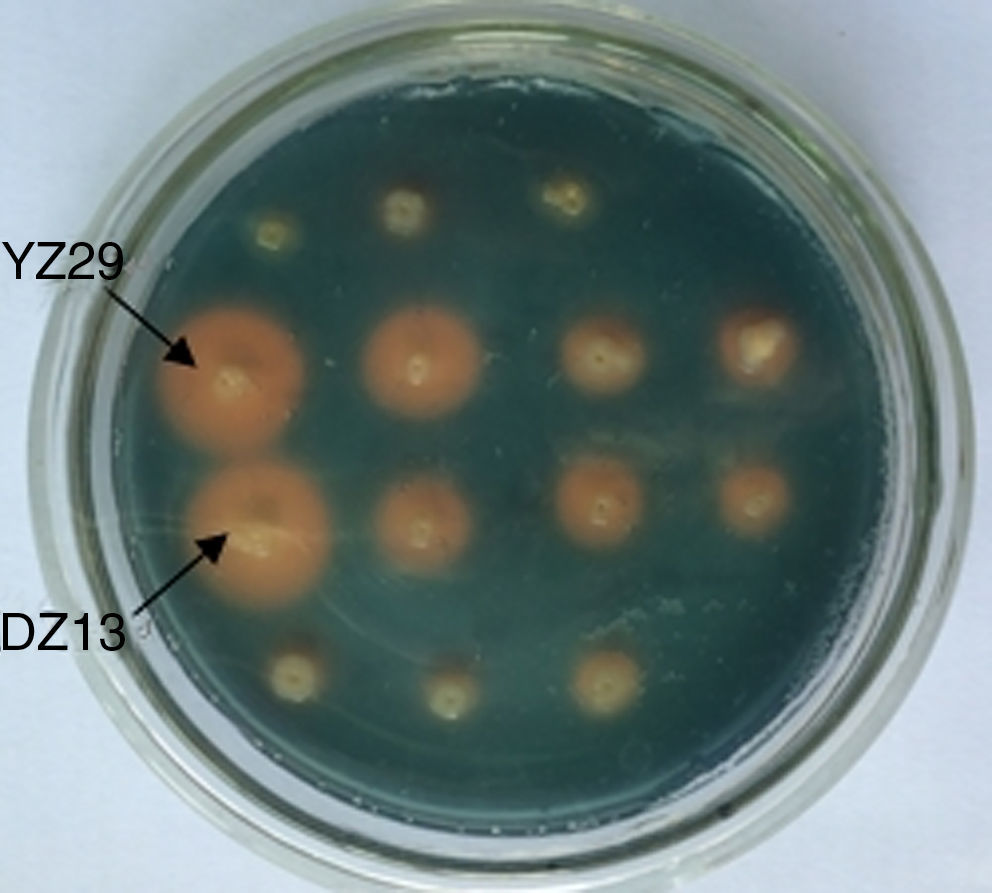
Siderophore-producing performance testThe two strains were isolated from the peanut rhizosphere soil by other researchers in our laboratory with the methods of Wang,11 and they have been detected to produce more siderophores than other isolates, including actinomycetes. To determine the siderophore-producing ability of these two strains, they were inoculated using sterile toothpicks on the CAS plates and cultivated at a constant temperature incubator at 37°C for 3 days to 5 days and were checked for the presence or absence of orange circles surrounding the bacteria.
Peanut cultivarsWeihua No. 8 (Arachis hypogaea L., vulgaris) was used in the pot assay in 2012. Baisha 1016 (A. hypogaea L., vulgaris) was used in the pot assay in 2013. Luhua No. 14 (A. hypogaea L., vulgaris) was used in the field assay. These three cultivars are all susceptible to iron deficiency.
Soil used in the assaysSoil used in the pot assay in 2012Calcareous soil was collected from the plough layer of a farmland in Jinan, East China (36°28′N, 116°45′E). The soil was composed of 18.53gkg−1 organic carbon, 100.75mgkg−1 NO3−, 4.04mgkg−1 NH4+, 22.89mgkg−1 rapidly available P, 96.31mgkg−1 rapidly available K, 2.318mgkg−1 available iron (DTPA-Fe), 1.6648mgkg−1 available zinc (DTPA-Zn), 0.7076mgkg−1 available copper (DTPA-Cu) and 3.32mgkg−1 available manganese (DTPA-Mn).
Soil used in the pot assay in 2013The soil used in the pot assay was also obtained from the plough layer of a farmland in Jinan, East China. The soil was composed of 19.89mgg−1 organic matter, 97.02mgkg−1 NO3−, 9.05mgkg−1 NH4+, 19.75mgkg−1 rapidly available P, 106.31mgkg−1 rapidly available K and 2.016mgkg−1 available iron (DTPA-Fe) and exhibited a strong calcareous reaction.
Soil used in the field trial in 2013The field trial was conducted in a farmland in Jinan City, East China (36°28′N, 116°45′E). The soil had the following composition: 21.93mgg−1 organic matter, 104.02mgkg−1 NO3−, 5.05mgkg−1 NH4+, 17.68mgkg−1 rapidly available P, 103.64mgkg−1 rapidly available K and 2.153mgkg−1 available iron (DTPA-Fe) and exhibited a strong calcareous reaction.
Preparation for the Inocula of YZ29 and DZ13To activate YZ29 and DZ13 preserved on agar slant culture medium, we inoculated the two strains into 10mL of liquid LB and cultivated on a rotary shaker at 180×g and 37°C for 12h. The seed solution was then inoculated into 200mL of liquid medium (LB medium in pot assays, bean sprouts medium in field assay) with 1% final concentration and fermented on a rotary shaker at 180×g and 37°C for 48h. The fermentation liquor was centrifuged at 8000×g for 10min, and the supernatant was discarded. Moderate sterile deionized water was added to the precipitate to obtain a 108cfumL−1 bacterial suspension.
Methods of applying the bacteriaSeed soakingScreened plump peanut seeds were dipped in bacterial suspension for 30min and dried in the shade before sowing.
Root irrigationBacterial suspension per plant was applied once directly into the soil near the roots after germination and before or after blossom (25mL per plant in pot assays, 200mL per plant in field assay).
Experimental designExperimental treatmentsThe following four treatments were used: no inoculation (C), YZ29 inoculation (Y), DZ13 inoculation (D) and dual inoculation of YZ29 and DZ13 (Y+D).
Pot assay in 2012The pot assay was performed in a greenhouse. Each treatment was applied to three pots. Ten seeds were sown in each pot (34cm×32cm), one seed per hole. After germination, in order to furthest reduce individual differences of plants, four seedlings with similar growth status were maintained in each pot. Pots were randomly arranged. Plants were watered every 1–2 days to maintain appropriate relative water content of the soil. Each pot was applied with equal volume of deionized water. During the whole growth period, no agrochemicals were applied. At each growth stage, one plant was sampled from each pot.
Pot assay in 2013The pot assay was performed in an open space. Inoculation treatments were the same as the assay in 2012. Each treatment was applied to four pots. Ten seeds were sown in each pot (34cm×32cm), one seed per hole. After germination, in order to furthest reduce individual differences of plants, four seedlings with similar growth status were maintained in each pot. Pots were randomly arranged. Plants were watered every 1–2 days to maintain appropriate relative water content of the soil. Each pot was applied with equal volume of deionized water. During the whole growth period, no agrochemicals were applied. At each growth stage, one plant was sampled from each pot.
