Although infections with NonTuberculous Mycobacteria have become less common in AIDS patients, they are important opportunistic infections after surgical procedures, likely because they are ubiquitous and not efficiently killed by many commonly used disinfectants. In Venezuela there have recently been many non-tuberculous mycobacteria soft tissue infections after minor surgical procedures, some apparently related to the use of a commercial disinfectant based on a Quaternary Ammonium Compound. We studied the activity of this and other quaternary ammonium compounds on different non-tuberculous mycobacteria by transforming the mycobacteria with a dnaA-gfp fusion and then monitoring fluorescence to gauge the capacity of different quaternary ammonium compounds to inhibit bacterial growth. The minimum inhibitory concentration varied for the different quaternary ammonium compounds, but M. chelonae and M. abscessus were consistently more resistant than M. smegmatis, and M. terrae more resistant than M. bovis BCG.
The advent of highly effective anti-retroviral therapy1,2 has led to a decrease in NonTuberculous Mycobacterial (NTM) infections in patients with the HIV/AIDS syndrome,3 but they are relatively common in patients with cystic fibrosis, and have recently become notorious as contaminant infections after major and minor surgery, particularly NTM of the M. abscessus complex.4,5 Although these low virulence bacteria are generally regarded as opportunistic agents requiring direct inoculation into a wound site, treating them is often difficult and requires prolonged multi-drug therapy, as they are intrinsically resistant to most antibiotics,6,7 including several of those used to treat M. tuberculosis.8 Their frequency as post-procedural contaminants is likely related to several factors: they are fairly ubiquitous in the environment, especially in soil9 and water10,11; they can form biofilms in tap water systems10–12; they are resistant to the antibiotics generally used for surgical prophylaxis8–13; and they appear to be able to survive exposure to many commonly used disinfectants.14,15 The recent popularity of conventional and non-conventional cosmetic procedures in Venezuela, often performed by diverse practitioners in less than optimal settings, has engendered a minor epidemic of NTM infections of the skin and soft tissues. Many of these cases were apparently caused by non-sterile techniques or the use of contaminated materials during surgery, liposuction, foreign body implantation, mesotherapy, acupuncture, or soft tissue augmentation, but incomplete disinfection of the skin prior to these procedures has also been implicated.16 The label of the disinfectant product most commonly used in medical settings in Venezuela stated that it would sterilize surgical instruments and anything else after only a 20-min exposure, and that it was tuberculocidal. These claims, based upon studies with M. smegmatis, appeared questionable, because the active ingredient is a QAC, a class of disinfectants regarded as having only mycobacteriostatic activity. As the dispute over the effectiveness of the product has provoked several civil suits, it seemed necessary to evaluate the activity of this product and other QACs against several NTM, including some clinical isolates.17 A number of fairly complicated methods have been proposed to evaluate the ability of disinfectants to reduce or eliminate bacteria from surfaces or surgical equipment.18 Our purpose, however, was to evaluate the activity of several QACs over a range of concentrations against different NTM known to represent a range of innate resistance.7,19 To accomplish this in a tractable format, we labeled four NTM and M. bovis BCG with green fluorescent protein (GFP) and tested the ability of different concentrations of the compounds to reduce bacterial growth, as measured by fluorescence. A similar assay was described previously.20
Materials and methodsStrains and plasmidsM. smegmatis mc2155 is a strain commonly used in the laboratory (LGM, IVIC), M. chelonae (LT 1529) and M. abscessus subspecies abscessus (LT 949) were clinical strains isolated in Caracas (Lab. TB, Inst. Biomedicina), M. bovis BCG (ATCC 35734) and M. terrae (ATCC 15755) was obtained from the American Type Culture Collection. The strains were grown at 37°C in Middlebrook 7H9 liquid media supplemented with 10% OAD (oleic acid 0.05%, bovine serum albumin 5%, dextrose 2%), 0.2% glycerol and 0.05% Tween 80 (7H9-OAD-Tw), or on Petri dishes containing Middlebrook 7H10 supplemented with 10% OAD and 0.2% glycerol. The species of the strains used were confirmed with the PRA method.21 Plasmids pYUB412 and pFPV2722,23 were the kind gifts of Dr. Lalita Ramakrishnan, and Drs. Martin Pavelka and William R. Jacobs Jr., respectively.
