The Provisional Act (Medida Provisória – MP) 2,186-16, of August 23, 2001, was the first legal framework to regulate access to Genetic Heritage (GH)1 and Associated Traditional Knowledge (ATK)2 in Brazil for purposes of scientific research, bioprospecting, and technological development. This MP was also responsible for the creation of the Genetic Heritage Management Council (CGen). However, this MP had very negative impact on scientific research, displeasing the academic community, which felt obstructed by bureaucratization and criminalized by administrative penalties, discouraging Research & Development (R&D) of Brazilian biodiversity resources.
The construction of a new legislation was complex, considering the different interests and visions among diverse sectors of civil society, represented by the academia, business sector, and holders of associated traditional knowledge, as well as the different sectors of government. Thus, it was almost 15 years before the publication of the “New Law on Biodiversity”, Law 13,123 of May 20, 2015, which came into force on November 17, 2016. However, regulation occurred only six months after the Law came into force, after extensive opposition, debates, and criticisms, through Decree No. 8772 of May 11, 2016. To facilitate compliance with the legislation and to assist the CGen, the Decree created the National System of Genetic Resource Management and Associated Traditional Knowledge (SisGen). Due to various bureaucratic and administrative issues, SisGen was made available to the public on November 6, 2017, which is almost one year after the Law came into force.
The new Law, whose scope is more comprehensive than previous legislation, involves research, technological development, and economic exploitation of finished product3 and reproductive material4 from access to GH and ATK. According to the new definitions of GH, access to GH,5 and research,6 the Law includes activities that were not contemplated by the MP, such as research related to molecular taxonomy, phylogeny, molecular epidemiology, and molecular ecology, as well as the use of information from public genetic sequence databases, such as GenBank.
It is important to emphasize that to comply with the legislation, an institution must first appoint a legal representative, who will be responsible for the institutional register and will alone have the power to represent it within SisGen. An institution may appoint more than one legal representative. After the validation of the institutional register by the Executive Secretariat of CGEN, the researchers of this institution will be able to register as applicants, which will be validated by the legal representative. Only after these procedures will the researchers be able to register their activities covered by the Law.
The replacement of the previous authorization (MP 2,186-16) by the current registry, which can be carried out during the research and technological development with GH and ATK in SisGen, resulted in significantly reduced bureaucratization of R&D in Brazil and is consequently one of the most positive changes in the Law. Nevertheless, a researcher needs to be very attentive to some cases that require prior registration, such as shipment of genetic heritage; application for intellectual property rights; marketing of an intermediate product; dissemination of results (final or partial); or even notification of a finished product or reproductive material developed from an access. Prior authorization will also be required for cases involving foreigners, in which access takes place in the border area and Brazilian jurisdictional waters, on the continental shelf, and in the exclusive economic zone.
Upon completing the SisGen electronic forms, the registration receipt or notification will automatically be issued. This document demonstrates that the user has provided the required information. In addition, the user may request a Certificate of Access Regulatory from CGen.
There are two possibilities for transportation of genetic heritage abroad: shipment and sending. “Shipment” is considered more critical because it involves transferring a sample of GH to an institution located outside Brazil for the purpose of access. In this case, it is necessary to sign a Material Transfer Agreement (MTA) between sender and recipient of the shipment abroad. “Sending” consists of transporting a sample from GH to provide services abroad, as part of research or technological development, in which the responsibility for the sample remains with whoever performs the access in Brazil. It is mandatory that, upon completion of the laboratory analyzes, the samples sent are destroyed or returned. In place of MTA, a legal instrument signed between the national institution responsible for the access and the partner or contracted institution will be required. In case of sample submitted for genetic sequencing, a legal instrument will not be mandatory, only the formal communication to the partner institution or contractor about obligations and prohibitions defined in the Law.
Another novelty of this legislation is the single paragraph of the article referring to the definitions used in the Law (Article 2), which ensures that any microorganism isolated in Brazil is part of the Brazilian genetic heritage. The purpose, in this case, is to resolve uncertainties and questions relating to its origin, about whether the microorganism is native or exotic, which was very frequent during the term of the previous legislation. In this context, biomedical researchers should take into consideration that research involving pathogens obtained from human samples (e.g. blood, urine, tissues) must meet the requirements of the Law, considering that this pathogenic microorganism is a genetic heritage. Thus, this type of research must be in accordance with Law 13,123, as well as with Resolution 466/2012 of the National Health Council, which establishes the ethical and scientific foundations for research with human beings.
