An increasing production of natural rubber (NR) products has led to major challenges in waste management. In this study, the degradation of rubber latex gloves in a mineral salt medium (MSM) using a bacterial consortium, a mixed culture of the selected bacteria and a pure culture were studied. The highest 18% weight loss of the rubber gloves were detected after incubated with the mixed culture. The increased viable cell counts over incubation time indicated that cells used rubber gloves as sole carbon source leading to the degradation of the polymer. The growth behavior of NR-degrading bacteria on the latex gloves surface was investigated using the scanning electron microscope (SEM). The occurrence of the aldehyde groups in the degradation products was observed by Fourier Transform Infrared Spectroscopy analysis. Rhodococcus pyridinivorans strain F5 gave the highest weight loss of rubber gloves among the isolated strain and posses latex clearing protein encoded by lcp gene. The mixed culture of the selected strains showed the potential in degrading rubber within 30 days and is considered to be used efficiently for rubber product degradation. This is the first report to demonstrate a strong ability to degrade rubber by Rhodococcus pyridinivorans.
Natural Rubber (NR) is a biopolymer that is produced from some plant species. The key composition of natural rubber latex is cis-1,4-poly(isoprene) that consists of isoprene as a monomer. In nature, polyisoprene can be divided into two groups, e.g. cis-poly(isoprene) and trans-poly(isoprene). The major source of natural rubber was produced from the Hevea brasiliensis. However, not only Hevea brasiliensis but also many plants can produce latex such as Ficus elastica, Ficus nitida and Euphorbia pulcherrima.1 The latex from the Hevea brasiliensis contains a polymer of cis-1,4-poly(isoprene) units and up to 90% of the dry weight of the latex is cis-1,4-poly(isoprene) and less than 10% are non-rubber constituents such as protein and carbohydrates.2,3
Global natural rubber production increased about 79.4% from 6.8 million metric tons in 2000 to 12.2 million metric tons in 2013.4 Natural Rubber is utilized daily in numerous applications such as agriculture, transportation, and various farming equipment, in addition, it is the major component in rubber tires.3 However, billions of discarded tires are currently stockpiled around the world and these are increasing exponentially and causing important environmental problems. At present, most rubber waste is eliminated by either burned or used as landfills but this process can cause serious pollution.5 Biodegradation of rubber wastes has been much of interest. Roles of microbes in degradation process are releasing of extracellular enzymes to cleave polymer chains into small molecules and these products are absorbed into the cell for use as carbon and energy source. Final process, CO2, H2O and other metabolic products are released and they can be used by other living organisms. Therefore, the biodegradation is an alternative way to degrade the rubber waste and could overcome the environmental problems.6–9
However, microbial degradation of polyisoprene rubber is a very slow process that taking months or even years. Microbial degradation of cis-1,4-poly(isoprene) rubber is currently being intensively investigated. Rubber-degrading organisms can be classified into two different groups; the first group is rubber-degrading organisms producing clear zones on natural rubber latex agar plates. Members of these groups generally belong to the mycelium-forming actinomycetes such as Actinoplanes, Streptomyces, Micromonospora and Rhodococcus.10–12 In contrast, the second group of rubber-degrading organisms is the microorganisms, which do not produce any clear zone, therefore, they require the direct contact with the rubber substrates. The examples of this group are nocardioform, actinomycetes, i.e. Gordonia sp., Mycobacterium sp. and Nocardia sp.10,13–15
Lcp (latex-clearing protein) encoded by lcp gene has been considered as a key enzyme in NR degradation by clearing-zone-forming gram-positive bacteria. While RoxA (rubber oxygenase) is a key enzyme in NR degradation found in the gram-negative bacterium Xanthomonas sp. strain 35Y. RoxA controlled by roxA gene is an extracellular protein secreted by this strain during growth on NR. Purified RoxA degraded cis-1,4-poly(isoprene) by oxidative cleavage at the double bonds, yielding 12-oxo-4,8-dimethyltrideca-4,8-diene-1-al as the main cleavage product; other minor cleavage products differed only in the number of repetitive isoprene units. In vitro experiments also revealed occurrence of two 18 O atoms in the reduced degradation product 12-hydroxy-4,8-dimethyltrideca-4,8-diene-1-ol, thereby disclosing a dioxygenase mechanism.16–18
Most efforts on the study of rubber biodegradation have been directed to using single bacterial strain which still gave poor results. It was hypothesized that the synergistic interaction among various microorganisms should accelerate the rubber degradation process. So in this study, the degradation of rubber latex gloves in a mineral salt medium (MSM) by a consortium, a mixed culture of the selected bacteria and a pure culture were compared. The weight loss, scanning electron microscopy (SEM) of the rubber glove substrate and viable cell counts were determined.
Materials and methodsIsolation of rubber degrading microorganisms from soil samples by enrichment culture techniqueThe rubber-degrading microorganisms were isolated from soil samples collected from rubber contaminated ground in Songkhla province, Thailand. Soil samples were mixed with pieces of natural rubber (NR) glove (0.5×0.5cm) incubated in sterile mineral salt medium (MSM) at 30°C, 150rpm. Mineral salt medium is composed of (g/L): Na2HPO4 9.0, KH2PO4 1.5, (NH4)2NO3 1.0, MgSO4 0.2, CaCl2 0.02, and Fe (III) NH4 0.0012. The medium was adjusted to an initial pH of 7.0 before sterilization.19 After a month of incubation, 0.5mL of those culture broths were transferred into MSM with latex and further incubated under the same conditions as described above for another month to encourage the growth of rubber degrading bacteria. The developed culture broth was then assigned as the natural soil consortium. The rubber degrading bacteria were also screened and isolated from the natural soil consortium using NR latex agar plates.
Screening of NR-degrading bacteriaThe degradation of the rubber gloves by the selected strains was investigated through plate assay. Latex overlay plates were prepared by spreading 5mL of solubilized latex concentrate as a thin film on MSM agar plates and then left to dry. All tested strains that grew well on the latex overlay agar were picked for further study.
