Production of a bioherbicide for biological control of weeds requires a series of steps, from selection of a suitable microbial strain to final formulation. Thus, this study aimed to select fungi for production of secondary metabolites with herbicidal activity using biological resources of the Brazilian Pampa biome. Phytopathogenic fungi were isolated from infected tissues of weeds in the Pampa biome. A liquid synthetic culture medium was used for production of metabolites. The phytotoxicity of fungal metabolites was assessed via biological tests using the plant Cucumis sativus L., and the most promising strain was identified by molecular analysis. Thirty-nine fungi were isolated, and 28 presented some phytotoxic symptoms against the target plant. Fungus VP51 belonging to the genus Diaporthe showed the most pronounced herbicidal activity. The Brazilian Pampa biome is a potential resource for the development of new and sustainable chemical compounds for modern agriculture.
Brazil hosts approximately 20% of the whole world's biological diversity, which can be employed as a resource for the development of new and sustainable ecosystem management tools and opportunities for bioprospecting.1 This large biodiversity is distributed within six biomes. Among those, the Pampa biome, which is restricted to part of Rio Grande do Sul State, presents distinct characteristics of vegetation, climate, and soil types, making it a unique ecosystem on the planet, capable of maintaining a high plant and animal diversity. However, Pampa is the least known Brazilian biome in terms of its biodiversity.2
Despite its importance, Brazilian microbial diversity is still considered largely unknown. Discovery of microorganisms for use as a source of commercially exploitable products may support programs focused on the application of biosynthetic or biodegradation processes.1,3 Fungi represent a part of the microflora of natural ecosystems and may be promising sources for the production of various compounds. It is estimated that there are about 150,300–263,900 fungal species in Brazil.4 Evidence showing that biological sources can provide natural products with phytotoxic activity opens a new perspective for the preservation of microbial species in the Pampa biome.
Phytopathogenic fungi produce toxins that may play a role in the development of plant diseases. Weeds are a significant problem in crop production, and their management is crucial for modern agriculture to avoid yield losses and to ensure food safety. Traditional chemical control options are limited due to ecodegradation, health hazards, and the development of herbicide resistance in weeds.5 Herbicide-resistant weeds are the main problem in weed control due to the number of weed biotypes resistant to herbicides that constantly increases by the continuous use of the same products for years.6 In the last 20 years, no chemical has been synthesized with a different mode of action than those discovered so far.7 Such compounds could present a considerable potential as models for developing herbicides with new modes of action.8,9
Based on these aspects, the main objective of this work was to isolate fungi from the Pampa biome for the production of bioactive molecules with herbicidal activity. Thirty-nine phytopathogenic fungi were isolated from plants of the Pampa biome. The production of metabolites was performed in liquid synthetic culture media. The phytotoxicity of the fungi was assessed via biological tests, and the most promising strain was identified by molecular analysis.
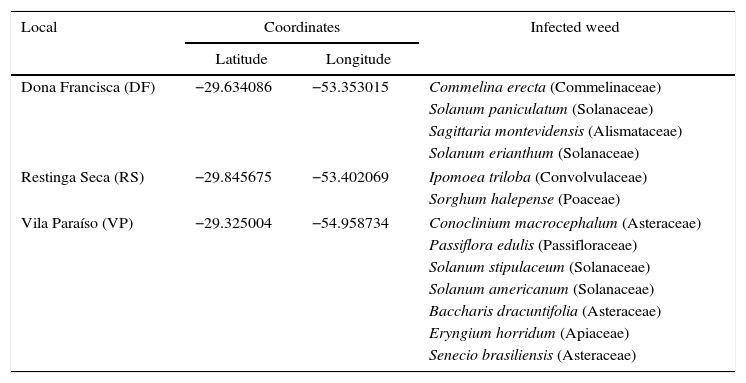
Materials and methodsIsolation and selection of microorganismsPhytopathogenic fungi were isolated from infected tissues of weeds of irrigated rice and rangelands at three locations of the Brazilian Pampa biome. Table 1 presents the locations of the collection sites as well as the weeds collected at each location. The strategy used for collection was based on selecting weeds with some symptoms of infection. Collection was carried out from December 2012 to April 2013 in different areas of the Pampa biome. The samples were stored packed in plastic bags and maintained at 4°C during transportation to the Laboratory of Bioprocesses where the isolation of fungi was carried out. Each infected tissue was transferred to a Petri dish containing potato dextrose agar (PDA) and incubated at 28°C for seven days in the dark. After this, each sample was subcultured three times to obtain a pure culture, which was transferred to a PDA slant in a test tube and stored at 4°C.