Regarding the shipment of microorganisms, the Law authorizes the transfer of the sample to third parties, with the condition that the MTA that accompanies the sample contains the same provisions as the original MTA, which should occur for all subsequent transfers. This was a major breakthrough, especially when the objective of the shipment is the deposit into international microbiological collections.
Foreign researchers will be able to access native biodiversity only if they are associated with public or private Brazilian scientific and technological research institutions, which must take responsibility for registering the activity. This requirement also applies to access samples of Brazilian genetic heritage deposited in ex situ collections or to genetic sequences obtained from samples of Brazilian genetic heritage deposited in public databases. Due to this requirement of association, foreign researchers, concerned about complying with Brazilian legislation, may give up to studying Brazilian biodiversity. To exemplify, the case of the description of a new species that needs the comparison with other Brazilian species deposited in biological collections, abroad or in Brazil, using molecular techniques. This situation would require the foreign researcher to have to look for a researcher in Brazil, who agrees to take the responsibility for registering the research (description of the new species), in order to get associated to him/her only for accessing this genetic heritage. Therefore, it is necessary to find alternatives and conduct adjustments in order to decrease the negative impacts that this requirement may cause.
In the current legislation, Associated Traditional Knowledge (ATK) encompasses all “information or practice of indigenous population, traditional community, or traditional farmers on the properties or direct or indirect uses associated with genetic heritage.” In addition, ATK is characterized in two ways: of identifiable origin – in which it is possible to link its origin to at least one indigenous population, traditional community, or traditional farmer; and of unidentifiable origin - when this linkage is not possible. In the case of ATK with identifiable origin, no research can be initiated before obtaining Prior Informed Consent (PIC).
Considering that in the new legislation, the Federal Government is the recipient of the benefit sharing, the National Fund for Benefit Sharing (FNRB), of a financial nature, was established. This Fund will receive the money from benefit sharing (Monetary Benefit Sharing) and fines, and aims to support actions and activities that acknowledge the value of genetic heritage and associated traditional knowledge, and promote its use in a sustainable way. To manage the resources of the FNRB, a Management Committee was created, and a National Benefit Sharing Program was established to promote conservation of biological diversity; recovery, creation, and maintenance of ex situ collections of genetic heritage samples; prospecting and training of human resources associated with the use and conservation of genetic heritage or associated traditional knowledge; and gathering and inventory of genetic heritage; etc.
When the economic exploitation comes from GH or ATK with unidentifiable origin, the Federal Government is indicated as the recipient of the benefit sharing to be deposited in the FNRB, which is set at 1% of the annual net revenue obtained from the exploitation of the product. However, this figure can be reduced to as much as 0.1% through a sectoral agreement. Nevertheless, when the economic exploitation comes from ATK of identifiable origin, the deposit in the FNRB will be 0.5% of the annual net revenue, in addition to the amount negotiated directly with the user. This act is intended to minimize problems of judicialization, by claiming that other traditional groups also hold the same knowledge.
In addition to the Monetary Benefit Sharing, the legislation also provides for Non-Monetary Benefit Sharing, which can be done by implementing projects for conservation or sustainable use of biodiversity or for protection and maintenance of associated traditional knowledge; technology transfer; distribution of the product in the public domain; training of human resources; free distribution of products in social interest programs, etc. Some of these Non-Monetary Benefit Sharing options may be more advantageous in some cases than the transfer of resources to the Fund.
The Law also establishes that when monetary resources deposited in the FNRB are derived from the economic exploitation of finished product and reproductive material obtained from access to GH coming from the ex situ collections that are accredited in SisGen, this resource will be destined to them. The Decree defined that these resources will be partially (between 60 and 80%) destined for the benefit of these collections. This is an achievement for the ex situ collections, considering that they play a fundamental role in the preservation and conservation of biodiversity, activities that involve high costs. On the other hand, there was another major change in the Law that excluded the requirement to deposit in ex situ collections an accessed GH sample, which in the case of shipment abroad, would guarantee the traceability of genetic resources and, therefore, the sovereignty of Brazil over its biodiversity. Even so, it is recommended that prior to shipment, a deposit be made in Brazilian biological collections. The future version 2 of SisGen (which is being developed) is expected to include specific fields for registering this information on a voluntary basis.
