Propolis and geopropolis are resinous products of bees showing antimicrobial effects. There is no data concerning their action against Pythium insidiosum – the causative agent of pythiosis, a pyogranulomatous disease of the subcutaneous tissue that affects mostly horses, dogs and humans. Fragments of 15 isolates of P. insidiodum were incubated with propolis and geopropolis extracts and evaluated for up to seven days to detect the minimal fungicidal concentration (MFC). Propolis inhibited three isolates at 1.0mgmL−1 after 24h and all other isolates at 3.4mgmL−1. Geopropolis led to more variable results, exerting predominantly a fungistatic action than a fungicidal one. Propolis was more efficient than geopropolis in inhibiting P. insidiosum since lower concentrations led to no growth after 24h. This effect may be due to propolis chemical composition, which has more active compounds than geopropolis. Propolis seemed to be a good candidate for in vivo studies, since treatment with conventional antifungal compounds is difficult in most of the cases, requiring extensive surgical debridement.
There has been an increased interest by the pharmaceutical industry in the search for natural products to maintain a healthy lifestyle, especially those with antimicrobial activity, due to bacterial and fungal resistance to antimicrobial drugs and side effects.1,2
Bee products have been widely investigated concerning their biological properties. Propolis is a balsamic and resinous product made by bees from different parts of plants, adding mandibular secretions, pollen and wax. Stingless bees may produce propolis as well as geopropolis. As to geopropolis, besides plant material, gland secretions, wax and pollen, some species of stingless bees add mud or clay to its composition, and it has been used in popular medicine in the treatment of respiratory diseases and dermatosis.3
The antimicrobial activity of propolis produced by Africanized honeybees has been extensively investigated,4–7 and in recent years there has been a great interest in the antibacterial properties of propolis and geopropolis produced by stingless bees. Geopropolis produced by Melipona compressipes fasciculata Smith exerted an antibacterial effect in vitro against Streptococcus mutans isolated from the oral cavity of young individuals of both gender, suggesting its use as an alternative for preventing dental caries.8 The antimicrobial action of geopropolis produced by Melipona fasciculata Smith in Maranhão State, northeast Brazil, was analyzed against Streptococcus mutans, Lactobacillus acidophilus and Candida albicans by the agar diffusion method, confirming its potential to control or prevent infections in the oral cavity.9
Although there is some data with respect to the antibacterial and antifungal activity of propolis and geopropolis, there is no data concerning their effects on Pythium insidiosum, a fungus-like organism belonging to Kingdom Stramenopila, Phylum Oomycota, which is the causative agent of pythiosis, a pyogranulomatous disease of the subcutaneous tissue that affects mostly horses, dogs and humans as well.10
Epidemiologically, pythiosis is related to human and animal contact with contaminated water, and zoospores constitute its infective form. This disease is life-threatening and diagnosis is time-consuming. Besides, treatment with conventional antifungal compounds is difficult in most of the cases, requiring extensive surgical debridement. The unsuccessful response to antifungal therapy is due to the absence of ergosterol in the plasmatic membrane, which is the main target of azoles, alilamines and polienes.10 Thus, we wish to present for the first time the effects of propolis produced by Africanized honeybees and geopropolis produced by M. fasciculata against Pythium insidiosum isolates.
Material and methodsExtract of propolis and geopropolisPropolis was collected in the Beekeeping Section, UNESP, Campus of Botucatu, São Paulo State, southeast Brazil (22° 53′ 25″S, 48° 27′ 19″W). Propolis sample was prepared as previously described.4,11 Briefly, 30g of propolis was ground and extracted with 100mL of 70% ethanol, in the absence of bright light, at room temperature and moderate shaking. After a week, the extract was filtered and the final concentration was calculated. Specific dilutions of extract of propolis at 1.0, 3.4, 7.0, 12.0 and 18mgmL−1 were prepared in Sabouraud (SAB) broth for inhibition assays.