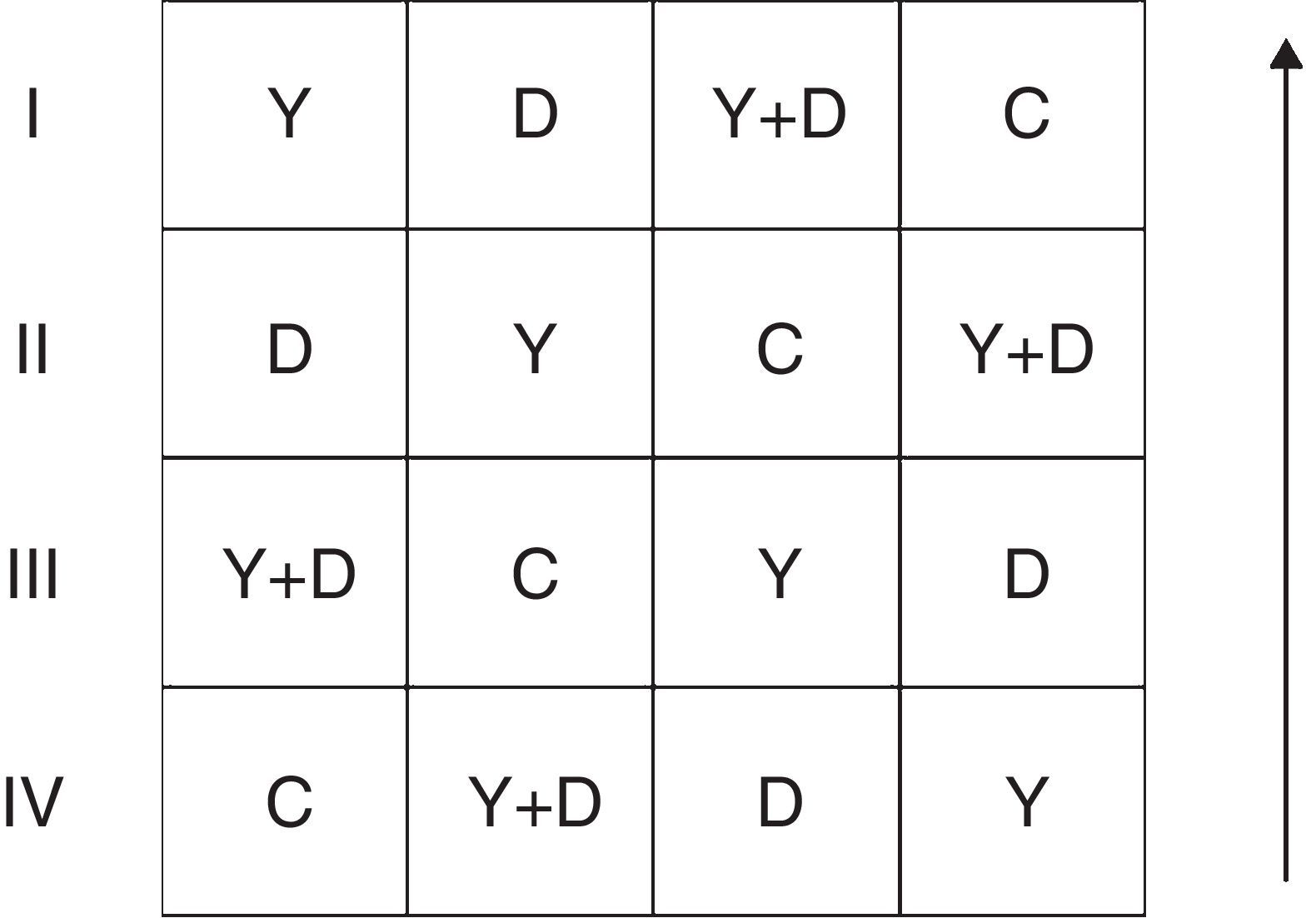
Field trialThe field trial was conducted in a farmland in Jinan City, East China (36°28′N, 116°45′E) from May to September 2013. Inoculation treatments were the same as the assay in 2012 and were conducted in a randomized complete block design. Each treatment had four replicates. Each replicate had an area of 15m2 and three peanut lines (approximately 225 holes, three seeds per hole). Row-to-row and plant-to-plant spacing were maintained at 30 and 20cm, respectively. The replicates were arranged as Fig. 1. During the whole growth period, no agrochemicals and additional artificial watering were applied to the farmland.
General arrangement of blocks in the field assay. I, II, III and IV represent the four replicates; the arrow represents the direction of peanut lines. C: control, received no inoculation; Y: received YZ29 inoculation; D: received DZ13 inoculation; Y+D: received YZ29 and DZ13 inoculation.
Chlorophyll was extracted by soaking 100mg of fresh young leaves in 25mL of mixed liquor of acetone and ethyl alcohol (1:1) at 50°C for 2–3h. Chlorophyll concentration was measured at 663 and 645nm with a spectrophotometer (Spectrumlab 22pc, Leng Quang Tech, Shanghai, China) using the Mackinney method.12
Determination of iron concentration in leavesIron was extracted by soaking 100mg of dry young leaves in 25mL of 1molmL−1 hydrochloric acid at room temperature for 24h. Iron concentrations of the extracted samples were measured using an atomic absorption spectrophotometer (NOVAA 300, Analytikjena, Jena, Germany).
Total nitrogen, phosphorus and potassium contents of plantsPlant shoots and roots of the peanut in each growth period were dried at 80°C for more than 72h and subsequently ground for nitrogen, phosphorus and potassium analyses. Sample liquid was prepared by the sulfuric acid hydrogen peroxide digestion method.13 The total potassium content of plant samples was analyzed using FP6430 flame spectrophotometer (Shanghai Analytical Instrument Factory, Shanghai, China). The total nitrogen and phosphorus contents of plant samples were analyzed using Bran+ Luebbe AutoAnalyzer 3 flow-injection analyser (Technicon Industrial Systems, Elmsford, NY, USA).
Determination of root activityIn each growth period, roots of all sampled peanut plants were harvested to determine root activity by the methylene blue adsorption method. Intact root samples were sequentially stained in three beakers containing 5mL of 0.075mgmL−1 methylene blue solution for 1.5min and then removed. The cleaned solution (0.5mL) in the three beakers was respectively diluted 15 times and subsequently measured at 660nm using a spectrophotometer (Spectrumlab 22pc, Leng Quang Tech, Shanghai, China).
Statistics of plant biomass and yield factorsIn each growth period, peanut plants from different treatments were harvested to determine dry weight. The roots were washed twice with deionized water, and then the plants were dried at 80°C to a constant weight. When peanut plants reached maturity, peanut pods from different treatments were harvested to determine the percentage of full pods and total weight of 100 fresh pods. The pods were dried at 80°C until the weight was constant, then percentage of full pods, kernel rate and yield were calculated.
Analysis of peanut qualityAt harvest time, peanuts from different treatments were harvested and dried at 80°C for more than 72h. Peanut kernels were ground to determine protein and crude fat. Protein content was measured using the Kjeldahl method.14 Crude fat content was determined with the Soxhlet extractor method.15
Analysis of bacterial community diversityThe terminal restriction fragment length polymorphism (T-RFLP) analysis method16 was used to analyze the bacterial community diversity of soil samples surrounding the roots of peanut plants that were subjected to different treatments in each growth period.
Detection of 16S rDNA sequences of soil samplesDNA of soil samples was extracted using a commercially available soil DNA extraction kit (Soil DNA Kit, Omega, USA). Genomic DNA samples were subjected to 16S rDNA amplification using fluorescent primers 27F-FAM (5′-FAM-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) and 1492R (Sangon Biotech Company, Shanghai, China). The conditions of PCR amplification were as follows: 94°C (5min); 30 cycles at 94°C (30s), 55°C (30s), and 72°C (1.5min); and 72°C (5min).17 Amplified DNA was purified using a commercially available kit (Common DNA Product Purification Kit, Tiangen, Beijing, China) before restriction digestion. PCR-amplified fragments were digested with the restriction enzymes Msp I, Hha I and Alu I at 37°C for 5h, and digestion was terminated at 65°C for 15min. Digestion products were subjected to short tandem repeat (STR) detection on an ABI 3730 DNA Sequencer (Applied Biosystems, Foster City, CA, USA).
Analysis of T-RFLP mapAnalysis of T-RFLP map was conducted based on Dunbar18 and Lukow19 methods. Bio-Dap software20 was used to determine the Shannon–Wiener diversity index, Margalef index and Simpson index to calculate the bacterial community of each soil sample. Based on the results of the T-RFLP map, species with speculated T-RF peaks were predicted using Web-based Phylogenetic Assignment Tool.21
Statistical analysisData were presented as mean±standard deviation from three separate experiments performed in duplicate. Statistical analysis was performed using the SPSS 19.0 software (SPSS, Chicago, USA). One-way analysis of variance (ANOVA) followed by Duncan test was used to compare group means. Differences were considered significant if p<0.05.
ResultsTest for siderophore productionSiderophore production of the strains YZ29 and DZ13 was tested. In both strains, orange circles visibly surrounded the bacterial colonies (Fig. 2), indicating that YZ29 and DZ13 were able to produce siderophores.
Identification of strainsMorphological characteristicsThe bacterial colonies of YZ29 were round, ivory yellow, slightly uplift and smooth, and the margin of the colony was soigne. The thalli of YZ29 were rod shaped, and its size was (0.5–0.7)μm×(1.2–2.0)μm. YZ29 could produce spores and was determined to be Gram-positive.
The bacterial colonies of DZ13 were irregular, filamentous and oyster white, and the surface was dry. The thalli of DZ13 were bacilliform, and its size was (0.7–0.9)μm×(1.2–1.5)μm. DZ13 could produce ovate spores and was found to be Gram-positive.
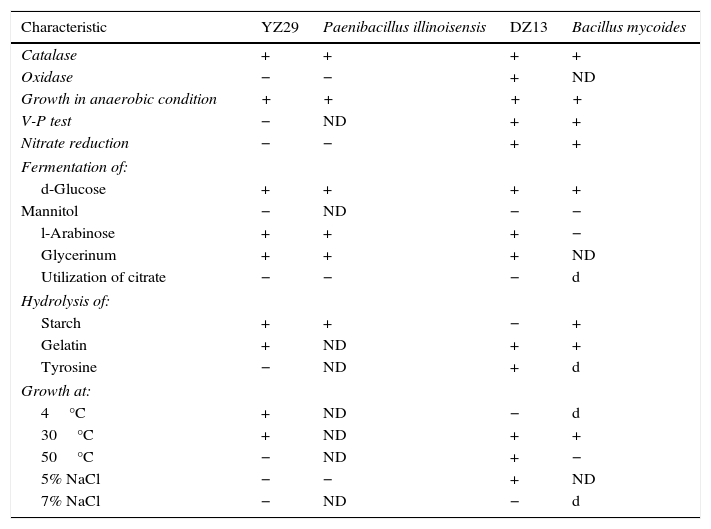
Physiological and biochemical characteristicsThe physiological and biochemical characteristics of YZ29 and DZ13 were determined (Table 1). The results were compared with Bergey's Manual of Determinative Bacteriology (eighth edition).