It is important to emphasize that the entire academic community should be aware of the one-year deadline, after SisGen took effect on November 6, 2017, to regularize, adjust, and reformulate the activities related to access to genetic heritage or associated traditional knowledge that were carried out during the term of MP 2186-16/2001 (between June 30, 2000 and the date of entry into force of the current Law, November 17, 2016). The Law provides that Regularization will be required for any activity that was performed contrary to MP, and within the scope of this legislation. Reformulation is necessary for all the requested authorization processes that were still in process on the date that the current Law came into force. The Adequacy will be necessary for the authorizations that were granted during the validity of MP. As for Regularization, the rules are more flexible, with exemption of 100% of the payment of fines for irregularities related to the previous rules for scientific research and technological development.
Additionally, it is important to point out that the dissemination of research results that were not registered in SisGen, even in scientific events, or the shipment made without previous registration, will represent infractions subject to fines. Therefore, it is recommended that the registration of activities that use Brazilian biodiversity, as a source of research and/or technological development, be carried out at the beginning of the activity to avoid fines that may be up to R$ 10,000,000 (around US$ 3,000,000), in the case of a legal entity.
The new legislation is based on a historical, ethical, and moral foundation that justifies the regulation of research, technological development, and economic exploitation of finished product and reproductive material, resulting from access to GH and ATK. Despite the opposition of some academics about government control over research involving Brazilian biodiversity, due to the resulting bureaucratization, it is important to clarify that this control was foreseen in the Federal Constitution of 1988, as well as in the Convention on Biological Diversity (CBD) and the Nagoya Protocol (supplementary agreement to the CBD), which aim to safeguard the conservation of biological diversity, the sustainable use of its components, and the rights of holders of associated traditional knowledge, as well as the fair and equitable sharing of the benefits arising from the utilization of genetic resources and associated traditional knowledge.
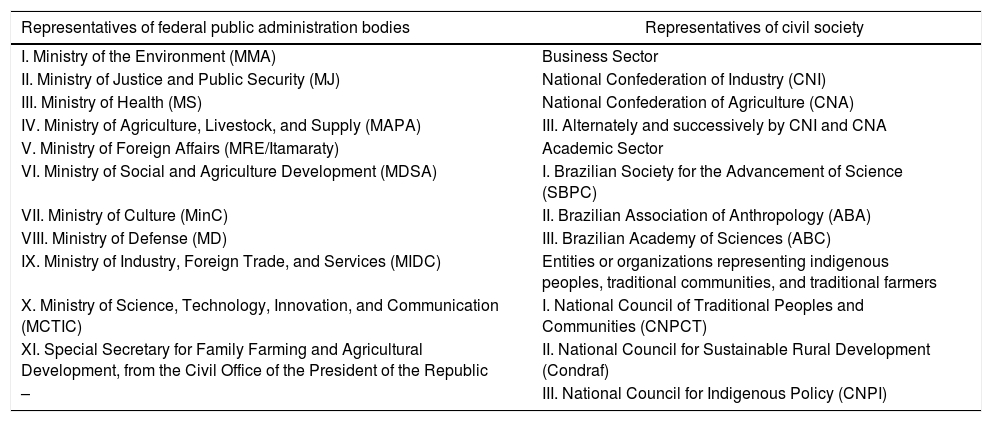
In this context, to enhance and guarantee the participation of civil society, representation of CGEN has been modified. According to the current legislation, CGEN is composed of 20 members, of which 11 are representatives of federal public administration bodies and 9 representatives from civil society, ensuring a balance between academia, business, and traditional populations (Table 1). This demonstrates a strengthened position of the holders of associated traditional knowledge in the current legislation who, represented by indigenous peoples, traditional communities, and traditional farmers, have an active voice in CGEN decisions.