Rubber glove degradation investigation of the individual tested bacteria, the mixed culture, and the natural soil consortiumLatex glove pieces were washed once with distilled water prior to drying in an oven at 60°C until obtained a constant weight before being used as a carbon source. Three experiments were designed to test the microbial activities for degrading rubber gloves. The first experiment was testing each individual isolated strain. The second experiment was testing the mixed cultures of high effective isolated strains (mixed cultures) and the third experiment tested the mixed cultures of all isolated strain (consortium). Each culture was incubated in MSM supplemented with dried latex glove pieces (0.6%, w/v) as a sole carbon source at 30°C, 150rpm for 4 weeks. Each experiment was performed in triplicate. The inoculated MSM without any latex glove pieces and the uninoculated MSM with latex glove pieces were used as biotic and abiotic control experiments, respectively. Viable cell counts of each culture were determined throughout the incubation time. The weight of latex gloves were measured before (W1) and after degradation (W2). Weight loss was calculated using equation: Weight loss=[(W1−W2)/W1]×100.
Scanning electron microscope (SEM)The surfaces of the NR glove film pieces were analyzed by scanning electron microscopy (SEM) to investigate the appearance changes after the degradation. The samples were washed with sterilized distilled water and then were fixed with 2% glutaraldehyde in 0.1M phosphate-buffered saline (PBS; pH 7.3). After that, the samples were washed with PBS and dehydrated in graded ethanol (50%, 60%, 70%, 80%, and 90% and absolute ethanol). The dehydrated samples were subjected to critical point drying with liquid CO2 according to the standard procedure. The samples were then mounted on aluminum specimen stubs by using electrically conductive carbon (PLANO, Wetzlar, Germany) and coated with a gold layer. Micrographs were recorded using scanning electron microscope (SEM-JSM5800LV; JEOL Ltd., Tokyo, Japan).
Fourier Transform Infrared (FTIR) spectroscopyThe FTIR (VERTEX 70; Bruker, Germany) analysis was done to detect the degradation of NR latex glove on the basis of changes in the functional groups. The correlation of absorption bands in the spectrum of an unknown compound with the known absorption frequencies was analyzed. The polymer pieces were mixed with KBr and made into a pellet, which was fixed to the FTIR sample plate. Spectra were taken in triplicate at 400–4000wavenumberscm−1 for each sample.
16S RNA gene analysisThe genomic DNA of isolate F5 was extracted using Power Soil DNA isolation kit (MO BIO Laboratories, a QIAGEN Company, USA). Molecular genetic identification was performed by amplification of 16S rRNA gene with bacterial universal primers 27F (5-AGAGTTTGATCCTGGCTCAG-3) and 1492R (5-CGGCTACCTTGTTACGACTT-3).20 The PCR reaction was carried out in a final volume of 50μL containing 10mM Tris–HCl (pH 8.3), 50mM KCl, 1.5mM MgCl2, each dNTP at a concentration of 0.2mM, 1.25IU of Taq polymerase, each primer at a concentration of 0.2mM and 1μL of the DNA template. PCR was performed according to the following program: 2min denaturation at 95°C, followed by 30 cycles of 1min denaturation at 95°C; 1min annealing at 55°C; 1min extension at 72°C; and a final extension step of 10min at 72°C. The PCR product was then analyzed and purified using an agarose Gel DNA Purification Kit (E.Z.N.A. Gel Extraction Kit; Omegabiotek, USA). The 16S rDNA gene was sequenced. A phylogenetic tree was constructed by means of MEGA, version 7.0, using a neighbor-joining algorithm, and the Jukes-Cantor distance estimation method with bootstrap analyses for 1000 replicates was performed.
Amplification of the lcp geneThe coding region of lcp from Rhodococcus pyridinivorans was amplified by using the primers lcpF (5-ATAATCCGAGCAGGCGCGA-3) and lcpR (5-TCGGGGATCTCGATGCTGA-3). PCR reaction contained 10mM Tris–HCl (pH 8.3), 50mM KCl, 1.5mM MgCl2, each dNTP at a concentration of 0.2mM, 1.25IU of Taq polymerase, each primer at a concentration of 0.2mM, and 1μL of the DNA template. PCR was performed according to the following program: 2min denaturation at 95°C, followed by 30 cycles of 1min denaturation at 95°C; 1min annealing at 55°C; 1min extension at 72°C; and a final extension step of 10min at 72°C.
CO2 emission testBiodegradation of latex glove was also determined by CO2 emission test. The released CO2 during the cultivation of cells in MSM in which contained rubber gloves as the sole carbon source was trapped in a solution of 1N Ba(OH)2 and was quantified by titrating of the remaining Ba(OH)2 with 0.1N HCl.21
ResultsIsolation of rubber-degrading microorganisms from soil samplesIn order to enrich the growth of rubber degrading bacteria, soil sample was incubated with rubber glove pieces for a month. The developed culture which was assigned as a natural soil consortium was grown on NR agar to isolate rubber degrading bacteria. Consortium PP demonstrated the best degrading ability causing the rubber weight loss up to 59%. Unfortunately, the degradation ability of consortium PP was un-stable causing the rubber weight loss varied from 30% to 59%. Therefore we attempt to construct a stable bacterial community by isolating rubber-degrading bacteria from this consortium, ten bacterial isolates, F1 to F10, obtained from a natural soil consortium were capable of growing on MSM agar containing NR as the sole carbon source. Isolate F5 was identified as Gram-positive and others were Gram-negative bacteria. All ten isolates did not produce clearing zone around the colonies indicated that they could degrade the latex concentrate by a direct contact with the rubber substrate.3 The degradation of rubber gloves were tested using each isolated strain compared with the mixed cultures of top 5 effective strains (F1, F2, F5, F6, F7) and the consortium of all 10 bacterial isolates (F1–F10).