Geographical coordinates of collection points as well as the infected weeds collected for isolation of fungi.
| Local | Coordinates | Infected weed | |
|---|---|---|---|
| Latitude | Longitude | ||
| Dona Francisca (DF) | −29.634086 | −53.353015 | Commelina erecta (Commelinaceae) |
| Solanum paniculatum (Solanaceae) | |||
| Sagittaria montevidensis (Alismataceae) | |||
| Solanum erianthum (Solanaceae) | |||
| Restinga Seca (RS) | −29.845675 | −53.402069 | Ipomoea triloba (Convolvulaceae) |
| Sorghum halepense (Poaceae) | |||
| Vila Paraíso (VP) | −29.325004 | −54.958734 | Conoclinium macrocephalum (Asteraceae) |
| Passiflora edulis (Passifloraceae) | |||
| Solanum stipulaceum (Solanaceae) | |||
| Solanum americanum (Solanaceae) | |||
| Baccharis dracuntifolia (Asteraceae) | |||
| Eryngium horridum (Apiaceae) | |||
| Senecio brasiliensis (Asteraceae) | |||
The growth of all phytopathogenic fungi isolated in the previous step was carried out in a liquid medium, aiming at the production of bioactive molecules with herbicidal action. For the pre-inoculum production, mycelium from each test tube containing one fungus was inoculated on PDA in a Petri dish and incubated for eight days at 28°C, which was sufficient for the fungal growth to cover the entire surface of the agar. Afterwards, the agar surface in the Petri dish was washed with 5mL of autoclaved water, and the suspension was transferred for fermentation.
The fermentations were carried out in 250-mL Erlenmeyer flasks containing 125mL of fermentation medium at 28°C, 120rpm for seven days (Innova 44R, New Brunswick). The medium was composed of (gL−1): glucose, 10.0; yeast extract, 7.5; peptone, 10.0; (NH4)2SO4, 2.0; FeSO4·7H2O, 1.0; MnSO4·H2O, 1.0; and MgSO4, 0.5, and the initial pH was adjusted to 6.0.10
After the fermentation, cells were separated by centrifugation at 4000rpm for 10min (Eppendorf, model 5804R), and the supernatant was filtered through a 0.45-μm polyvinylidene fluoride (PVDF) membrane. The filtered sample was used to evaluate its bioherbicidal activity in the bioassay. Each of the 39 fungi was considered a different bioherbicide.
The activity of the bioherbicides obtained from the phytopathogenic fungi was demonstrated using cucumber (Cucumis sativus L., variety Wisconsin), a target plant frequently used in bioassays of herbicides. A completely randomized design, composed of 39 treatments (each selected strain was considered one treatment) and four repetitions, where each repetition represented a tray containing 20 propylene vessels with a volume of 200mL of a commercial substrate (MacPlant®) without any treatment, was employed. Three seeds were sown in each vessel, and after the emergence, only one plant was maintained in each vessel and transferred at the seedling stage to a greenhouse located at the Federal University of Santa Maria. The seeds used in the experiment were obtained from a local market and did not undergo any treatment before seeding.
A volume of 30mL of fermented broth was applied at the same time to each bioassay using a garden sprayer. Control assays were performed using the culture medium instead of the fermented broth. After 21 days, plant injury was visually estimated as a percentage of growth reduction in comparison with the untreated controls, where 100% represented complete plant death and 0% represented no effect.11 In addition, other parameters were also investigated, including (i) the height of the plant; (ii) the length of the root; (iii) fresh weight of the aerial and root parts; and (iv) dry weight of the aerial and root parts, which were evaluated after the application of a bioherbicide.
All treatments were normalized by dividing the value obtained in the treatment by the value obtained in the control test. Based on the plant development, the following results were obtained: “−” for inhibition between 0 and 0.95, “N” for a zero or non-significant effect (0.95–1.05), and “+” for a growth effect (higher than 1.05). All data were subjected to the analysis of variance (ANOVA) and to Tukey's test (p<0.05) to compare the means.