Physiological and biochemical characteristics of YZ29 and DZ13.
| Characteristic | YZ29 | Paenibacillus illinoisensis | DZ13 | Bacillus mycoides |
|---|---|---|---|---|
| Catalase | + | + | + | + |
| Oxidase | − | − | + | ND |
| Growth in anaerobic condition | + | + | + | + |
| V-P test | − | ND | + | + |
| Nitrate reduction | − | − | + | + |
| Fermentation of: | ||||
| d-Glucose | + | + | + | + |
| Mannitol | − | ND | − | − |
| l-Arabinose | + | + | + | − |
| Glycerinum | + | + | + | ND |
| Utilization of citrate | − | − | − | d |
| Hydrolysis of: | ||||
| Starch | + | + | − | + |
| Gelatin | + | ND | + | + |
| Tyrosine | − | ND | + | d |
| Growth at: | ||||
| 4°C | + | ND | − | d |
| 30°C | + | ND | + | + |
| 50°C | − | ND | + | − |
| 5% NaCl | − | − | + | ND |
| 7% NaCl | − | ND | − | d |
Note: Paenibacillus illinoisensis and Bacillus mycoides are type strains. +, positive; −, negative; d, 11%–89% strains are positive; ND, not detected. Analysis of 16S rRNA gene sequences.
Each characteristic of YZ29 was the same as in the type strain Paenibacillus illinoisensis. Some characteristics of DZ13, such as fermentation of l-Arabinose, hydrolysis of starch and growth at 50°C, were contrary to that of the type strain Bacillus mycoides. The aforementioned differences may be due to the different growth environments.
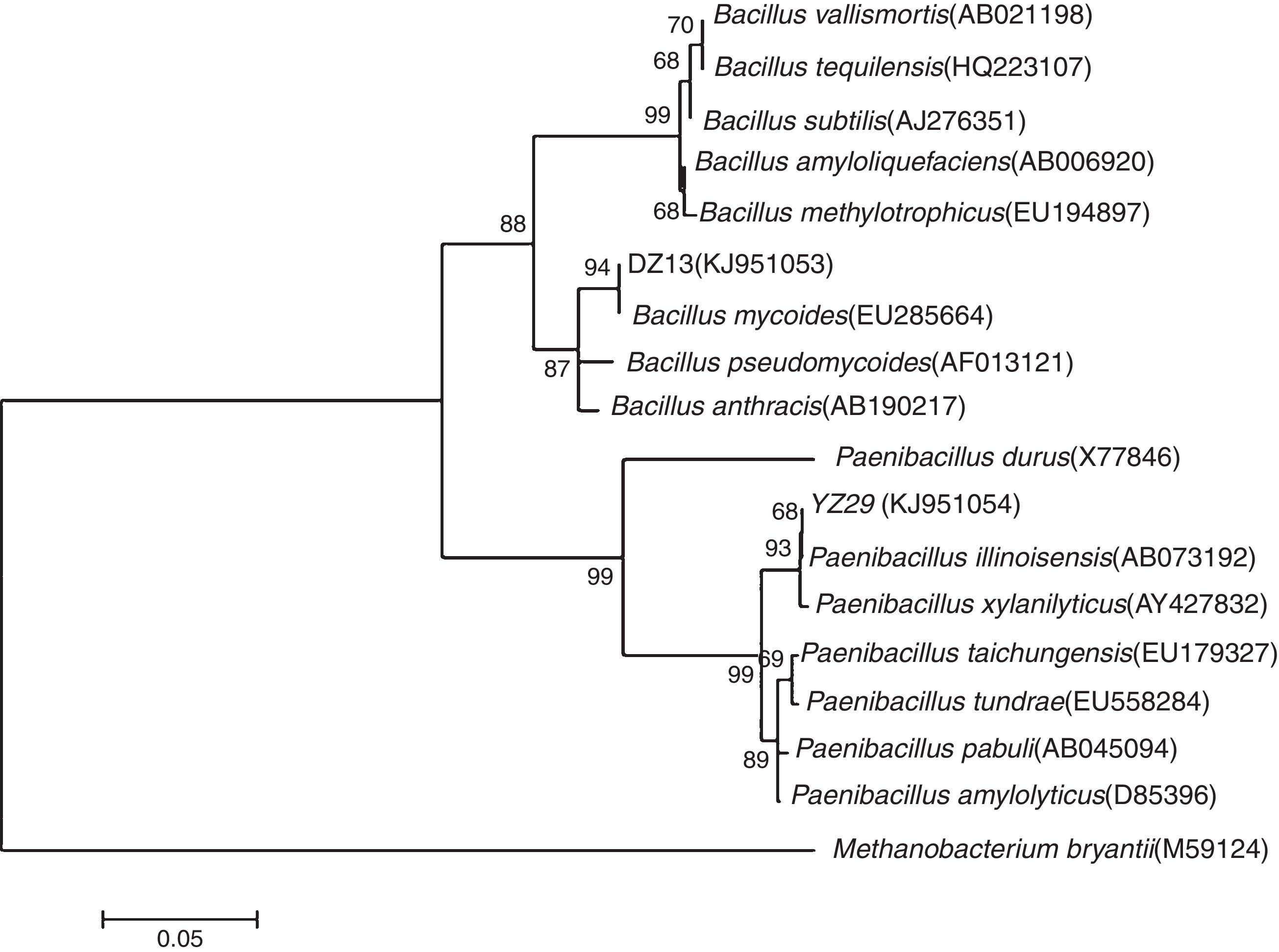
Analysis of 16S rRNA gene sequencesThe 16S rRNA gene sequences of YZ29 and DZ13 were submitted to GenBank and got accession numbers KJ951054 and KJ951053, respectively. A phylogenetic tree was created based on 16S rRNA gene sequences (Fig. 3). YZ29 and P. illinoisensis (AB073192) were in the same minimum branch. Moreover, the sequence similarity of 16S rRNA gene sequences of YZ29 and P. illinoisensis (JQ579623) was 99% based on sequence alignment with BLAST. DZ13 and B. mycoides (EU285664) were in the same minimum branch, and the sequence similarity of 16S rRNA gene sequences of DZ13 and B. mycoides (EU285664) was 97% based on sequence alignment with BLAST. Considering the aforementioned results of the morphological characteristics and physiological and biochemical characteristics, YZ29 and DZ13 were identified as P. illinoisensis and Bacillus sp., respectively.
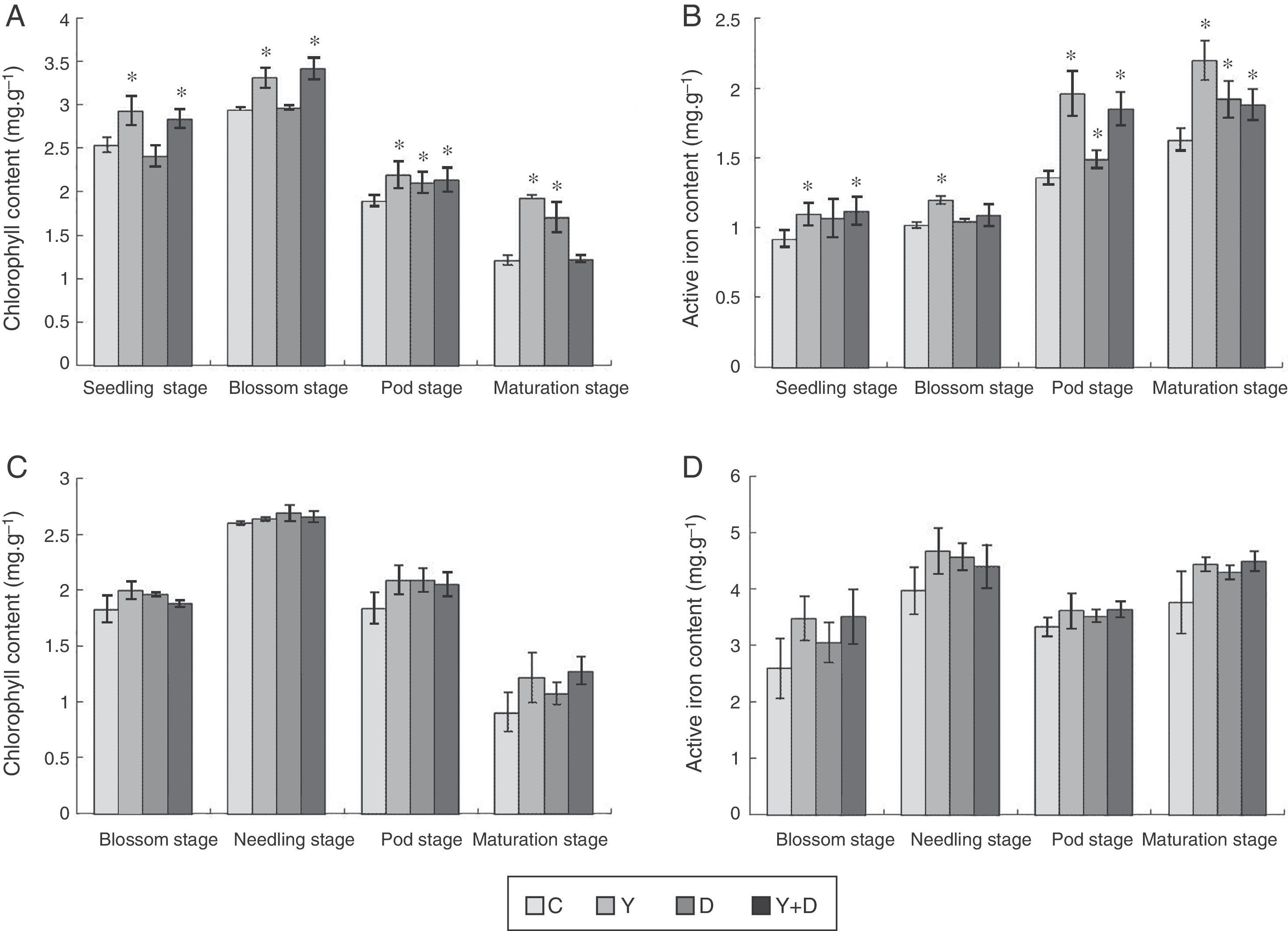
Chlorophyll and active iron contents in leavesIn the potted plants, chlorophyll content in the three inoculated treatments were compared with that in the control plants at the four stages (Fig. 4A). At the seedling and blossom stages, significant increase (p<0.05) was observed in the chlorophyll content in Y-treated and Y+D-treated plants compared with the control. At the pod stage, the chlorophyll content in all of the four treatments decreased compared with the blossom stage, whereas, chlorophyll content in the three inoculated treatments was significantly higher than that in the control (p<0.05). At the maturation stage, the chlorophyll content in Y-treated and D-treated plants was still markedly higher (p<0.05) compared with the control plants. Strikingly, the chlorophyll content in D-treated plants was lower than that in Y-treated plants at the seedling and blossom stages (Fig. 4A).