Geopropolis was produced by M. fasciculata and collected in Palmeirândia, Maranhão State, northeast Brazil (2° 39′S, 44° 55′W). Ecosystems of this region include mangroves, flooding fields, lagoons, forests and babassu fields. Geopropolis samples were kept at 4°C before extraction, ground and macerated in 100mL of ethanol 70% at room temperature, under moderate shaking.9 After 24h, the extract was filtered and submitted to solvent evaporation. Geopropolis was dissolved in 1% dimethyl sulfoxide (DMSO), which did not affect the pathogen growth (data not shown). Geopropolis extract was diluted at 3.4, 5.0, 7.0, 12.5 and 18mgmL−1 in SAB broth for assays.
Both extracts of propolis and geopropolis were new and prepared for the assays.
Chemical analysis of propolis and geopropolisThe chemical composition of propolis and geopropolis was analyzed by Dr. Vassya Bankova, in the Institute of Organic Chemistry with Centre of Phytochemistry, Bulgaria, using gas chromatography–mass spectrometry (GC–MS).12,13 Briefly, analysis was performed with a Hewlett Packard Gas Chromatograph 5890 Series II Plus linked to a Hewlett Packard 5972 mass spectrometer system equipped with a 23m long, 0.25mm id, 0.5μm film thickness HP5-MS capillary column. The temperature was programmed from 100°C to 310°C at a rate of 5°Cmin−1. Helium was used as a carrier gas, flow rate 0.7mLmin−1. Split ratio 1:80, injector temperature 280°C. The ionization voltage was 70eV. The identification was accomplished using computer searches on a NIST98 MS data library.
Pythium insidiosum susceptibility to propolis and geopropolis extractsFifteen Pythium insidiosum isolates were used for sensitivity tests: an isolate from the first human case of pythiosis in Brazil (B-01), and 14 obtained from equine pythiosis (Eq-2 to Eq-15). All isolates were obtained from the Middle West region of São Paulo State, Brazil, and maintained in the Laboratory of Medical Mycology of the Department of Microbiology and Immunology, UNESP, Campus of Botucatu.
Isolates were inoculated into plates containing SAB agar at 35°C for 7 days. Afterwards, standardized fragments (5mm) were taken and put into microtubes containing SAB broth with different concentrations of propolis or geopropolis in a final volume of 1.0mL, to obtain the minimal fungicidal concentration (MFC) to hyphal growth. Control contained only SAB broth. After 24h at 35°C under moderate shaking, to prevent precipitation of the extract and the pathogen, fragments were plated individually in SAB agar and incubated at 35°C for seven days. All experiments were performed in quintuplicate.
The susceptibility of Pythium insidiosum isolates to propolis or geopropolis was determined by measuring the diameter (mm) of radial growth of the colony at 24, 48 and 168h (7 days) of incubation. All cultures were photographed and the colonies diameter was achieved using the software Image J (image processing and analysis in JAVA; http://rsbweb.nih.gov/ij), as described by Pires et al.16 Diameters at the angles 0, 45, 90 and 135° of each plate were recorded in mm.
MFC was determined by no growth of Pythium insidiosum over time. The absence of growth at 24h but growing after 48 and 168h, using the same concentration of propolis or geopropolis, was considered as a fungistatic action.
ResultsChemical composition of propolis and geopropolisBenzoic acid, dihydrocinnamic acid, coumaric acid, caffeic acid, prenyl-p-coumaric acid, flavonoids, artepillin C, trihydroxymethoxy flavonon, tetrahydroxy flavonon, and triterpenes were the main compounds found in propolis composition. Carbohydrates and their derivatives, triterpenes, anacardic acid, alkylresorcinols, and sugar alcohols were the major constituents of geopropolis identified by GC–MS.
Sensitivity tests on Pythium insidiosum isolatesAfter 24h, three isolates of Pythium insidiosum (B-01, Eq-7 and Eq-14) were inhibited by propolis at 1.0mgmL−1. All other isolates (n=12) were inhibited at 3.4mgmL−1 (Table 1; Figs. 1 and 2).