Fungal identificationThe most promising fungus for the production of a bioherbicide was identified. Fungal DNA was extracted from aliquots of growth in the liquid medium using the ZR Fungal/Bacterial DNA MiniPrep kit (Zymo Research). After extraction of total DNA, the internal transcribed spacer 1 (ITS1)-5.8S rDNA-ITS2 region of nuclear ribosomal DNA was amplified with primers ITS1 and ITS4. The reaction of amplification was carried out according to Baldoni et al.12 Amplification of the correct fragment was verified by electrophoresis in a 1.5% agarose gel with 1× Tris–borate–ethylenediaminetetraacetic acid (TBE) buffer. The DNA fragments were stained with BlueGreen Loading Dye I® (LGC Biotecnologia, Cotia, Brazil) and analyzed in ultraviolet light. The products of polymerase chain reaction (PCR) were purified using the GenElute PCR Clean-Up Kit® (Sigma, St. Louis, MO, USA) following the manufacturer's instructions. Sequencing of the samples was carried out using the ABI PRISM 3100 Genetic Analyzer (Applied Biosystems). The sequenced fragments were analyzed by the program Staden Package 2.0.0b to obtain a consensus sequence.13 The consensus sequence was deposited to GenBank (accession number KU523580), and a comparative search of GenBank sequences was carried out using the BLASTn tool. The additional sequences retrieved from GenBank included those of Brazilian species described for this genus.14 For the identification of the fungus, all the sequences were aligned using the program BioEdit v. 7.2.5 with the ClustalW algorithm.15
The phylogeny was reconstructed by maximum likelihood based on the analysis of the ITS region using MEGA 5.0.16 A total of 1000 bootstrap replicates were used for the reconstruction. The Kimura two-parameter nucleotide-substitution model was used with ModelTest run with uniform rates and partial deletion (95%) parameters.17 The sequences of Diaporthe ambigua (KC343015) and Diaporthella corylina (KC343004) were used as the outgroups.18,19
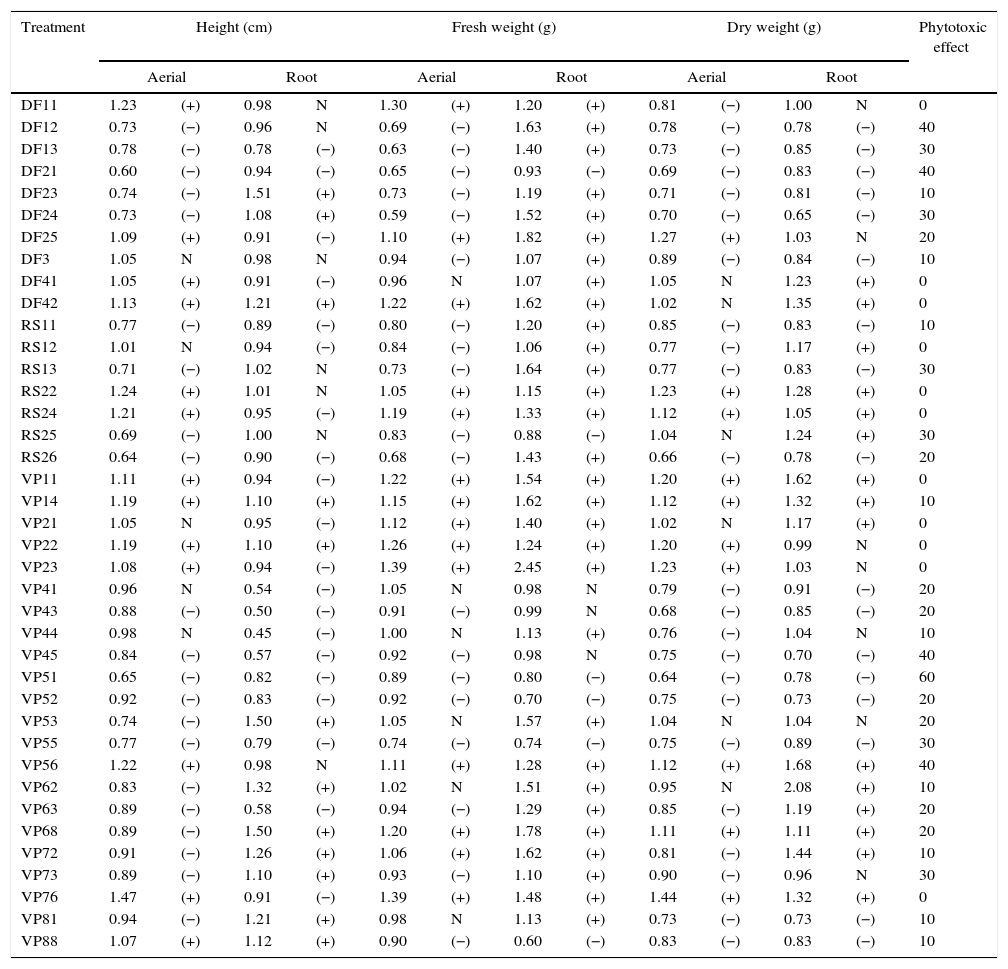
Results and discussionIn this work, 39 phytopathogenic fungi were isolated from weeds of the Pampa biome. Table 2 presents the results obtained in the bioassays. Twenty-eight fungi showed phytotoxic effects against the target plant, and the most pronounced effect was shown by fungus VP51 with an activity of 60%. Other fungi (DF12, DF21, VP45, and VP56) also showed activity at the level of 40%. These same fungi also produced good results in the growth inhibition of aerial parts of the target, with a reduction of the height and fresh weight around 35–40% relative to the control. The growth inhibition of the root part was less pronounced in comparison with the aerial part. Generally, the inhibition was around 20%, and it did not result in the plant death. The low herbicidal activity might be related to the fact that the active metabolite was significantly diluted in the crude extract. However, it is important to consider that a bioherbicide may not necessarily cause the same effect on plants as a chemical herbicide. Bioherbicides have the potential to provide a competitive advantage for growing seedlings through the infection and delay of the growth of weed seedlings.20
Inhibitory effect of the fermented broth obtained after fermentation of each isolated fungus on the target plant (C. sativus).