Chlorophyll and active iron contents in the leaves of different treatments in each growth period. (A) Chlorophyll content of leaves in each growth period in the pot assay in 2013. (B) Active iron contents of leaves in each growth period in the pot assay in 2013. (C) Chlorophyll content of leaves in each growth period in the field trial. (D) Active iron contents of leaves in each growth period in the field trial. Data were presented as mean±standard deviation. * indicates significant difference comparing with the control group (p<0.05). C: control, received no inoculation; Y: received YZ29 inoculation; D: received DZ13 inoculation; Y+D: received YZ29 and DZ13 inoculation.
Furthermore, the active iron content of leaves in the three inoculated treatments in pots were compared with that in the control plants in the whole growth period (Fig. 4B). YZ29 significantly increased the active iron content in leaves by 19.57%, 17.65%, 44.12% and 34.97% (p<0.05), respectively, compared with the control in the four growth periods. Besides, DZ13 enhanced the active iron content in leaves by 16.30%, 2.94%, 9.56% and 17.79%, respectively, compared with the control. In addition, the active iron contents in the leaves of Y-treated plants were obviously higher than those of D-treated plants at the blossom, pod and maturation stages, indicating that YZ29 had a higher capacity than DZ13 in promoting iron uptake (Fig. 4B).
However, under field conditions, no significant differences (p>0.05) were detected in terms of chlorophyll content and active iron content of peanut leaves in the four treatments (Fig. 4C and D).
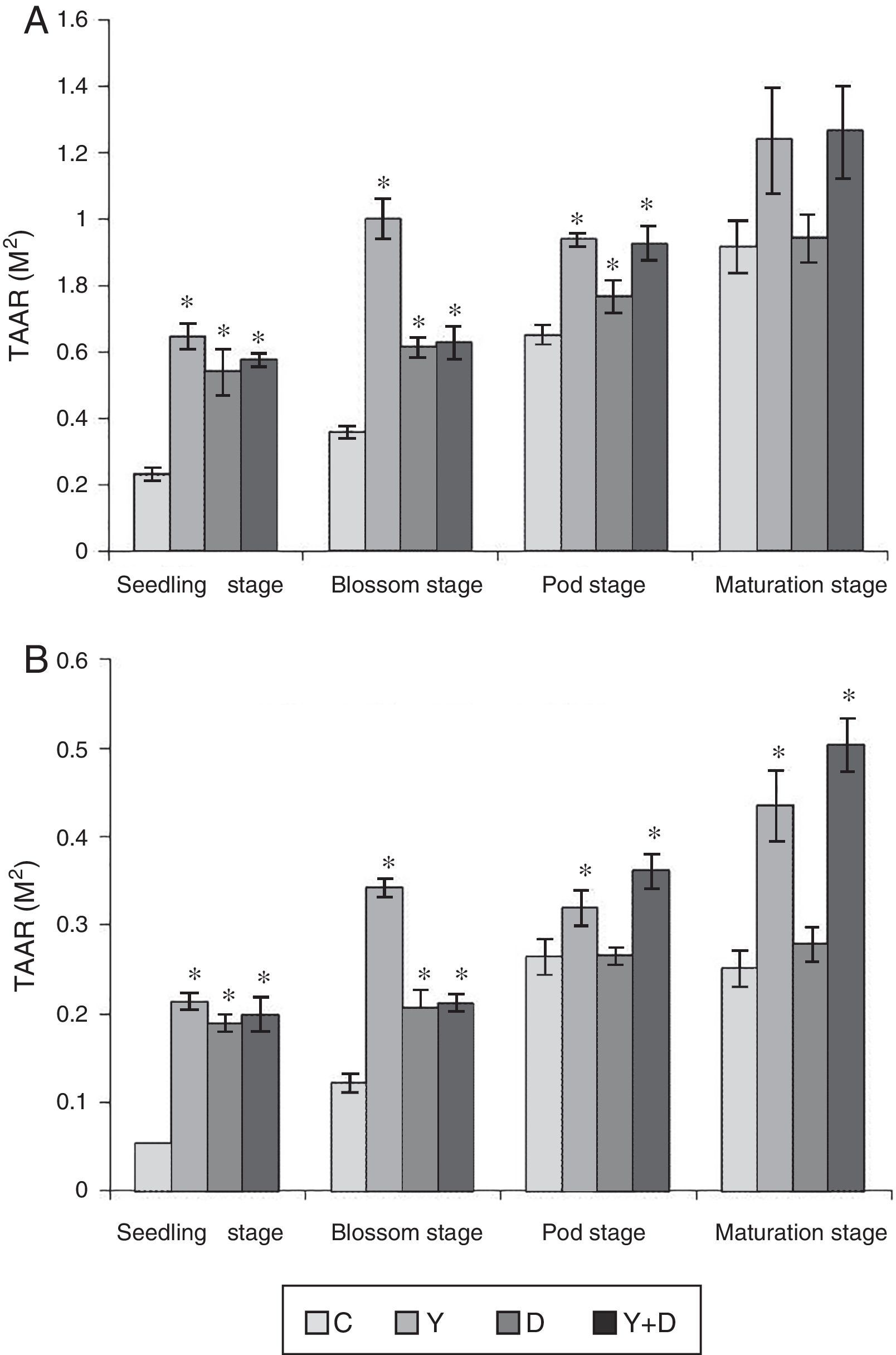
Root activityRoot total absorption area can directly reflect the root system ability to absorb moisture and nutrients and root active absorption area can reflect the root activity. In the first pot assay, root activities, which include the total absorption area (TAAR) and active absorption area (AAAR) were measured in the four growth periods (Fig. 5).
Root activities in different growth periods of peanuts in the pot assay in 2012. (A) Total absorption area of peanut roots and (B) active absorption area of peanut roots. TAAR, total absorption area; AAAR, active absorption area. Data were presented as mean±standard deviation. * indicates significant difference comparing with the control group (p<0.05). C: control, received no inoculation; Y: received YZ29 inoculation; D: received DZ13 inoculation; Y+D: received YZ29 and DZ13 inoculation.
TAAR and AAAR were both higher in Y-treated, D-treated, and Y+D-treated plants than that in the control plants. At the seedling and blossom stages, both of TAAR and AAAR were the highest (0.647m2, 0.214m2, 1.001m2, and 0.342m2) in Y-treated plants and significantly increased (p<0.05) by 180.09%, 296.30%, 179.61% and 180.33% compared with the control, respectively. At the pod stage, both of TAAR and AAAR were significantly higher in Y-treated and Y+D-treated plants than those in the other two treatments. TAAR in Y-treated plants significantly increased (p<0.05) by 43.93% compared with the control; AAAR in Y+D-treated plants significantly increased (p<0.05) by 36.74% compared with the control. At the maturation stage, no significant differences (p>0.05) were observed in TAAR in the four treatments. However, AAAR in Y-treated and Y+D-treated plants were significantly higher (0.435m2 and 0.504m2) than that in the other two treatments and significantly increased (p<0.05) by 73.31% and 100.80%, respectively, compared with the control plants. As a result, YZ29 and DZ13 enhanced the root activity, which may contribute to a stronger ability to absorb moisture and nutrients, and the effect of YZ29 was better than that of DZ13.