Effects of different concentrations of propolis and geopropolis (mgmL−1) on the radial growth of Pythium insidiosum isolates (mm), after 24, 48 and 168h of incubation at 35°C on SAB agar. Results are expressed as mean of the diameter (mm) of quintuplicate of each concentration. Concentrations above 3.4mgmL−1 of propolis and 12.0mgmL−1 of geopropolis are not expressed, since there was no growth of P. insidiosum.
| Pythium insidiosum isolates | Propolis | Geopropolis | Control | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24h | 48h | 168h | 24h | 48h | 168h | 24h | 48h | 168h | |||||||||||||
| 1.0 | 3.4 | 1.0 | 3.4 | 1.0 | 3.4 | 3.4 | 5.0 | 7.0 | 12.5 | 3.4 | 5.0 | 7.0 | 12.5 | 3.4 | 5.0 | 7.0 | 12.5 | ||||
| B-01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.4 | 0 | 0 | 0 | 30.6 | 0 | 0 | 0 | 3.5 | 21.2 | 90.0 |
| Eq-2 | 1.4 | 0 | 13.6 | 0 | 66.1 | 0 | 2.5 | 0 | 0 | 0 | 16.0 | 0 | 0 | 0 | 45.4 | 0 | 0 | 0 | 8.3 | 25.2 | 78.7 |
| Eq-3 | 5.6 | 0 | 30.6 | 0 | 88.4 | 0 | 17.0 | 0 | 0 | 0 | 35.1 | 7.5 | 1.4 | 0 | 90.0 | 32.0 | 18.0 | 0 | 18.5 | 35.6 | 90.0 |
| Eq-4 | 19.8 | 0 | 39.4 | 0 | 90.0 | 0 | 26.2 | 0 | 0 | 0 | 49.0 | 5.2 | 0 | 0 | 90.0 | 18.0 | 0 | 0 | 20.7 | 40.4 | 90.0 |
| Eq-5 | 2.8 | 0 | 29.9 | 0 | 90.0 | 0 | 11.2 | 4.6 | 0 | 0 | 36.1 | 25.4 | 0 | 0 | 84.0 | 72.0 | 0 | 0 | 11.6 | 36.6 | 90.0 |
| Eq-6 | 1.8 | 0 | 5.8 | 0 | 18.0 | 0 | 7.2 | 1.3 | 0 | 0 | 22.6 | 5.7 | 0 | 0 | 72.0 | 18.0 | 0 | 0 | 16.5 | 33.1 | 90.0 |
| Eq-7 | 0 | 0 | 0 | 0 | 0 | 0 | 5.6 | 2.1 | 0 | 0 | 18.8 | 9.0 | 0 | 0 | 51.3 | 36.0 | 0 | 0 | 19.0 | 38.6 | 90.0 |
| Eq-8 | 14.2 | 0 | 27.7 | 0 | 90.0 | 0 | 14.0 | 13.3 | 5.0 | 0 | 40.9 | 36.9 | 23.8 | 0 | 90.0 | 88.5 | 75.9 | 0 | 15.1 | 27.6 | 90.0 |