| Treatment | Height (cm) | Fresh weight (g) | Dry weight (g) | Phytotoxic effect | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aerial | Root | Aerial | Root | Aerial | Root | ||||||||
| DF11 | 1.23 | (+) | 0.98 | N | 1.30 | (+) | 1.20 | (+) | 0.81 | (−) | 1.00 | N | 0 |
| DF12 | 0.73 | (−) | 0.96 | N | 0.69 | (−) | 1.63 | (+) | 0.78 | (−) | 0.78 | (−) | 40 |
| DF13 | 0.78 | (−) | 0.78 | (−) | 0.63 | (−) | 1.40 | (+) | 0.73 | (−) | 0.85 | (−) | 30 |
| DF21 | 0.60 | (−) | 0.94 | (−) | 0.65 | (−) | 0.93 | (−) | 0.69 | (−) | 0.83 | (−) | 40 |
| DF23 | 0.74 | (−) | 1.51 | (+) | 0.73 | (−) | 1.19 | (+) | 0.71 | (−) | 0.81 | (−) | 10 |
| DF24 | 0.73 | (−) | 1.08 | (+) | 0.59 | (−) | 1.52 | (+) | 0.70 | (−) | 0.65 | (−) | 30 |
| DF25 | 1.09 | (+) | 0.91 | (−) | 1.10 | (+) | 1.82 | (+) | 1.27 | (+) | 1.03 | N | 20 |
| DF3 | 1.05 | N | 0.98 | N | 0.94 | (−) | 1.07 | (+) | 0.89 | (−) | 0.84 | (−) | 10 |
| DF41 | 1.05 | (+) | 0.91 | (−) | 0.96 | N | 1.07 | (+) | 1.05 | N | 1.23 | (+) | 0 |
| DF42 | 1.13 | (+) | 1.21 | (+) | 1.22 | (+) | 1.62 | (+) | 1.02 | N | 1.35 | (+) | 0 |
| RS11 | 0.77 | (−) | 0.89 | (−) | 0.80 | (−) | 1.20 | (+) | 0.85 | (−) | 0.83 | (−) | 10 |
| RS12 | 1.01 | N | 0.94 | (−) | 0.84 | (−) | 1.06 | (+) | 0.77 | (−) | 1.17 | (+) | 0 |
| RS13 | 0.71 | (−) | 1.02 | N | 0.73 | (−) | 1.64 | (+) | 0.77 | (−) | 0.83 | (−) | 30 |
| RS22 | 1.24 | (+) | 1.01 | N | 1.05 | (+) | 1.15 | (+) | 1.23 | (+) | 1.28 | (+) | 0 |
| RS24 | 1.21 | (+) | 0.95 | (−) | 1.19 | (+) | 1.33 | (+) | 1.12 | (+) | 1.05 | (+) | 0 |
| RS25 | 0.69 | (−) | 1.00 | N | 0.83 | (−) | 0.88 | (−) | 1.04 | N | 1.24 | (+) | 30 |
| RS26 | 0.64 | (−) | 0.90 | (−) | 0.68 | (−) | 1.43 | (+) | 0.66 | (−) | 0.78 | (−) | 20 |
| VP11 | 1.11 | (+) | 0.94 | (−) | 1.22 | (+) | 1.54 | (+) | 1.20 | (+) | 1.62 | (+) | 0 |
| VP14 | 1.19 | (+) | 1.10 | (+) | 1.15 | (+) | 1.62 | (+) | 1.12 | (+) | 1.32 | (+) | 10 |
| VP21 | 1.05 | N | 0.95 | (−) | 1.12 | (+) | 1.40 | (+) | 1.02 | N | 1.17 | (+) | 0 |
| VP22 | 1.19 | (+) | 1.10 | (+) | 1.26 | (+) | 1.24 | (+) | 1.20 | (+) | 0.99 | N | 0 |
| VP23 | 1.08 | (+) | 0.94 | (−) | 1.39 | (+) | 2.45 | (+) | 1.23 | (+) | 1.03 | N | 0 |
| VP41 | 0.96 | N | 0.54 | (−) | 1.05 | N | 0.98 | N | 0.79 | (−) | 0.91 | (−) | 20 |
| VP43 | 0.88 | (−) | 0.50 | (−) | 0.91 | (−) | 0.99 | N | 0.68 | (−) | 0.85 | (−) | 20 |
| VP44 | 0.98 | N | 0.45 | (−) | 1.00 | N | 1.13 | (+) | 0.76 | (−) | 1.04 | N | 10 |
| VP45 | 0.84 | (−) | 0.57 | (−) | 0.92 | (−) | 0.98 | N | 0.75 | (−) | 0.70 | (−) | 40 |