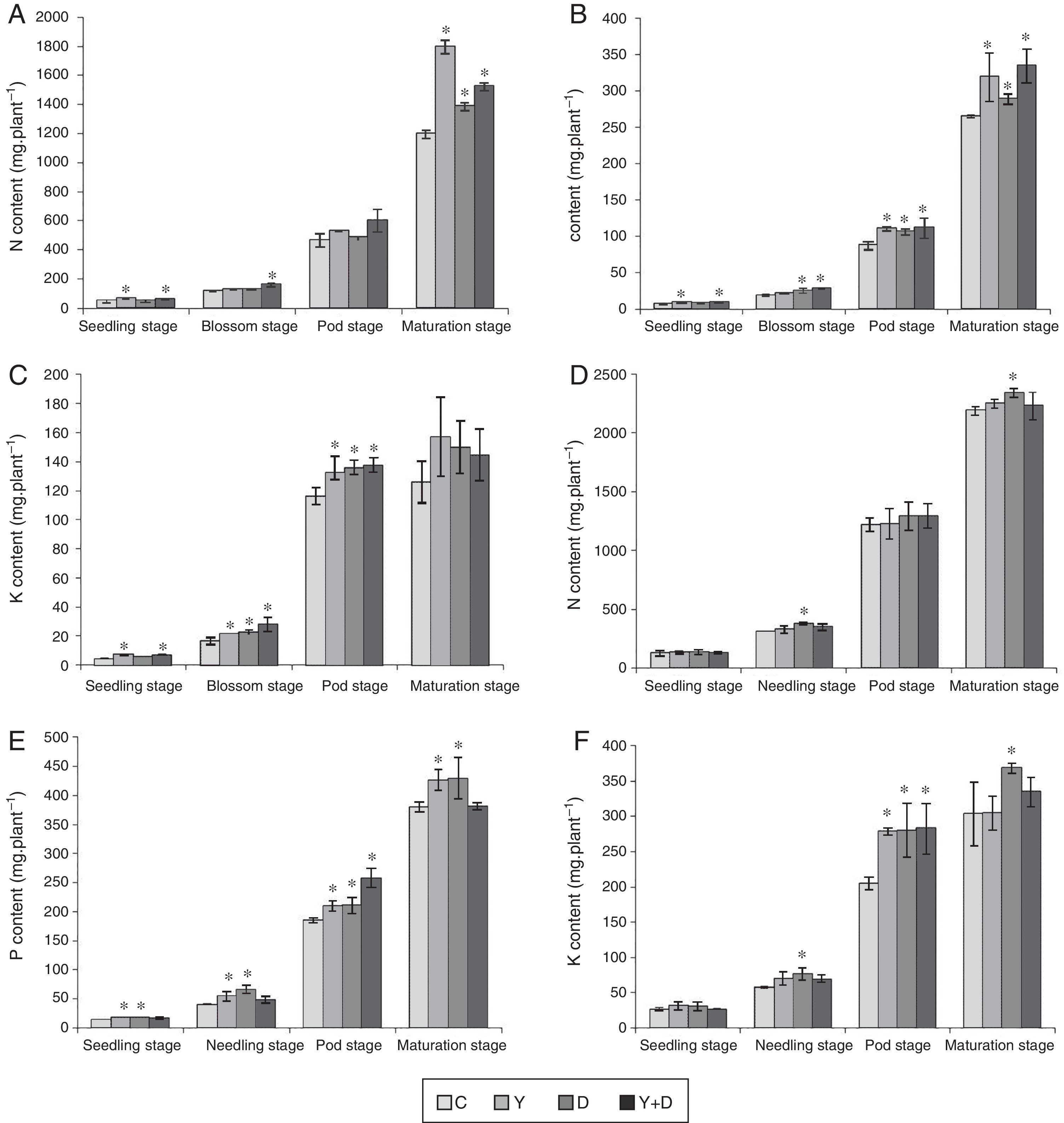
Total nitrogen, phosphorus and potassium contents of plantsIn the potted plants, nutrient accumulation of peanut in the three inoculated treatments varied compared with that in the control treatment (Fig. 6A–C). The nutrient accumulation of the four treatments ultimately exhibited the same pattern with chlorophyll concentration and active iron content in leaves.
Total nitrogen, phosphorus and potassium accumulation of peanut plants of different treatments in each growth period. (A) Total nitrogen accumulation of peanut plants of different treatments in each growth period in the pot assay in 2013. (B) Total phosphorus accumulation of peanut plants of different treatments in each growth period in the pot assay in 2013. (C) Total potassium accumulation of peanut plants of different treatments in each growth period in the pot assay in 2013. (D) Total nitrogen accumulation of peanut plants of different treatments in each growth period in the field trial. (E) Total phosphorus accumulation of peanut plants of different treatments in each growth period in the field trial. (F) Total potassium accumulation of peanut plants of different treatments in each growth period in the field trial. Data were presented as mean±standard deviation. * indicates significant difference comparing with the control group (p<0.05). C: control, received no inoculation; Y: received YZ29 inoculation; D: received DZ13 inoculation; Y+D: received YZ29 and DZ13 inoculation.
At the seedling stage, significant differences (p<0.05) were observed in nitrogen, phosphorus and potassium accumulation of Y-treated and Y+D-treated plants compared with the control plants. Nitrogen, phosphorus and potassium accumulation of Y-treated plants was the highest at the seedling stage and increased by 41.53%, 32.01% and 70.88% compared with the control (C) plants, respectively. At the blossom stage, nitrogen, phosphorus and potassium accumulation of Y+D-treated plants significantly increased (p<0.05) (by 39.10%, 56.61% and 68.52%, respectively) compared with the control plants. At the pod stage, pronounced differences (p<0.05) were observed in phosphorus and potassium accumulation of Y-treated, D-treated and Y+D-treated plants compared with the control plants. At the maturation stage, nitrogen and phosphorus accumulation of Y-treated plants were significantly higher (p<0.05) and increased by 50.21% and 20.38%, respectively, compared with the control. In summary, the nutrient accumulation of Y-treated plants was higher than that of D-treated plants. Therefore, YZ29 strain was more effective in promoting nutrient absorption in peanut than DZ13.
In the field trial, YZ29 and DZ13 also affected the nutrient accumulation of peanut (Fig. 6D–F). At the seedling stage, significant difference (p<0.05) was observed only in phosphorus accumulation of Y-treated and D-treated plants compared with the control and increased by 27.94% and 30.28%, respectively, compared with the control plants. At the needling stage, nitrogen, phosphorus and potassium accumulation of D-treated plants were significantly higher (p<0.05) than those of the control plants and were enhanced by 21.90%, 63.90% and 33.52%, respectively, compared with the control. At the pod stage, phosphorus and potassium accumulation of Y+D-treated plants were the highest and markedly (p<0.05) increased by 39.36% and 37.79% compared with the control plants. At the maturation stage, nitrogen, phosphorus and potassium accumulation of D-treated plants were the highest and significantly (p<0.05) enhanced by 6.67%, 13.17% and 21.59%, respectively, compared with the control plants.