| Eq-9 | 16.7 | 0 | 28.7 | 0 | 90.0 | 0 | 20.3 | 20.1 | 5.1 | 0 | 38.9 | 39.3 | 17.4 | 0 | 90.0 | 90.0 | 54.0 | 0 | 19.9 | 39.1 | 90.0 |
| Eq-10 | 15.7 | 0 | 26.3 | 0 | 90.0 | 0 | 9.7 | 0 | 0 | 0 | 28.6 | 0 | 0 | 0 | 72.0 | 0 | 0 | 0 | 18.1 | 28.8 | 90.0 |
| Eq-11 | 13.5 | 0 | 27.4 | 0 | 90.0 | 0 | 13.3 | 6.3 | 2.8 | 0 | 28.8 | 21.9 | 14.6 | 0 | 90.0 | 90.0 | 90.0 | 0 | 14.7 | 29.1 | 90.0 |
| Eq-12 | 4.9 | 0 | 10.8 | 0 | 42.9 | 0 | 2.8 | 0 | 0 | 0 | 11.8 | 2.7 | 0 | 0 | 53.4 | 19.3 | 0 | 0 | 6.4 | 14.5 | 58.5 |
| Eq-13 | 2.6 | 0 | 9.9 | 0 | 36.0 | 0 | 13.0 | 4.9 | 0 | 0 | 27.2 | 18.6 | 7.0 | 0 | 90.0 | 70.4 | 33.7 | 0 | 18.5 | 35.4 | 90.0 |
| Eq-14 | 0 | 0 | 0 | 0 | 0 | 0 | 4.5 | 0 | 0 | 0 | 12.4 | 1.1 | 0 | 0 | 70.7 | 15.1 | 0 | 0 | 10.9 | 23.9 | 90.0 |
| Eq-15 | 6.3 | 0 | 21.1 | 0 | 87.9 | 0 | 0 | 0 | 0 | 0 | 16.4 | 6.6 | 1.5 | 0 | 83.4 | 55.5 | 17.5 | 0 | 15.6 | 29.5 | 90.0 |
Human B-01 isolate in plates containing only Sabouraud medium – control (A and C) and 1mgmL−1 of propolis (B and D) after 48 and 168h, respectively. The diameter of colonies growth was measured in mm and obtained by the software Image J (image processing and analysis in JAVA; http://rsbweb.nih.gov/ij/).
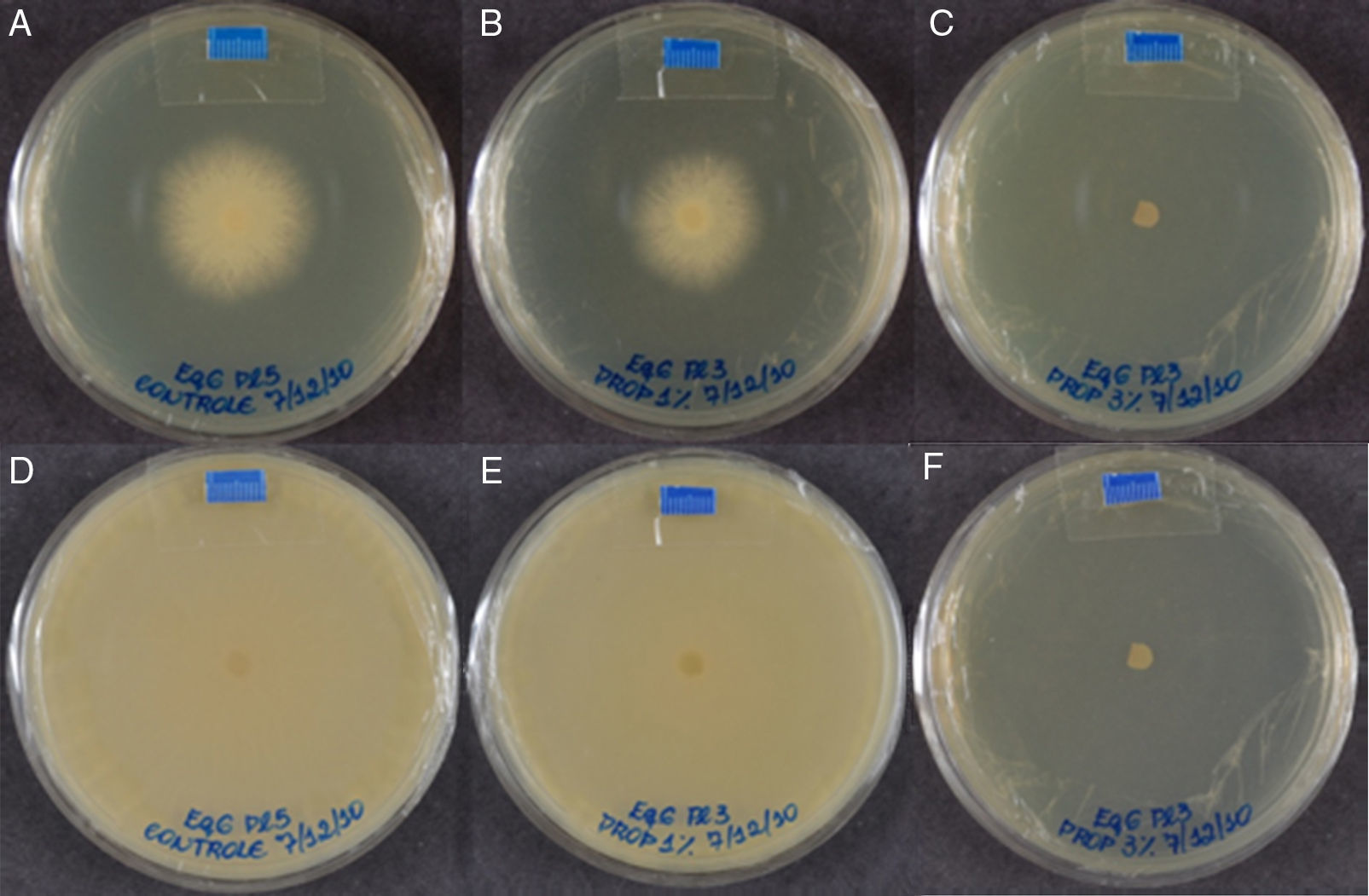
Equine isolate in plates containing only SAB medium – control (A and D), 1.0mgmL−1 of propolis (B and E) and 3.4mgmL−1 of propolis (C and F) after 48 and 168h, respectively. The diameter of colonies growth was measured in mm and obtained by the software Image J (image processing and analysis in JAVA; http://rsbweb.nih.gov/ij/).