| VP51 | 0.65 | (−) | 0.82 | (−) | 0.89 | (−) | 0.80 | (−) | 0.64 | (−) | 0.78 | (−) | 60 |
| VP52 | 0.92 | (−) | 0.83 | (−) | 0.92 | (−) | 0.70 | (−) | 0.75 | (−) | 0.73 | (−) | 20 |
| VP53 | 0.74 | (−) | 1.50 | (+) | 1.05 | N | 1.57 | (+) | 1.04 | N | 1.04 | N | 20 |
| VP55 | 0.77 | (−) | 0.79 | (−) | 0.74 | (−) | 0.74 | (−) | 0.75 | (−) | 0.89 | (−) | 30 |
| VP56 | 1.22 | (+) | 0.98 | N | 1.11 | (+) | 1.28 | (+) | 1.12 | (+) | 1.68 | (+) | 40 |
| VP62 | 0.83 | (−) | 1.32 | (+) | 1.02 | N | 1.51 | (+) | 0.95 | N | 2.08 | (+) | 10 |
| VP63 | 0.89 | (−) | 0.58 | (−) | 0.94 | (−) | 1.29 | (+) | 0.85 | (−) | 1.19 | (+) | 20 |
| VP68 | 0.89 | (−) | 1.50 | (+) | 1.20 | (+) | 1.78 | (+) | 1.11 | (+) | 1.11 | (+) | 20 |
| VP72 | 0.91 | (−) | 1.26 | (+) | 1.06 | (+) | 1.62 | (+) | 0.81 | (−) | 1.44 | (+) | 10 |
| VP73 | 0.89 | (−) | 1.10 | (+) | 0.93 | (−) | 1.10 | (+) | 0.90 | (−) | 0.96 | N | 30 |
| VP76 | 1.47 | (+) | 0.91 | (−) | 1.39 | (+) | 1.48 | (+) | 1.44 | (+) | 1.32 | (+) | 0 |
| VP81 | 0.94 | (−) | 1.21 | (+) | 0.98 | N | 1.13 | (+) | 0.73 | (−) | 0.73 | (−) | 10 |
| VP88 | 1.07 | (+) | 1.12 | (+) | 0.90 | (−) | 0.60 | (−) | 0.83 | (−) | 0.83 | (−) | 10 |
Fig. 1 shows some effects on aerial parts, such as yellowing, leaf spots, and blight. The most pronounced effects on aerial parts were obtained with fungi VP76, DF24, and VP51, which are presented in Fig. 1(A–C), respectively, where it is possible to compare their effects with that of the control (Fig. 1D). The blasting symptom seen in Fig. 1A and C was also noticed by Chung et al.21 when evaluating the potential of a bioherbicide from Plectosporium tabacinum for growth inhibition of Sagittaria trifolia. Berner et al.22 reported that one of the phytotoxic symptoms caused by fungi of the genus Cercosporella was small brown spots on leaves.
Leaf spots (Fig. 1B) were observed in the first 72h after the application of the DF24 bioherbicide. These plants presented mild, irregularly distributed lesions, having a dark green to dark brown color, which were limited to the sprayed leaves. The yellowing around the spots became widespread in the leaf blade, forming necrosis from the tips and edges of the sheet. The leaves that emerged after the inoculation were free of the disease. The leaf spot effects, followed by yellowing, were also described by Yandoc et al.23 who analyzed the effects of the fungi Bipolaris sacchari and Drechslera gigantea on the control plant Imperata cylindrical.