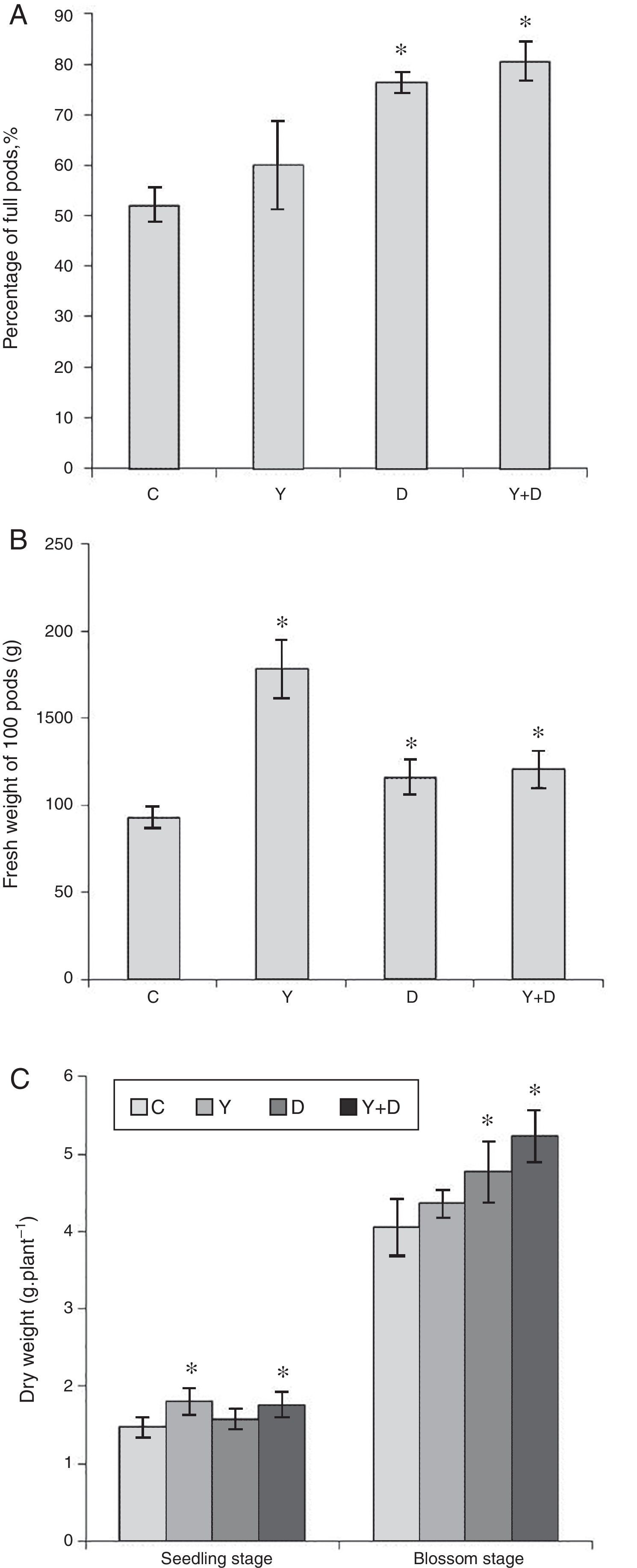
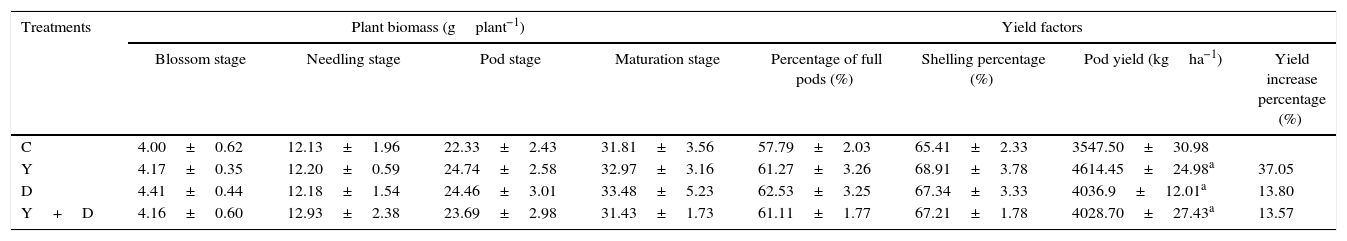
Growth characteristicsIn the pot assay in 2012, percentage of full pods and total weight of 100 fresh pods were measured at the maturation stage. Percentage of full pods in D-treated and Y+D-treated plants were significantly higher (p<0.05) than that in the control plants, and enhanced by 46.28% and 54.27%, respectively (Fig. 7A). The fresh weight of 100 fresh pods were significantly higher in the three inoculated treatments compared with that in the control, and increased by 91.71%, 24.87% and 29.81%, respectively (Fig. 7B).
Biomass and yield factors in the pot assays. (A) Percentage of full pods in different treatment groups after harvest in the pot assay in 2012. (B) Fresh weight of 100 pods in different treatment groups after harvest in the pot assay in 2012. (C) Dry weight of peanut plants in different treatment groups in the seedling and blossom stages in the pot assay in 2013. Data were presented as mean±standard deviation. * indicates significant difference comparing with the control group (p<0.05). C: control, received no inoculation; Y: received YZ29 inoculation; D: received DZ13 inoculation; Y+D: received YZ29 and DZ13 inoculation.
In the pot assay in 2013, the plant dry matter was measured at the seedling and blossom stages of dual inoculation of YZ29 and DZ13 (Fig. 7C). At the seedling stage, the dry weight of the whole plants in the Y-treated and Y+D-treated plants were significantly higher (p<0.05) (1.80gplant−1 and 1.76gplant−1, respectively) than the control, and increased by 22.45% and 19.73%, respectively. The Y+D-treated plants at the blossom stage exhibited the highest dry weight (5.23gplant−1) and was significantly (p<0.05) higher than that in the control plants.
In the field trial, no significant differences were observed in the plant biomass, percentage of full pods and shelling percentage among the four treatments. The pod yield in the three inoculated treatments was significantly higher (p<0.05) than that in the control, and the yield in the Y-treated plants increased by 37.05% compared with the control (Table 2).
Differences of plant biomass and yield factors after harvest in the field trial.
| Treatments | Plant biomass (gplant−1) | Yield factors | ||||||
|---|---|---|---|---|---|---|---|---|
| Blossom stage | Needling stage | Pod stage | Maturation stage | Percentage of full pods (%) | Shelling percentage (%) | Pod yield (kgha−1) | Yield increase percentage (%) | |
| C | 4.00±0.62 | 12.13±1.96 | 22.33±2.43 | 31.81±3.56 | 57.79±2.03 | 65.41±2.33 | 3547.50±30.98 | |
| Y | 4.17±0.35 | 12.20±0.59 | 24.74±2.58 | 32.97±3.16 | 61.27±3.26 | 68.91±3.78 | 4614.45±24.98a | 37.05 |
| D | 4.41±0.44 | 12.18±1.54 | 24.46±3.01 | 33.48±5.23 | 62.53±3.25 | 67.34±3.33 | 4036.9±12.01a | 13.80 |
| Y+D | 4.16±0.60 | 12.93±2.38 | 23.69±2.98 | 31.43±1.73 | 61.11±1.77 | 67.21±1.78 | 4028.70±27.43a | 13.57 |
Note: Data are presented as mean±standard deviation. C, control, received no inoculation; Y, YZ29 inoculation; D, DZ13 inoculation; Y+D, YZ29 and DZ13 inoculation.
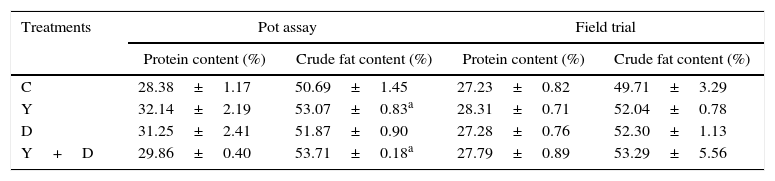
In the pot assay in 2013, the protein content of kernels in Y-treated, D-treated, and Y+D-treated plants increased by 13.25%, 9.18% and 5.21%, respectively, compared with the control. The crude fat contents of kernels in Y-treated and Y+D-treated plants were significantly higher than (p<0.05) that of the control (C) treatment and increased by 4.70% and 5.96%, respectively, compared with that of the control (Table 3).
Differences of protein and crude fat contents of peanut kernels after harvest in the pot assay in 2013 and field trial.
| Treatments | Pot assay | Field trial | ||
|---|---|---|---|---|
| Protein content (%) | Crude fat content (%) | Protein content (%) | Crude fat content (%) | |
| C | 28.38±1.17 | 50.69±1.45 | 27.23±0.82 | 49.71±3.29 |
| Y | 32.14±2.19 | 53.07±0.83a | 28.31±0.71 | 52.04±0.78 |
| D | 31.25±2.41 | 51.87±0.90 | 27.28±0.76 | 52.30±1.13 |
| Y+D | 29.86±0.40 | 53.71±0.18a | 27.79±0.89 | 53.29±5.56 |
Note: Data are presented as mean±standard deviation. C, control, received no inoculation; Y, YZ29 inoculation; D, DZ13 inoculation; Y+D, YZ29 and DZ13 inoculation.
Under field conditions, the effects of YZ29 and DZ13 on protein and crude fat contents of peanut kernels were not significant (p>0.05) (Table 3). The protein contents in kernels in Y-treated, D-treated, and Y+D-treated plants increased by 3.97%, 0.18% and 2.06%, respectively, compared with the control plants. The crude fat contents in Y-treated, D-treated and Y+D-treated plants increased by 4.69%, 5.21%, and 7.20%, respectively, compared with the control treatment. Collectively, YZ29 and DZ13 was able to enhance the quality of peanut kernels under the potted and field conditions.
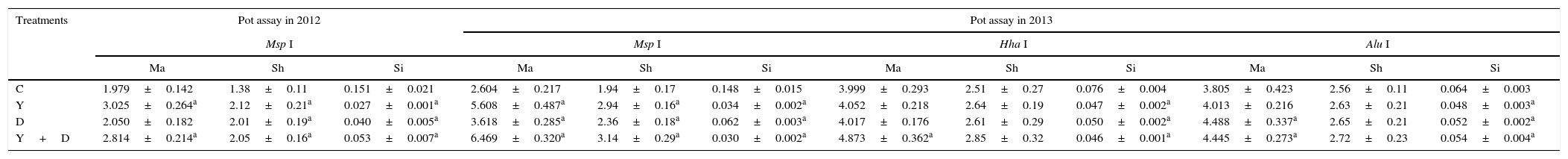
Structure of microbial community in soilYZ29 and DZ13 affected the structure of microbial community in soil surrounding the roots of peanut plants. Under pot and field conditions, the species diversity of soil surrounding the peanut roots in the three inoculated treatments significantly increased compared with the control (Table 4). Margalef and Shannon–Wiener diversity indexes were higher, and the Simpson index was lower in the three inoculated treatments than those in the control (Table 5).In the pot assay in 2012, the soil samples surrounding the peanut roots at the pod stage were digested by Msp I digestion. Margalef and Shannon–Wiener diversity indexes in Y-inoculated and Y+D-inoculated treatments were significantly higher than those in the control (p<0.05), and they were the highest in the Y-inoculated treatment. The Simpson index was significantly lower in the three inoculation treatments than in the control (p<0.05), and it was the lowest in the Y-inoculated treatment (Table 5). The amount of beneficial bacteria, such as Azoarcus sp., Rhizobium sp., Rhodobacter sp. and Frankia sp., also increased in the Y-inoculated treatment compared with the control (Table 4). In the pot assay in 2013, when soil samples were digested by Msp I digestion, there were significant differences in the Margalef, Shannon–Wiener diversity, and Simpson indexes between the three inoculation treatments and the control (p<0.05); when soil samples were digested by Hha I and Alu I, Simpson index was significantly lower in the three inoculation treatments than in the control (p<0.05, Table 5). The amount of Bacillus sp., Paenibacillus sp., Burkholderia sp. and Frankia sp. in the Y+D-inoculated treatment increased (Table 4).Under field conditions, when soil samples were digested by Msp I and Alu I, Margalef index was significantly higher in the three inoculation treatments than in the control (p<0.05); when soil samples were digested by Hha I, Simpson index was significantly lower in the three inoculation treatments than in the control (p<0.05). There were significant differences in the three diversity indexes between the D-inoculated treatment and the control whichever the soil samples were digested by (p<0.05, Table 5). Furthermore, the amount of Bacillus sp., Pseudomonas sp. and Microbacterium sp. in the D-inoculated treatment was distinctly increased compared with the control (Table 4).