Federal public administration and civil society represented in CGEN.
| Representatives of federal public administration bodies | Representatives of civil society |
|---|---|
| I. Ministry of the Environment (MMA) | Business Sector |
| II. Ministry of Justice and Public Security (MJ) | National Confederation of Industry (CNI) |
| III. Ministry of Health (MS) | National Confederation of Agriculture (CNA) |
| IV. Ministry of Agriculture, Livestock, and Supply (MAPA) | III. Alternately and successively by CNI and CNA |
| V. Ministry of Foreign Affairs (MRE/Itamaraty) | Academic Sector |
| VI. Ministry of Social and Agriculture Development (MDSA) | I. Brazilian Society for the Advancement of Science (SBPC) |
| VII. Ministry of Culture (MinC) | II. Brazilian Association of Anthropology (ABA) |
| VIII. Ministry of Defense (MD) | III. Brazilian Academy of Sciences (ABC) |
| IX. Ministry of Industry, Foreign Trade, and Services (MIDC) | Entities or organizations representing indigenous peoples, traditional communities, and traditional farmers |
| X. Ministry of Science, Technology, Innovation, and Communication (MCTIC) | I. National Council of Traditional Peoples and Communities (CNPCT) |
| XI. Special Secretary for Family Farming and Agricultural Development, from the Civil Office of the President of the Republic | II. National Council for Sustainable Rural Development (Condraf) |
| – | III. National Council for Indigenous Policy (CNPI) |
The new law, although containing several advances, still causes a series of concerns and generates some controversies. For example, its scope, which includes basic research that has no economic potential, such as taxonomy, epidemiology, molecular ecology, etc. Another question is if exotic species, when they are spontaneous or domesticated populations, are subject to the Law or not.
Several of these concerns and other questions that require clarification or adjustments can be addressed in the CGEN Sectoral boards, which are permanent and aim to make proposals from the sectors based on technical discussions. In 2017, the sectoral board of traditional knowledge holders and the sectoral board of the academia were established. The latter was proposed by the Brazilian Society for the Advancement of Science (SBPC) together with Brazilian Academy of Sciences (ABC) and Brazilian Association of Anthropology (ABA) and is composed by specialists representing the Brazilian Society of Microbiology (SBM), the Brazilian Botanical Society (SBB), the Brazilian Society of Zoology (SBZ) and ABA, as well as specialists in biotechnology, consequently all the aspects and scope of the academic areas affected by the Law are contemplated. Therefore, it is important that the academia join forces to propose the necessary adjustments to make the new legislation more efficient and less bureaucratic.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank the Vice-Presidency of Research and Biological Collections (VPCCB) of Fiocruz for financial support.
Information on genetic origin of plant, animal, microbial, or species of other nature species, including substances derived from the metabolism of these living beings.
This text is partly based on previous publications by the authors: Oliveira, D.R., da Silva, M., 2016. Regulamentada a Nova Lei da Biodiversidade: Desafios e perspectivas para P&D no Brasil. Jornal da Ciência Notícias – SBPC, 15/06/16. da Silva, M., 2017. A Lei da Biodiversidade: sua origem e seu impacto na pesquisa e no desenvolvimento tecnológico com patrimônio genético e conhecimento tradicional associado, em: Nader, H.B., de Oliveira,F., Mossri, B.B. (Orgs.), A ciência e o poder legislativo: relatos e experiências. SBPC, São Paulo, pp. 184–194. Oliveira, D.R., da Silva, M., Carmo, F., Angeli, R. 2017. Cumprindo as exigências da Nova Lei da Biodiversidade – Lei 13.123/2015. Chamada à comunidade científica para a regularização e cadastramento de atividades envolvendo patrimônio genético e conhecimento tradicional associado. Jornal da Ciência Notícias – SBPC, 27/10/17.
Information or practice of indigenous population, traditional community, or traditional farmers on the properties or direct or indirect uses associated with genetic heritage.
Product whose nature does not require any type of additional productive process, derived from access to genetic heritage or to associated traditional knowledge, in which the component of genetic heritage or associated traditional knowledge is one of the main elements that add value to the product, which is available for use by the final consumer, being either a person or company.
Plant propagation or animal reproductive material of any genus, species, or crop derived from sexual or asexual reproduction.
Research or technological development carried out on a sample of genetic heritage.
Experimental or theoretical activity carried out on genetic heritage or associated traditional knowledge, with the objective of producing new knowledge, through a systematic process of knowledge construction that generates and tests hypotheses and theories, describes and interprets the fundamentals of phenomena and observable facts.