Similar symptoms were reported by other authors when a fermented broth was used for weed control. Inhibition of growth was also a phytotoxic effect of bacterial isolates on weeds, as observed by Weissmann et al.24 Gronwald et al.25 obtained a reduction of 31% in plant height, reporting that the inhibition of growth was an important factor in determining the action of a bioherbicide. Walker and Tilley26 also observed a reduction in the dry weight of Senna obtusifolia treated with a broth fermented by Myrothecium verrucaria.
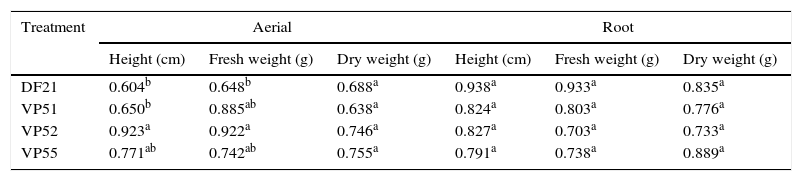
To determine the most potential treatment for the production of bioherbicides, some fungi (DF21, VP51, VP52, and VP55) showing the highest herbicidal activity and the highest inhibition of the height and weight of plants were screened. To determine if there were differences among these fungi, the data from Table 2 were analyzed by ANOVA, followed by Tukey's test (p<0.05). The results are compiled in Table 3. For the aerial part, fungi DF21 and VP51 showed the most pronounced inhibitory effects on the plant height, which were statistically different from those shown by the others. Regarding fresh weight, the effect shown by fungus DF21 was statistically different from those shown by the others, whereas there were no verified significant differences in the effects on dry weight, as well as root parts. Based on these results, it can be inferred that the fungi showed effects mainly