Differences of T-RFs on the T-RFLP map of soil samples at the pod stage.
| Treatments | Number of effective T-RFsa | Added effective fragment size (bp) and species they may represent compared with the control | ||
|---|---|---|---|---|
| The pot assay in 2012 | Msp I | C | 10 | |
| Y | 18 | Azoarcus sp. (83), Capnocytophaga sp. (83, 86), Bacteroides sp. (96), Rhizobium sp. (126), Agrobacterium sp. (128), Paracoccus sp./Rhodobacter sp. (130), Desulfotomaculum sp./Frankia sp. (140), 66 | ||
| D | 14 | Bacillus sp./Paenibacillus sp./Halobacillus sp. (148), Heliorestis sp. (288), Francisella sp./Nannocystis sp. (484), Microcystis sp. (500) | ||
| Y+D | 17 | Bacteroides sp. (96), Heliorestis sp. (288), Francisella sp./Nannocystis sp. (484), Buchnera sp. (501), 61, 66, 98 | ||
| The pot assay in 2013 | Msp I | C | 11 | |
| Y | 19 | Microcystis sp./Oceanospirillum sp./Saccharothrix sp. (129), Bacillus sp./Eubacterium sp. (136), Bacillus sp./Paenibacillus sp./Rubrobacter sp. (138), Burkholderia sp./Eubacterium sp./Frankia sp. (141), Francisella sp./Nannocystis sp. (484), 66, 92, 467 | ||
| D | 15 | Azoarcus sp./Capnocytophaga sp. (83), Rhizobium sp. (126), Bacillus sp./Eubacterium sp. (136), 62 | ||
| Y+D | 24 | Bacillus sp./Eubacterium sp. (136), Bacillus sp./Paenibacillus sp./Rubrobacter sp. (138), Burkholderia sp./Eubacterium sp./Frankia sp. (141), Cyclobacterium sp./Nocardioides sp. (142), Geotoga sp./Propioniferax sp. (199), Nevskia sp./Sporomusa sp. (290), Burkholderia sp. (426), 62, 66, 92, 224, 436, 467 | ||
| Hha I | C | 13 | ||
| Y | 15 | Clostridium sp. (66), Bacillus sp./Paenibacillus sp./Thermoactinomyces sp. (238) | ||
| D | 16 | Bacteroides sp./Flavobacterium sp./Persicobacter sp. (96), Planctomyces sp. (126), 130 | ||
| Y+D | 18 | Clostridium sp. (66), Bacteroides sp. (98), Lactobacillus sp. (174), Halobacteroides sp. (551), Azoarcus sp./Clostridium sp./Desulfotomaculum sp. (571) | ||
| Alu I | C | 12 | ||
| Y | 14 | Clostridium sp. (66), Lactobacillus sp. (273) | ||
| D | 16 | Cytophaga sp. (83), Fusobacterium sp./Pyrenomonas sp. (131), Lactobacillus sp. (273), 126 | ||
| Y+D | 14 | Clostridium sp. (66), Pediococcus sp./Rubrivivax sp. (274) | ||
| The field assay in 2013 | Msp I | C | 19 | |
| Y | 27 | Agrobacterium sp. (128), Bacillus sp./Corynebacterium sp./Rhodomicrobium sp./Streptomyces sp. (161), Microbacterium sp. (172), Francisella sp./Nannocystis sp. (484), 66, 168, 421, 467 | ||
| D | 29 | Agrobacterium sp. (128), Bacillus sp./Pseudomonas sp./Thermomonospora sp. (146), Microbacterium sp. (172), Francisella sp./Nannocystis sp. (484), 61, 66, 168, 421, 436, 467 | ||
| Y+D | 26 | Microbacterium sp. (172), Francisella sp./Nannocystis sp. (484), Achromatium sp./Eubacterium sp./Methylomicrobium sp. (487), Marchantia sp./Skeletonema sp. (502), 66, 168, 467 | ||
| Hha I | C | 14 | ||
| Y | 17 | Salinicoccus sp. (63), Acholeplasma sp. (221), Thermomonospora sp. (379) | ||
| D | 19 | Salinicoccus sp. (63), Bacteroides sp. (98), Acholeplasma sp. (221), Clostridium sp./Eubacterium sp./Flavobacterium sp./Lactobacillus sp. (233), Corynebacterium sp. (378) | ||
| Y+D | 18 | Salinicoccus sp. (63), Bacteroides sp. (98), Acinetobacter sp./Actinobacillus sp./Azoarcus sp./Burkholderia sp./Nitrosospira sp./Pseudomonas sp. (207), Aeromonas sp./Photobacterium sp. (572) | ||
| Alu I | C | 14 | ||
| Y | 21 | Clostridium sp. (66), Eubacterium sp. (81), Mesorhizobium sp./Rhizobium sp./Rhodomicrobium sp. (141), Achromatium sp./Azoarcus sp. (152), Streptococcus sp. (168), Bacteroides sp./Paenibacillus sp./Thermoactinomyces sp. (244), 285 | ||
| D | 20 | Clostridium sp. (66), Mesorhizobium sp./Rhizobium sp./Rhodomicrobium sp. (141), Streptococcus sp. (168), Amycolatopsis sp./Brevibacillus sp./Butyrivibrio sp. (198), Rhizobium sp./Rubrobacter sp. (204), Lactobacillus sp. (231) | ||
| Y+D | 20 | Clostridium sp. (66), Eubacterium sp. (81), Mesorhizobium sp./Rhizobium sp./Rhodomicrobium sp. (141), Streptococcus sp. (168), Amycolatopsis sp./Brevibacillus sp./Butyrivibrio sp. (198), Abiotrophia sp. (215) | ||
Note: Ap=ni/N×100, where ni represents the peak area of one distinct T-RF and N is the sum of all peak areas in the T-RFLP map. Species with bold numbers cannot be speculated by T-RFLP Phylogenetic Assignment Tool. C, control, received no inoculation; Y, YZ29 inoculation; D, DZ13 inoculation; Y+D, YZ29 and DZ13 inoculation.
Bacterial diversity of soil samples at the pod stage.
| Treatments | Pot assay in 2012 | Pot assay in 2013 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Msp I | Msp I | Hha I | Alu I | |||||||||
| Ma | Sh | Si | Ma | Sh | Si | Ma | Sh | Si | Ma | Sh | Si | |
| C | 1.979±0.142 | 1.38±0.11 | 0.151±0.021 | 2.604±0.217 | 1.94±0.17 | 0.148±0.015 | 3.999±0.293 | 2.51±0.27 | 0.076±0.004 | 3.805±0.423 | 2.56±0.11 | 0.064±0.003 |
| Y | 3.025±0.264a | 2.12±0.21a | 0.027±0.001a | 5.608±0.487a | 2.94±0.16a | 0.034±0.002a | 4.052±0.218 | 2.64±0.19 | 0.047±0.002a | 4.013±0.216 | 2.63±0.21 | 0.048±0.003a |
| D | 2.050±0.182 | 2.01±0.19a | 0.040±0.005a | 3.618±0.285a | 2.36±0.18a | 0.062±0.003a | 4.017±0.176 | 2.61±0.29 | 0.050±0.002a | 4.488±0.337a | 2.65±0.21 | 0.052±0.002a |
| Y+D | 2.814±0.214a | 2.05±0.16a | 0.053±0.007a | 6.469±0.320a | 3.14±0.29a | 0.030±0.002a | 4.873±0.362a | 2.85±0.32 | 0.046±0.001a | 4.445±0.273a | 2.72±0.23 | 0.054±0.004a |
| Treatments | Field assay in 2013 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Msp I | Hha I | Alu I | |||||||
| Ma | Sh | Si | Ma | Sh | Si | Ma | Sh | Si | |
| C | 5.354±0.431 | 2.90±0.30 | 0.033±0.001 | 3.890±0.378 | 2.58±0.21 | 0.058±0.003 | 3.664±0.125 | 2.53±0.10 | 0.059±0.003 |
| Y | 7.092±0.593a | 3.23±0.38 | 0.029±0.003 | 4.203±0.271 | 2.76±0.32 | 0.046±0.003a | 4.729±0.362a | 2.83±0.24 | 0.046±0.002a |
| D | 7.745±0.632a | 3.34±0.27 | 0.027±0.002a | 4.582±0.294a | 2.80±0.19 | 0.049±0.002a | 4.853±0.382a | 2.80±0.18a | 0.054±0.001 |
| Y+D | 6.649±0.721a | 3.19±0.16 | 0.028±0.001a | 4.409±0.374 | 2.78±0.31 | 0.048±0.002a | 4.401±0.378a | 2.71±0.28 | 0.058±0.003 |
Note: Data are presented as mean±standard deviation. Ma, Margalef index; Sh, Shannon–Wiener index; Si, Simpson index; C, control, received no inoculation; Y, YZ29 inoculation; D, DZ13 inoculation; Y+D, YZ29 and DZ13 inoculation.