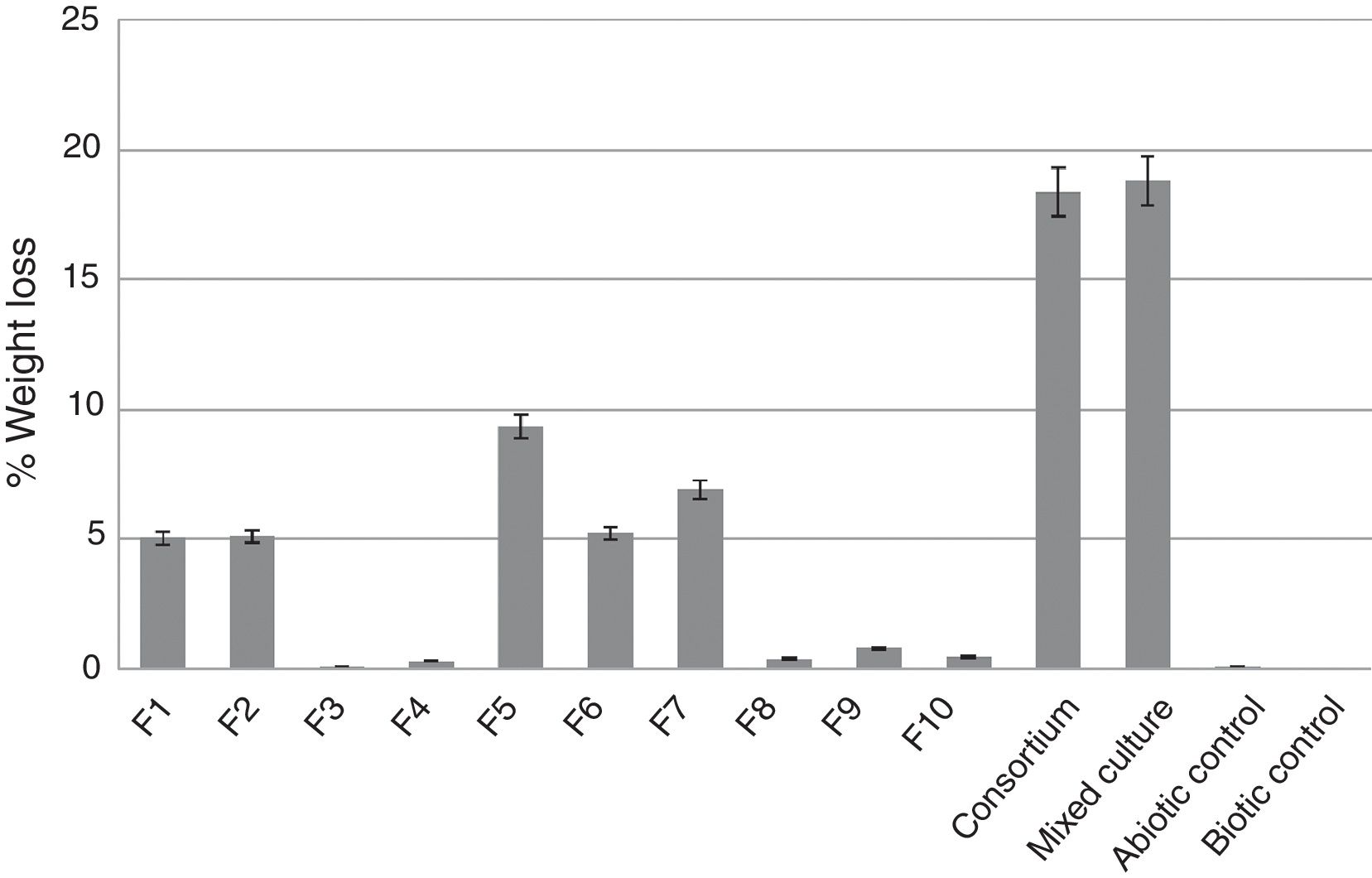
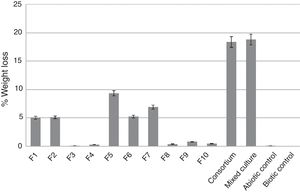
Weight loss of latex glove piecesThe weight loss of latex glove pieces after one-month incubation was around 0.5–9% when using individual culture of F1–F10 and among 10 isolates, F5 gave the best weight loss of 9.36% (Fig. 1). The highest weight loss of 18.82% was obtained from the mixed culture of top 5 effective strains (F1, F2, F5, F6, F7), while 18.38% was obtained from the consortium (F1–F10) compared to only 0.2% of the abiotic control (Fig. 1).
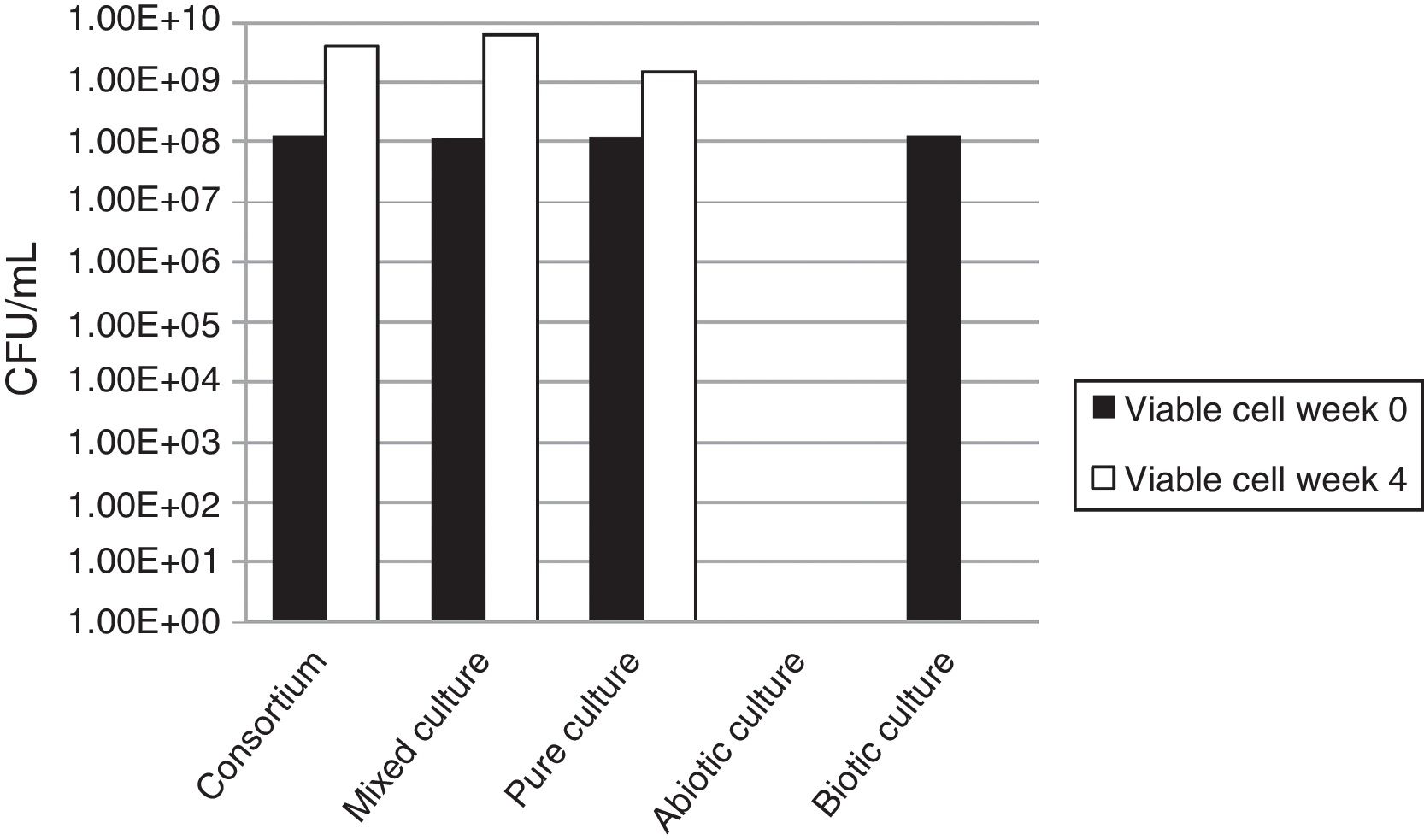
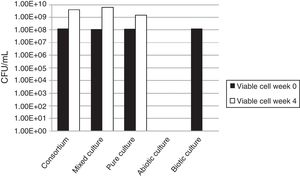
Viable cell countsThe increased viable cell counts through the end of the incubation time in all tested bacterial groups indicated that the bacteria had the ability to degrade natural rubber and used rubber gloves as the sole carbon source (Fig. 2). In spite of the adhesive growth behavior, an increase in the number of cells suspended in the medium during cultivation occurred. The bacterial population of the mixed cultures showed the biggest increase from 1.2×108CFU/mL to 6.2×109CFU/mL after 1 