on the aerial parts of the target, and the most prominent wasVP51. This fungus demonstrated considerable phytotoxicity and affected morphology of the target. For this reason, it was selected for molecular identification.
Comparison of mean among the effects of treatments on the aerial and root parts of C. sativus.
| Treatment | Aerial | Root | ||||
|---|---|---|---|---|---|---|
| Height (cm) | Fresh weight (g) | Dry weight (g) | Height (cm) | Fresh weight (g) | Dry weight (g) | |
| DF21 | 0.604b | 0.648b | 0.688a | 0.938a | 0.933a | 0.835a |
| VP51 | 0.650b | 0.885ab | 0.638a | 0.824a | 0.803a | 0.776a |
| VP52 | 0.923a | 0.922a | 0.746a | 0.827a | 0.703a | 0.733a |
| VP55 | 0.771ab | 0.742ab | 0.755a | 0.791a | 0.738a | 0.889a |
Different letters (a,b) in the column represent a significant difference at 95% (p<0.05 – Tukey test).
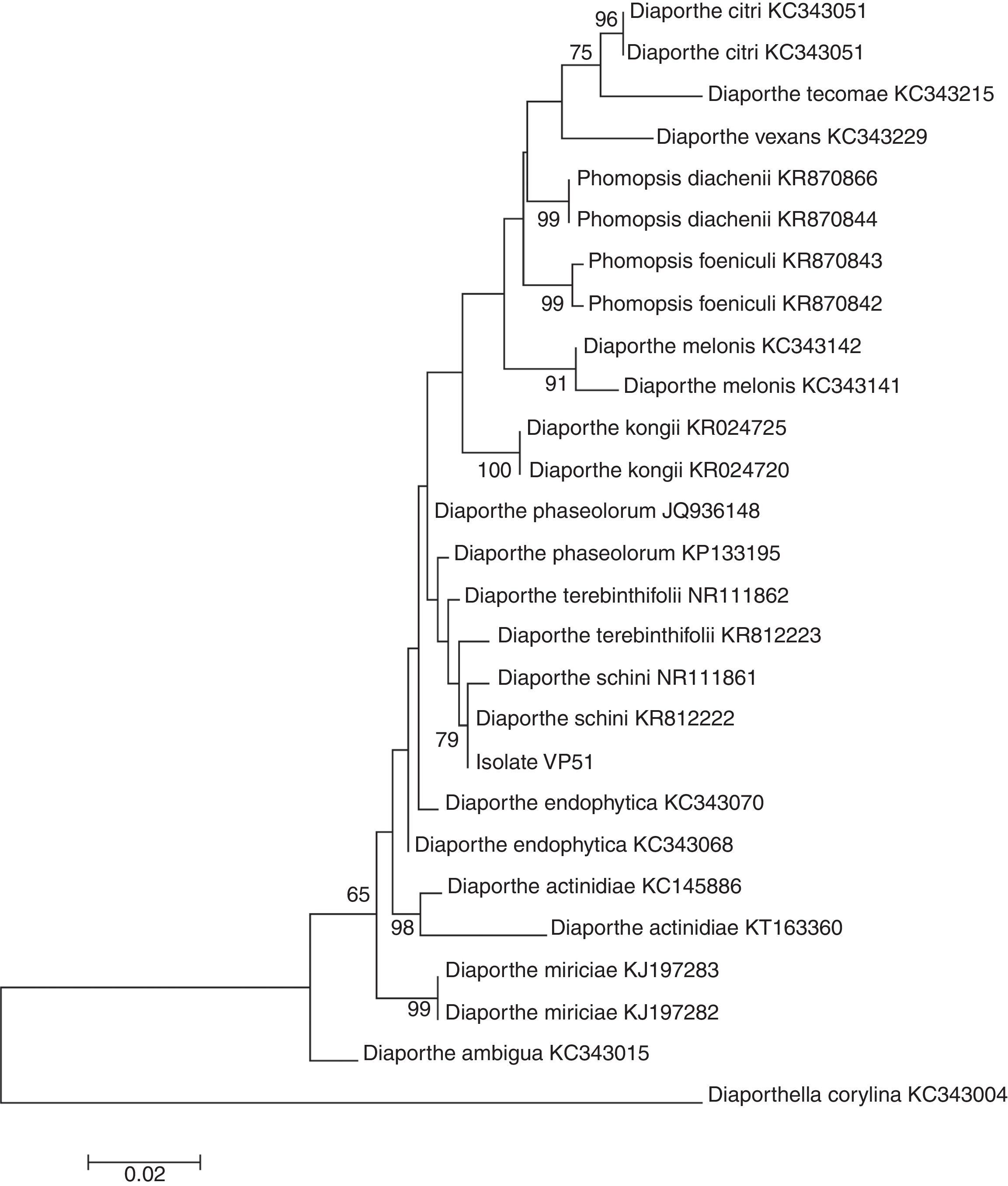
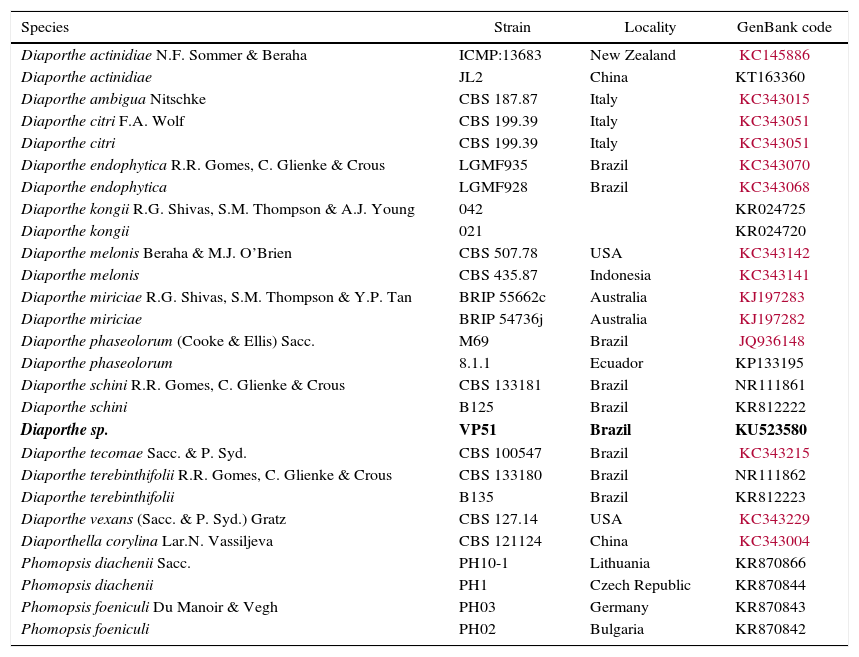
The molecular analysis of the ITS1-5.8S-ITS2 region of VP51 showed its high similarity to the species Diaporthe schini (100%), D. terebinthifolii (99%), and D. phaseolorum (99%) (Table 4), among the nucleotide sequences available in the National Center for Biotechnology Information (NCBI) database. However, no significant divergences among these species were found in this region to allow identification of VP51 at the species level. The phylogenetic clade formed did not support the species separation (low bootstrap values) (Fig. 2).