Siderophores produced by microorganism have the potential ability to relieve iron-deficiency chlorosis of peanut effectively.4,6 In this study, iron nutrient uptake of peanut and plant growth were evaluated after the inoculation of two siderophore-producing strains using potted experiments and a field trial.
In the pot experiment, a linear correlation was observed between active iron and chlorophyll contents in leaves in the growth period. Iron is important for the synthesis of chlorophyll. The formation of chlorophyll synthesis precursor 5-amino ketones pentanoic acid (ALA) requires iron, and chlorophyll formed by ALA also requires iron in the synthesis process.22 However, a declined trend in the chlorophyll content of the four inoculated treatments and an increasing trend in the active iron of leaves were observed at the blossom stage. We speculated that given the lack of chlorophyll in plants at the blossom stage, more active chlorophyll synthetic reaction was required for the normal plant growth. In the second pot experiment, visual inspection showed that the degree of yellowing in the new leaves of peanut in the control treatment was more serious than in the inoculated treatments at the pod stage, and significant differences of chlorophyll and active iron contents in leaves were detected between the inoculated treatments and control treatment, indicating that the siderophores secreted by YZ29 and DZ13 ameliorated the symptoms. Similar results have been observed in mung bean inoculated by the ion of P. fluorescens strain GRP3A.23 Therefore, after the inoculation of YZ29 and DZ13, the chlorophyll and active iron contents of peanut were distinctly affected under pot conditions. However, we found that in the field trial, there were no significant differences in active iron and chlorophyll contents in leaves, between the inoculated treatments and control treatment. We speculated that in the field conditions, many complex and uncontrollable environmental factors, such as rain, climate and soil microbial environment, might weaken the effect of YZ29 and DZ13, thus leading to the non-significant differences of the indicators in leaves between the inoculated treatments and control treatment. In our further study, we will conduct larger scale field trial to confirm the effect of YZ29 and DZ13. Furthermore, both TAAR and AAAR were significantly higher in the three inoculated treatments at the seedling and blossom stages, compared with the control. The intensity of plant root activity is closely related to the entire plant life activities, and the peanut root absorption area and root vitality are closely associated with the growth and development of the ground part in plant. Developed roots and strong root activity will contribute to the robust plant growth.24 Thereby, these results suggested that YZ29 and DZ13 were able to enhance peanut root activity.
In the present study, the concentration of nitrogen, phosphorus and potassium increased in the peanut plants with bacterial inoculation at some certain growth stages, compared with the controls. Nitrogen, phosphorus and potassium are three essential plant nutrient elements and play critical roles in the metabolism and growth of plants. Nitrogen is an important component of chlorophyll, and chlorophyll content is closely related to nitrogen abundance.25 Potassium is the activator of multiple enzymes in plants. It does not only promote photosynthesis, but also enhance nitrogen plant metabolism.26 Iron is the necessary element of ferritin and molybdo-ferredoxin in nitrogen-fixing microorganisms,27 thus, it is also one of the indispensable elements of symbiotic nitrogen fixation in leguminous plants. Collectively, the increased concentration of nitrogen, phosphorus and potassium may be due to increased chlorophyll concentration and enhanced root activity, thereby promoting peanut growth. Similar studies have also been reported earlier by Leong and Dey.28,29
Peanut plant biomass and pod yield are the final responses that determine whether siderophores produced by YZ29 and DZ13 can promote iron nutrient absorption and peanut growth. Under potted conditions, the plant dry matter at the seedling and blossom stages significantly increased in the plants with bacterial dual-inoculation, compared with the controls. The definite enhancement of plant dry matter may result from the improved uptake of iron, nitrogen, phosphorus and potassium. Furthermore, in the field assay, pod yield in the Y-treated plants increased by 37.5% compared with the control. A previous similar study has reported that P. fluorescens PGPR2 increased pod yield by 28.3% compared with the control.29 Moreover, in this study, a significant increase (p<0.05) was observed in the crude fat content of kernels in Y-treated and Y+D-treated plants compared with the control under potted conditions. Similarly, P. fluorescens has been demonstrated to improve the crude fat content of peanut kernels.30 Therefore, after the inoculation of YZ29 and DZ13, peanut plant biomass, pod yield and crude fat content of kernels were obviously increased, which may be due to the siderophores produced by YZ29 and DZ13.
Under potted conditions, the structure of microbial community in soil surrounding the roots of peanut plants was changed after bacterial inoculation. The amount of a set of beneficial bacteria, such as Rhizobium sp., Bacillus sp., Streptomyces sp., Lactobacillus sp., Azoarcus sp. and Pseudomonas sp. Previous studies have reported the effects of the aforementioned bacteria on plant. For instance, Rhizobium and Azoarcus provide nitrogen to leguminous plants by nitrogen fixation. Bacillus and Pseudomonas strains produce antibiotics, thereby consequently preventing and curing plant diseases and promoting plant growth.31–34 Furthermore, Streptomyces sp. promote the biodegradation of soil humic acids.35 A set of Lactobacillus strains can improve soil fertility36 and prevent plant diseases.37 In this study, several reasons may result in the improvement of microorganism richness and diversity in the soil surrounding roots of peanut plants. On the one hand, the root activity of peanut was enhanced, resulting in the improvement of soil enzyme activity and increase of microbial population in the soil. 38 On the other hand, root metabolic activity was improved with enhanced root activity, which affected the species and quantity of root exudates, thus, the species and quantity of microorganism in the soil varied.39,40 Similar studies have also been previously reported by other researchers.41,42 Taken together, it was demonstrated that the structure of microbial community in soil surrounding the roots of peanut plants was affected after the inoculation of YZ29 and DZ13.
Although environmental factors, such as rain, climate and previous crop, were complex under field conditions, the uptake of iron nutrient and other nutrients in peanut was still improved. Besides, plant biomass and pod yield was increased after the inoculation of YZ29 and DZ13. These results suggested the positive effect of YZ29 and DZ13 on iron nutrient uptake and plant growth of peanut, and the two strains may be used as candidates to be made as bacterial manure.
Reports about the effect of microbial siderophores on the growth of peanut in soil are available. As early as 1986, Jurkevitch et al. reported that chlorophyll content of peanut plants was significantly higher in the separated iron pigment-treated soil and bacterial suspension-treated soil than the FeEDDHA-treated and control plants in pots.43 In 2004, Dey reported that siderophore-producing P. fluorescens PGPR1, PGPR2 and PGPR4 increased the nitrogen and phosphorus contents in soil, and increased pod yield in calcareous soil in pot and field trials.29 But, the aforementioned studies did not focus on the change in iron content in peanut plants, which was an important indicator to investigate the effect of microbial siderophores on iron nutrition of plants. Moreover, previous studies only evaluated the growth of peanut in one growth period. By contrast, the present study analyzed the changes in iron content in peanut leaves and evaluated the growth of peanut in the whole growth period. However, this study has some limitations. The types of siderophores produced by these two strains is needed to be determined, and extracellular ferric reductase activity of the microbial strains is needed to be studied, which will be further investigated and reported later.
In conclusion, after the inoculation of two siderophore-producing stains YZ29 and DZ13, iron and chlorophyll contents of peanut leaves, peanut root activity, total nitrogen, phosphorus and potassium accumulation of plants, quality of peanut kernels and plant biomass were improved, compared with the controls. The two strains are promising to be made into bacterial manure. In addition, the present study is the first to explore the effect of siderophores produced by P. illinoisensis on iron absorption. The effect of siderophores produced by Bacillus and Paenibacillus in calcareous soils on iron nutrition uptake of peanut is also first to be reported. One of our on-going studies aims to obtain purified siderophores produced by the two strains and then investigate their effects on plant, elucidate their structures, and apply the purified siderophores to growing media of peanut to evaluate the status of plants.
Conflicts of interestThe authors declare that they have no conflict of interest.
This work was supported by the National Natural Science Foundation of China (Grant No. 31100005) and the Science and Technology Major Projects of Shandong Province (2015ZDXX0502B02). We would like to thank the financial support by STANLEY GROUP and also thank the professor Fengzhen Liu in Plant genetics and breeding department of Shandong Agricultural University, who provided help for peanut cultivation.