month incubation with rubber gloves, the bacterial population in the consortium had increased from 1.2×108CFU/mL to 4.0×109CFU/mL while the pure culture showed the smallest bacterial population increase from 1.2×108CFU/mL to 1.5×109CFU/mL. The production of biomass from rubber also implies the presence of oxidative degradation of rubber polymer.
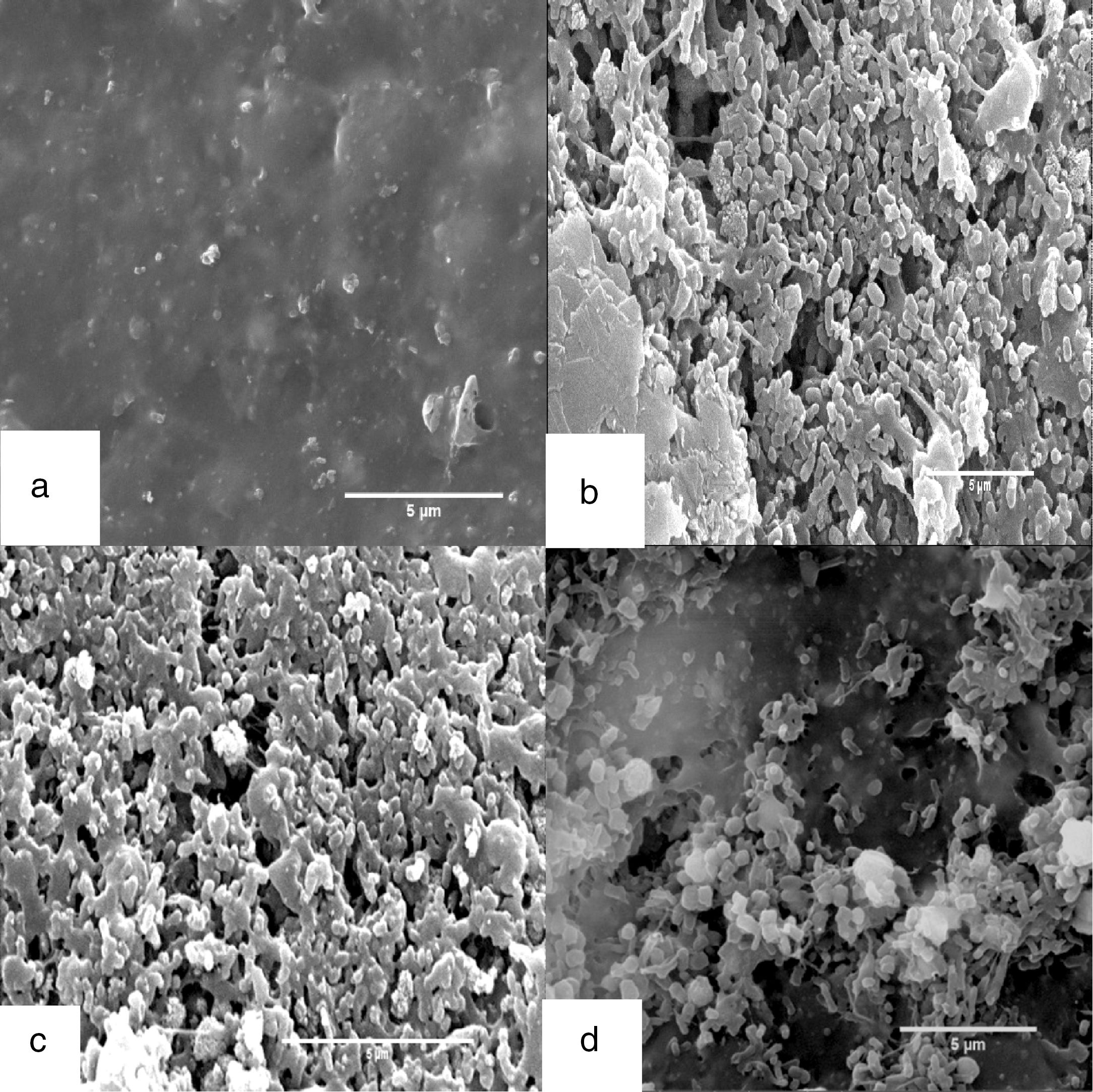
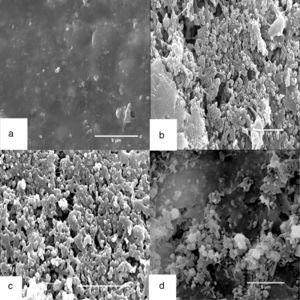
SEMThe biodegradation of rubber gloves was also observed by SEM. After 4 weeks of incubation with the consortium, mixed culture and F5, the surfaces of rubber gloves were covered with bacterial colonization compared to control (Fig. 3a–d). Significant changes were irregular crashes, surface erosions, and holes in variable sizes on the surface of latex glove pieces. Interestingly, isolate F5 (Fig. 3d) cells embedded and colonized into the rubber matrix. The adhesive growth attachment of consortium and mixed culture on rubber surfaces can be detected as early as 7 days of incubation while when using single strain of F5, bacterial colonization occurred only after 10 days of incubation.