Geopropolis effects were more variable. After 24h, only two isolates (B-01 and Eq-15) were inhibited at 3.4mgmL−1; however, this effect was only fungistatic, since growth of such isolates was seen after 48h. Using 5.0mgmL−1, a fungistatic effect was also observed for the isolates Eq-3, Eq-4, Eq-12, Eq-14 and Eq-15 compared to the same concentration at 24h. For 7.0mgmL−1, a fungistatic action was seen for Eq-3, Eq-13 and Eq-15 (Table 1; Figs. 3 and 4).
Human B-01 isolate growth in plates containing only SAB medium – control (A and D), 3.4mgmL−1 of geopropolis (B and E) and 5mgmL−1 of geopropolis (C and F) after 48 and 168h, respectively. The diameter of colonies growth was measured in mm and obtained by the software Image J (image processing and analysis in JAVA; http://rsbweb.nih.gov/ij/).
Equine isolate growth in plates containing only SAB medium – control (A and D), 5mgmL−1 of geopropolis (B and E) and 7mgmL−1 of geopropolis (C and F) after 48 and 168h, respectively. The diameter of colonies growth was measured in mm and obtained by the software Image J (image processing and analysis in JAVA; http://rsbweb.nih.gov/ij/).
No growth was seen after 7 days using 3.4mgmL−1 of propolis and 12.5mgmL−1 of geopropolis.
DiscussionCurrently, there is a great need to discover new antifungal compounds with high efficacy and low toxicity. There are few antifungal compounds for treatment of mycoses in comparison to antibacterial ones, and the main mechanism of action of antifungal agents is the inhibition of ergosterol synthesis, or the binding to this molecule, disrupting the fungal cell membrane. However, such compounds show toxicity to the host, due to the phylogenetic relationship between fungi and animals.14 Antifungal agents targeting on fungal cell wall have been evaluated as well, mainly by inhibiting β-glucans synthesis, such as the echinocandins, which have a lower toxicity to the host but an extremely high cost of treatment.