Specimens included in this study. Accession Genbank numbers in bold referred to the ITS sequences obtained from Diaporthe sp. in Pampa bioma, Southern Brazil.
| Species | Strain | Locality | GenBank code |
|---|---|---|---|
| Diaporthe actinidiae N.F. Sommer & Beraha | ICMP:13683 | New Zealand | KC145886 |
| Diaporthe actinidiae | JL2 | China | KT163360 |
| Diaporthe ambigua Nitschke | CBS 187.87 | Italy | KC343015 |
| Diaporthe citri F.A. Wolf | CBS 199.39 | Italy | KC343051 |
| Diaporthe citri | CBS 199.39 | Italy | KC343051 |
| Diaporthe endophytica R.R. Gomes, C. Glienke & Crous | LGMF935 | Brazil | KC343070 |
| Diaporthe endophytica | LGMF928 | Brazil | KC343068 |
| Diaporthe kongii R.G. Shivas, S.M. Thompson & A.J. Young | 042 | KR024725 | |
| Diaporthe kongii | 021 | KR024720 | |
| Diaporthe melonis Beraha & M.J. O’Brien | CBS 507.78 | USA | KC343142 |
| Diaporthe melonis | CBS 435.87 | Indonesia | KC343141 |
| Diaporthe miriciae R.G. Shivas, S.M. Thompson & Y.P. Tan | BRIP 55662c | Australia | KJ197283 |
| Diaporthe miriciae | BRIP 54736j | Australia | KJ197282 |
| Diaporthe phaseolorum (Cooke & Ellis) Sacc. | M69 | Brazil | JQ936148 |
| Diaporthe phaseolorum | 8.1.1 | Ecuador | KP133195 |
| Diaporthe schini R.R. Gomes, C. Glienke & Crous | CBS 133181 | Brazil | NR111861 |
| Diaporthe schini | B125 | Brazil | KR812222 |
| Diaporthe sp. | VP51 | Brazil | KU523580 |
| Diaporthe tecomae Sacc. & P. Syd. | CBS 100547 | Brazil | KC343215 |
| Diaporthe terebinthifolii R.R. Gomes, C. Glienke & Crous | CBS 133180 | Brazil | NR111862 |
| Diaporthe terebinthifolii | B135 | Brazil | KR812223 |
| Diaporthe vexans (Sacc. & P. Syd.) Gratz | CBS 127.14 | USA | KC343229 |
| Diaporthella corylina Lar.N. Vassiljeva | CBS 121124 | China | KC343004 |
| Phomopsis diachenii Sacc. | PH10-1 | Lithuania | KR870866 |
| Phomopsis diachenii | PH1 | Czech Republic | KR870844 |
| Phomopsis foeniculi Du Manoir & Vegh | PH03 | Germany | KR870843 |
| Phomopsis foeniculi | PH02 | Bulgaria | KR870842 |
Therefore, this result is not sufficient to provide full identification, and at this time, it is only possible to say that the fungus belongs to the genus Diaporthe. Gomes et al.14 suggested redefining the classification of the species within the genus Diaporthe based on morphological and cultural characteristics, the mating type, and DNA sequences to obtain a satisfactory delineation of the species within the genus Diaporthe.
The genus Diaporthe (anamorph: Phomopsis) belongs to the phylum Ascomycota, subphylum Pezizomycotina, class Sordariomycetes, order Diaporthales characterized as sexual fungi. However, some fungi in this genus present asexual forms, leading to difficulty in identifying members of this genus at the species level. Diaporthe spp. are often described as producers of enzymes and secondary metabolites27 with the potential as antibiotics,28 fungicides,29 and anticancer agents,30 as well as that for preventing herbivory and for biological control of weeds.31,32 Ethyl 2,4-dihydroxy-5,6-dimethylbenzoate, phomopsilactone,33 phomopxanthone A and B,34 taxol,30 phomopsichalasin,35 lactones,29 nonenolides, phomonol, phomotone,36 and phomophene are some of the compounds produced by members of the genus.
Some of the compounds listed above showed herbicidal activity. Phomentrioloxin B caused small necrotic spots on a number of plant species, whereas gulypyrone A caused leaf necrosis on Helianthus annuus plantlets.30 Cimmino et al.8 tested several compounds produced in liquid culture by Phomopsis sp. (teleomorph: Diaporthe gulyae) for the control of the annual weed Carthamus lanatus.
ConclusionsIn this work, 39 fungi were isolated from the Pampa biome with the goal of obtaining biomolecules with herbicidal activity against weeds. Twenty-eight fungi caused some phytotoxic symptoms, but the most pronounced effects were obtained with fungi DF21, VP51, VP52, and VP55. Among those, VP51 showed the highest herbicidal activity and was subjected to molecular identification. The nucleotide sequence of VP51 was compared with sequences available in the NCBI database, and the fungus was identified as belonging to the genus Diaporthe, members of which have already been reported as producers of bioherbicides.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank CAPES - Coordination for the Improvement of Higher Education Personnel and CNPQ - National Council for Scientific and Technological Development for the scholarships and the State Department of Development and Investment Promotion (SDPI-RS) for the financial support of this work.