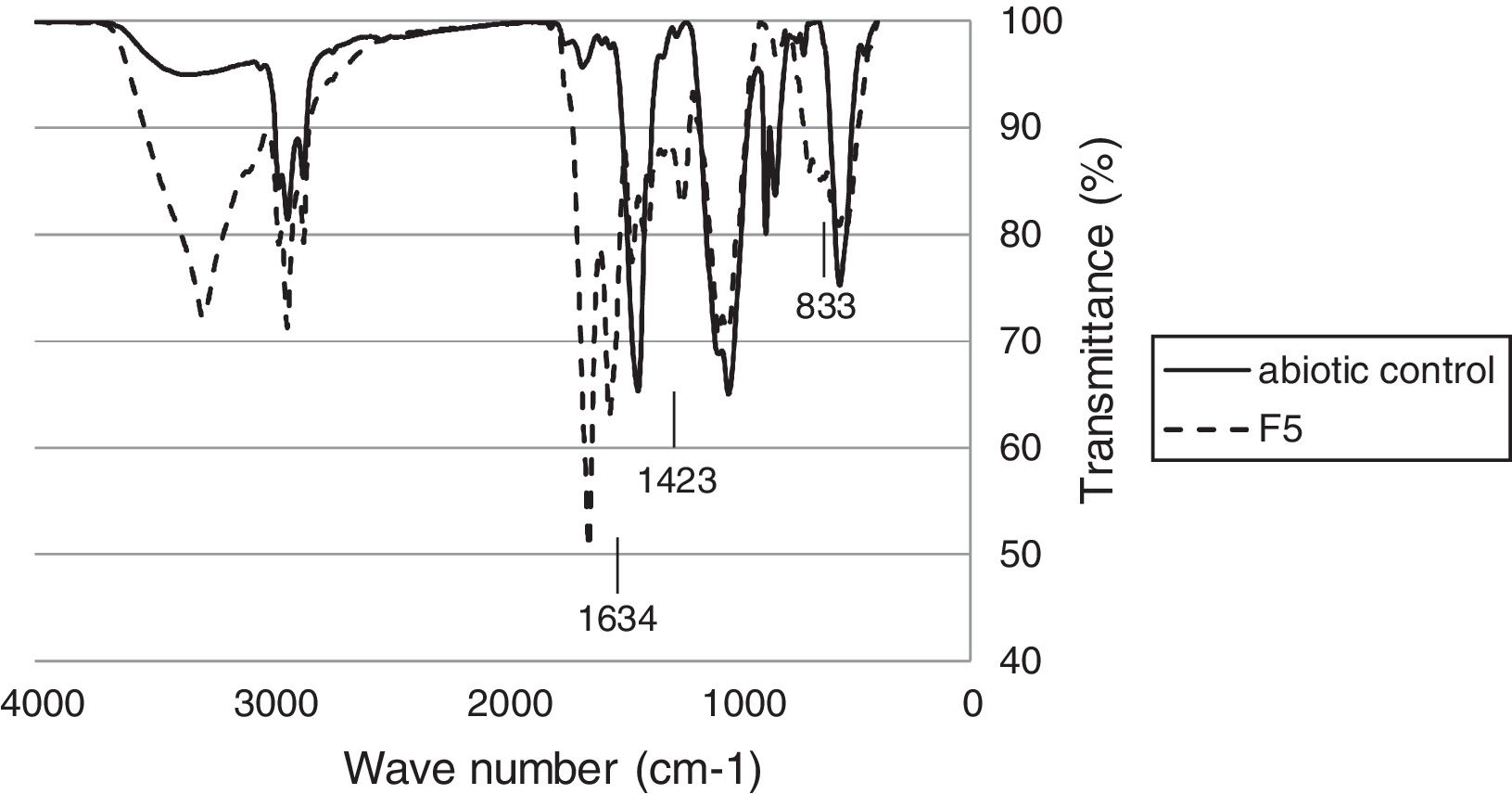
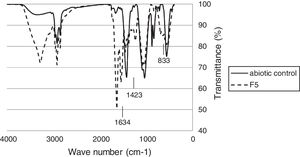
Fourier Transform Infrared spectroscopy testFTIR analysis of the rubber glove pieces after incubated with F5 showed various spectra changes compared to the control (Fig. 4). In the transmittance area corresponding to the cis-1,4 double bonds in the polyisoprene chain, a relative decrease of the (CH2) band at 833cm−1 was observed for the tested sample compared to that of the abiotic control. The FTIR peak decreased at 1423cm−1 in latex glove pieces treated with isolate F-5 culture, indicating the break of functional groups like CC, methyl and ester bonds. Moreover an increase in the intensity of the peak at 1634cm−1 showed the presence of aldehydes (CO) groups in the case of test pieces as compared to control. These indicated that F5 used latex gloves as a carbon source during cultivation.