Pythium insidiosum is an aquatic organism classified in the Stramenopila Kingdom and Oomycetes Class. Besides several differences with true fungi, one of the most important, that implies directly in treatment, is the absence of ergosterol in its cell membrane, the main target of most antifungal agents.10 Thus, there is no effective antifungal therapy against Pythium insidiosum and pythiosis is difficult to treat.
Dória et al. evaluated the effects of intravenous regional limb perfusion administration of amphotericin B in horses to treat pythiosis after surgical excision and thermocautery, resolving infection with manageable side effects.15 Pires et al. reported that photodynamic therapy was effective both in vitro and in vivo in the inactivation of Pythium insidiosum, representing a new approach for treating pythiosis.16
Propolis produced by Africanized bees has been intensively investigated regarding its antimicrobial action. Several studies have suggested that the main compounds responsible for its antimicrobial activity are flavonoids and phenolic acid esters.4,17 The main constituents of our propolis sample were identified: benzoic acid, dihydrocinnamic acid, coumaric acid, caffeic acid, prenyl-p-coumaric acid, flavonoids, artepillin C, trihydroxymethoxy flavonon, tetrahydroxy flavonon, and triterpenes, among others. Moreover, the main vegetal source of propolis in Botucatu, São Paulo State, Brazil is Baccharis dracunculifolia DC, followed by Araucaria angustifolia (Bert.) O. Kuntze and Eucalyptus citriodora Hook.18
Here, the lowest concentration of propolis was able to inhibit 20% (3/15) of the isolates at 24h. The fungicidal activity of propolis produced by Apis mellifera has been observed also for other microorganisms, such as Candida tropicalis and Candida albicans as well as dermatophytes of the genus Trichophyton.11,19 Lustosa et al. observed that propolis displayed fungistatic and fungicidal activities against yeasts that cause onychomycosis.20 Propolis also increased the fungicidal activity of macrophages against Paracoccidioides brasiliensis and the fungicidal activity of human monocytes against Candida albicans.21,22
Saccharomyces cerevisiae was employed as a model to study genetics and cell biology, aiming to understand the mechanism of action of propolis produced by Apis mellifera on fungi, observing that the extract was able to induce cell death by apoptosis and secondary necrosis.23 Fungal cells in the initial development phase (phase lag) and in the stationary phase were more resistant, whereas cells in the exponential phase were much more sensitive to propolis. In addition, propolis induced accumulation of reactive oxygen species during apoptosis.
The major components of geopropolis extract were hexoses, glucitol, glucuronyl acid, inositol, and triterpenes.12 The antimicrobial activity of geopropolis produced by M. fasciculata in different regions of Maranhão State, Brazil was analyzed against Streptococcus mutans, L. acidophilus and Candida albicans by the agar diffusion method and by minimal bactericidal concentration. It was observed that the extract of geopropolis collected in Palmeirândia showed a higher antimicrobial activity and the highest flavonoids content.9
Regarding Pythium insidiosum evaluation, geopropolis seemed to exert a fungistatic activity rather than a fungicidal one after 48h, with MFC concentrations higher than propolis.
There are few studies regarding natural products on Pythium insidiosum. Garlic extract showed antimicrobial effects in vitro against 17 strains of Pythium insidiosum isolated from horses, with MIC values ranging from 0.39 to 6.25mgmL−1.24 In Thailand, the disease is endemic for humans and the effects of traditional medicinal plants (Alyxia schlechteri and Clausena harmandiana roots) were investigated, showing that some compounds were able to inhibit the mycelia growth of the pathogen.25,26
In conclusion, propolis was more efficient in inhibiting mycelia growth of Pythium insidiosum while geopropolis showed a fungistatic effect. This effect may be due to the propolis chemical composition, which has more active compounds than geopropolis. Since propolis exhibited a better response, further experiments should be carried out both in vitro and in vivo, as treatment with conventional antifungal agents is still problematic.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP – 2010/50064-7 and 2010/01028-8). Authors wish to thank Mr. Manuel Barros for donating geopropolis sample.