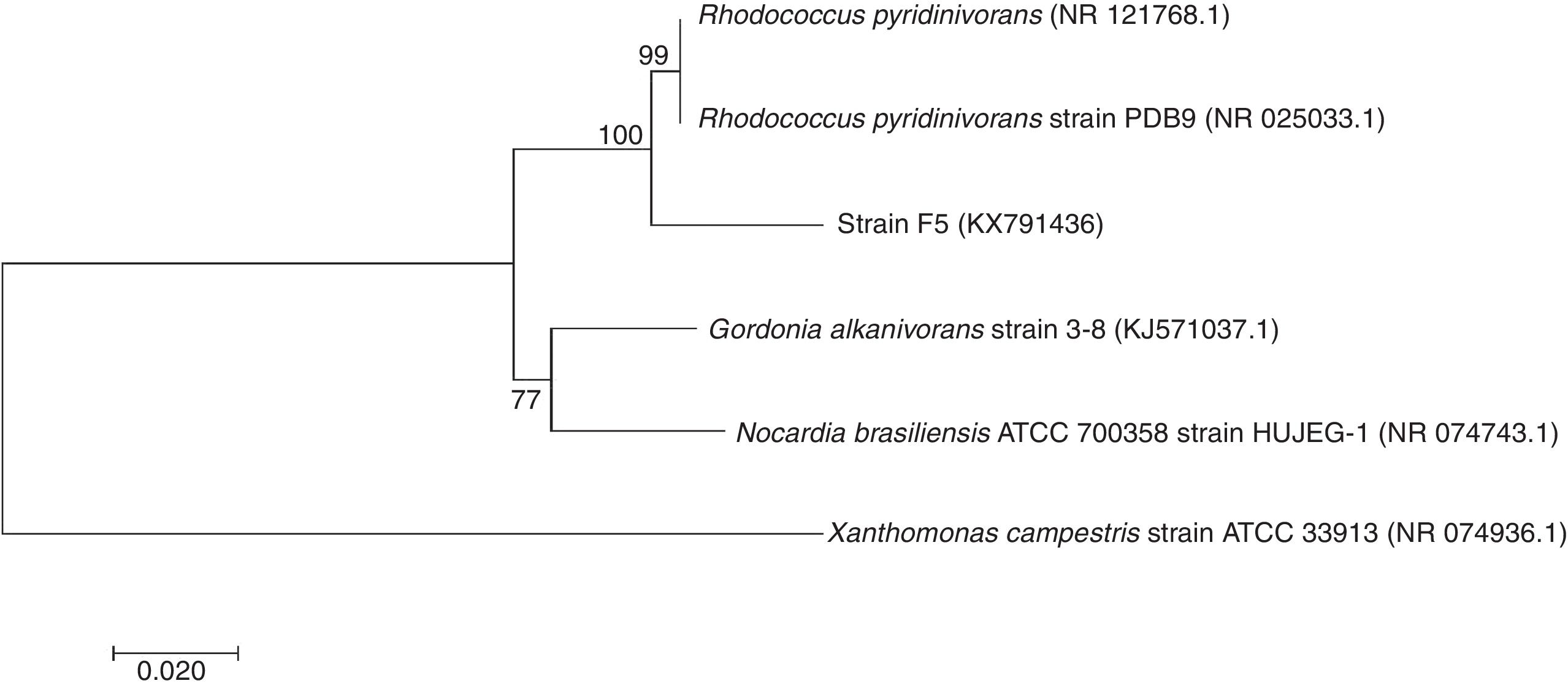
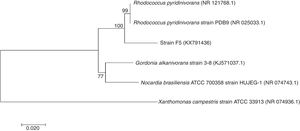
Molecular identification using 16S rRNA gene sequence comparisonThe sequencing result of 1.470kb nucleotides of 16S rRNA gene of strain F5 was aligned and compared with other bacteria available in the NCBI GenBank. The phylogenetic analysis of the 16S rRNA sequence showed that the strain F5 belongs to genus Rhodococcus sp. having 99% similarities with Rhodococcus pyridinivorans and was submitted to GenBank under accession number KX791436 (Fig. 5).
Molecular identification and phylogenetic analysis using lcp gene sequence comparisonThe PCR product of lcp gene was sequenced retrieving 1.293kb nucleotides. The sequences were then aligned and compared to the database of the NCBI GenBank. The phylogenetic analysis of the lcp sequences showed that 99% similarities with an lcp gene from Rhodococcus pyridinivorans. The presence of lcp gene confirmed the biodegradation process in this species. Further investigation is required to confirm the reaction mechanism of this enzyme.
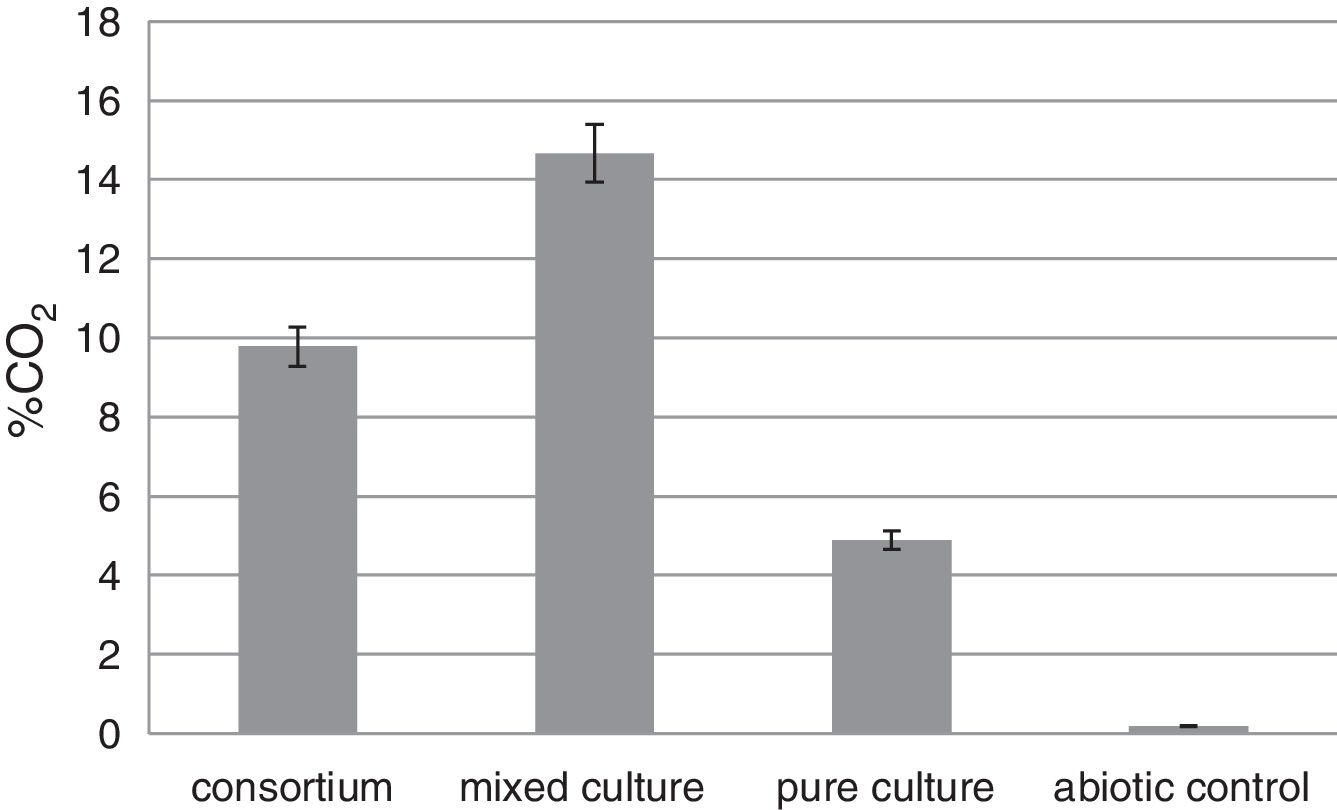
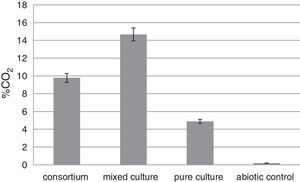
CO2 emission testThe CO2 evolved from the biodegradation was measured to compare the ability to degrade rubber gloves of tested bacteria. The mixed culture released 14.67% CO2, 9.78% from the consortium and 4.89% from the pure culture F5 (Fig. 6). The highest CO2 release was related to the highest weight loss of latex glove experiment and confirmed the best degradation occurred when the mixed culture was incubated with rubber gloves.
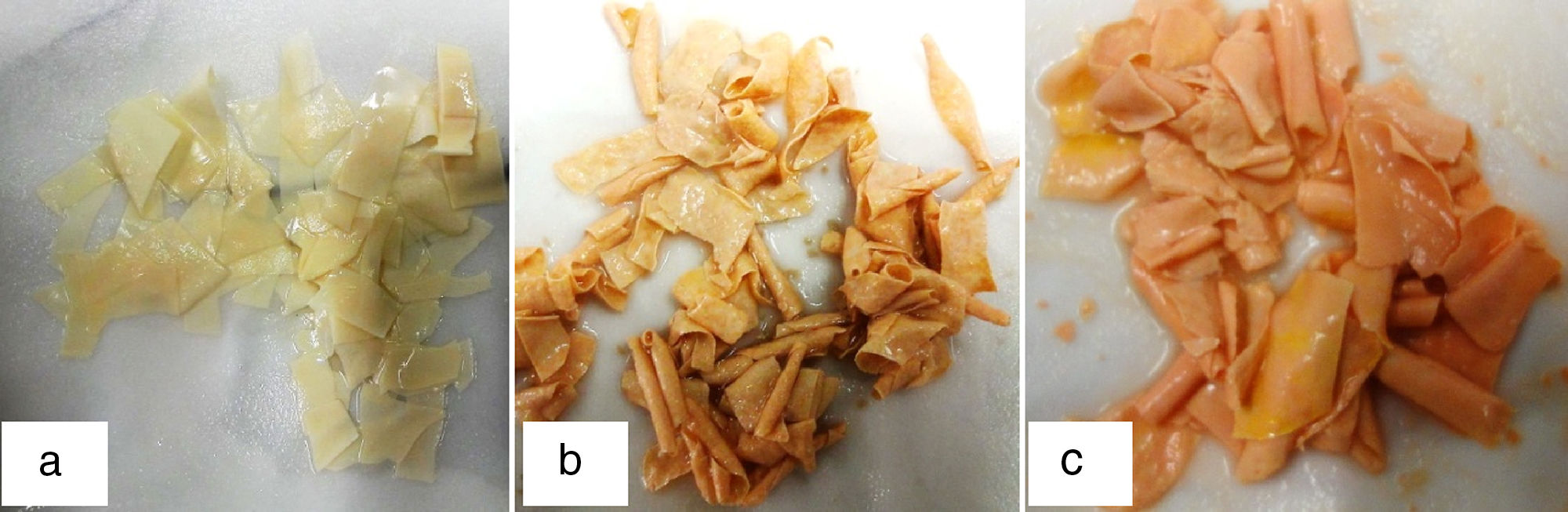
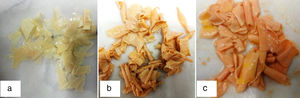
DiscussionsThe presence of rubber wastes currently results in environmental pollution. The ability of microorganisms to degrade rubber wastes has received considerable attention. Recently different kinds of environmental pollutants were reported to be effectively degraded by microbial consortia.23–27 The synergetic interaction of microorganisms in this study points out an alternative approach to perform rubber degradation. It was clearly shown that using mixed culture and the consortium resulted in better degradation. The obvious changes in rubber appearances were observed and the color of rubber pieces became orange (Fig. 7). However, the decrease of rubber degrading property of the consortium after successive transfer in NR medium was observed. The instability of degrading ability might due to the loss of any bacterial population in the consortium during transfer. The rubber degrading property could be re-stored again after a re-cultivation with a long incubation time which allows microorganisms to adapt. The efficiency of consortium degraded rubber gloves in broth culture was shown by weight loss and the deterioration of rubber pieces; it might be due to the breakdown of long ester bonds into low molecular weight fragments by one organism making access easier for some enzymes produced by the other. The rate of rubber degradation was high in case of treatment with microbial consortium as compared to the individual strains. Our results are in conformity with those previously reported and show that the significant changes in surface morphology occur due to the microbial degradability of treated polymers. A direct contact via a biofilm and the embedding of cells into rubber gloves may be a possible mechanism, as a report of Gordonia and some other actinomycetes suggests.19
The FTIR-ATR spectroscopy was applied to detect the changes in the functional groups of the polymer. The FTIR spectra of the NR latex gloves recorded in the transmission and ATR mode corresponded to the cis-1,4 double bonds in the polyisoprene chains in the region of 700–900cm−1. The decreased spectra of cis-1,4 double bonds, methyl and ester bonds in the polyisoprene chains, and the appearance of ketone and aldehyde groups from FTIR analysis indicated that an oxidative attack at the double bonds might be the first metabolic steps of the biodegradation process.28–30 After microbial treatment, FTIR analysis of the rubber gloves indicated some reduction of the molecular sizes and the formation of new peaks with the breakdown of some bonds. The increased spectra of CO stretching at 1634cm−1, indicated that some of the unsaturated bonds (CC) in the NR were possibly converted to CO.
The primary degradation reaction of cis-1,4-isoprene is an initial exposure the rubber to bacterial enzymes that can oxidize some double bonds and break the long chain hydrocarbon linkages to produce smaller more degradable molecules. These enzymes are either excreted by clear zone forming rubber degrading bacteria or attached to the bacterial cell walls in the group of bacteria require direct growth on the rubber surface.31,32 Tsuchii and his team determined the degradation products of vulcanized and non-vulcanized rubber using Nocardia sp. 835A.22 The authors concluded that natural rubber is degraded by an oxygenase reaction, and may also involve the scission of isoprene at the unsaturated double bond, leading to the formation of aldehydes or ketones at each end of the split isoprene chains.
Tsuchii and Takeda also described the rubber degrading activity in the culture medium using Xanthomonas sp. strain 35Y by attempting to cleave rubber with crude enzyme, and pointed out that the cleavage reaction was enzyme-catalyzed process.28 This evidence revealed that the enzyme reaction could be considered to be a two-step reaction. In the first step, the high molecular weight rubber polymer was degraded and converted to medium molecular weight (MW) hydrocarbon intermediates. The extracellular crude enzyme from strain 35Y had a high substrate specificity for cis-1,4-polyisoprene compounds and attacked high MW polymer chains rather than lower MW oligomers, 12-oxo-4,8-dimethyltrideca-4,8-diene-1-al was identified as one of the first degradation products. Moreover, Linos and his team confirmed Tsuchii's theories on the rubber cleavage mechanism by a Gordonia strain and found that not only aldehyde and ketone groups were detected but also the decrease in the number of cis-1,4-isoprene bond during the process, in addition, two different new functional groups were detected after being treated with bacteria.31 These results indicated that an oxidative enzyme attacked at the double bond in the first metabolic step of the biodegradation process.31,32 According to all the evidences in the biochemical degradation reactions of polyisoprene rubber to the identification of metabolites, Bode and his team has established the “hypothetical pathway of rubber degradation” presenting the first step as the oxidative cleavage at the isoprene double bonds, with the formation of cis-1,4-isoprene oligomers such as 12-oxo-4,8-dimethyltrideca-4,8-diene-1-al and the presence of keto and aldehyde groups at the end of the split chains.7 These low molecular weight oligomers are consequently utilized by bacteria and the aldehyde group is oxidized to the corresponding carboxylic acid. The acid is activated to a coenzyme A (CoA) ester and then passes though the β-oxidation cycle.7,33 However, further investigation and evidences on the biochemical analysis, metabolite or end products of rubber are required.
Apart from the FTIR and weight loss analysis, carbon dioxide evolution has commonly been used to evaluate biodegradability of rubber; microbial degradation of polymeric material can be evaluated by entrapping CO2 evolution as a result of mineralization. In the present study, the quantity of CO2 released in the test flasks containing rubber gloves as the only source of carbon was significantly higher than in control flasks without rubber gloves, providing the evidence of mineralization of rubber polymer. In most of the published studies conducting rubber degradation, Actinomycetes are reported to be the main group of rubber degrading microbes. The latex clearing protein (Lcp) encoded by lcp gene was first reported in Streptomyces sp. K30 and also later found in other gram-positive rubber degrader including Nocardia farcinica E1, Nocardia nova SH22a, Nocardia sp. NVL3 Actinoplanes sp. OR16 and Gordonia westfalica Kb1.17,29,30,34–36 To our knowledge, we are the first group to use bacterial consortium to degrade rubber and also the first study to demonstrate an ability to degrade rubber by Rhodococcus pyridinivorans. Genus Rhodococcus has been proved to be a promising option for the bioremediation of many xenobiotic compounds.37–40 Watcharakul et al. firstly reported the ability of R. rhodochrous in rubber degradation and also revealed some different properties of Lcp protein of R. rhodochrous RPK1 that has not been described previously in other rubber-degrading bacteria.12 The detection of lcp gene in R. pyridinivorans F5 in this study confirmed the ability of this strain in degrading rubber gloves. Since R. pyridinivorans F5 did not produce a clearing zone around the colony on NR overlay plate, this result supports the hypothesis of the study of Linh et al. that the Lcp itself is not responsible for clearing zone formation.31 It will be necessary to further investigate the biochemical and biophysical properties of this enzyme to fully understand the variation of the enzyme properties and to verify the role of Lcp in rubber degradation.
ConclusionsIn the present study the consortium, mixed culture and pure culture were able to degrade rubber gloves in which significant changes can be detected within 30 days. The determination of the weight loss, the increase in viable cell counts, FTIR, CO2 evolution test and the SEM has confirmed that the natural rubber degradation process occurred. The structure analysis from FTIR confirmed that the structure of the rubber was changed after treatment with the bacteria and led to the deterioration of the polymer. Rhodococcus pyridinivorans strain F5 demonstrated the highest rubber degrading activity among all single strains used. The presence of lcp gene encode for latex degrading enzyme in Rhodococcus pyridinivorans F5 supports the essential function of lcp in poly(cis-1,4 isoprene) degradation. Mixed culture of the selected strains reached the highest rubber degradation after one-month incubation and showed the potential to be used efficiently for rubber degradation. Further studies are necessary to elucidate roles of each strain in the mixed culture and consortium. The results obtained on batch biodegradation of rubber gloves using a consortium will be useful in application of continuous removal of rubber wastes. This has an advantage when the process is applied in the controlled environment conditions, where pure culture might not be able to utilize the secondary metabolites.
Financial supportThis project was funded by Research Assistant scholarships, Faculty of Science, Prince of Songkla University the Faculty of Science and also the Research grant from Prince of Songkla University.
Conflicts of interestThe authors declare no conflicts of interest.
We are really grateful to Dr. Brian Hodgson for his unconditional support and great advices. We are thankful to Dr. Nicholas Hodgson and Mrs. Anna Chatthong for English language checking and editing.