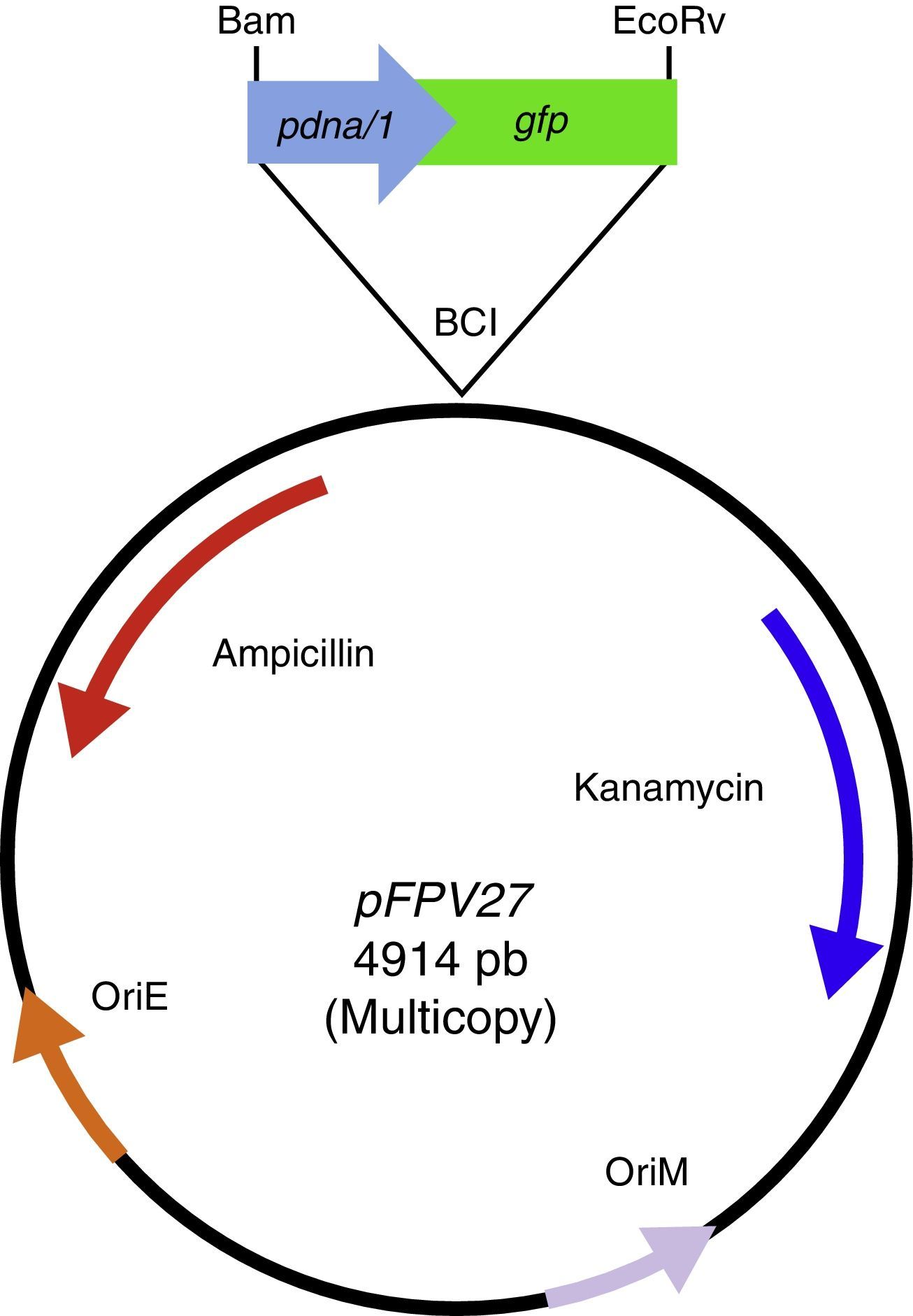
Cloning of the dnaA-gfp fusionReagents for DNA manipulations were obtained from commercial sources and used according to routine protocols. For the construction of plasmid pdnaA-gfp, the dnaA promoter region from M. tuberculosis (582bp) was amplified from a cosmid24 containing the rpmH–dnaA region, using primers GLRA3 (5′ cta tgt ctg gca aca t) and GLRA4 (5′ tat ctc cct ggt tct cgt ta), and ligated upstream of gfp into the BamHI and EcoRV sites of pFPV27 (Fig. 1). The chromosomal integrating version of the dnaA-gfp fusion, on plasmid pintdnaA-gfp, was obtained by cloning a BamHI–BclI fragment from pdnaA-gfp into the BclI site of pYUB412.22 Plasmids pdnaA-gfp and pintdnaA-gfp were electroporated into the mycobacterial strains and plated onto kanamycin (pdnaA-gfp) or hygromycin (pintdnaA-gfp) selective media. GFP-expressing mycobacteria were visualized for fluorescence using a Nikon Eclipse 600 fluorescence microscope.
Monitoring bacterial growth by fluorescenceA colony of mycobacteria from solid media was inoculated into 5mL of 7H9 OAD-Tw, with kanamycin 50μg/mL when the strains contained plasmid pdnaA-gfp, or hygromycin 100μg/mL for strains with integrating plasmid pintdnaA-gfp, in a 50mL conical tube, and grown with agitation at 37°C until reaching exponential phase, then transferred to 50mL of the same media and grown in 500mL flasks. For the initial studies comparing fluorescence vs. growth, samples were taken periodically to measure the optical density and fluorescence every 3h for M. smegmatis, every 6h for M. abscessus and every 24h for M. chelonae, M. bovis BCG and M. terrae. Optical density at 600nm (OD600) was measured in a 96 well spectrophotometer (Molecular Devices Spectramax 340), and fluorescence (485nm excitation and 535nm emission) was measured in a 96 well plate fluorimeter (Molecular Devices SpectraMax, GeminisXS). Aliquots were also diluted and plated on 7H10-OAD plates to determine colony-forming units (CFU).
DisinfectantsThe disinfectants evaluated were: cetyltrimethyl ammonium bromide (CTAB), dodecyl trimethyl ammonium bromide (DTAB), cetylpyridinium chloride (HPC) and dimethyl benzyl lauryl ammonium bromide (DBLAB – marketed under a commercial product name http://www.pharmcast.com/Patents/Yr2002/August2002/082702/6441045_Disinfectant082702.htm). The disinfectants were prepared by dissolving CTAB, HPC, or DTAB in 50mL volumes of 7H9-OAD-Tw to obtain 4% solutions of each. DBLAB, as the commercial product, was diluted with 7H9-OAD-Tw to a concentration of 4%. All disinfectants solutions were kept at 4°C until used.
Studies with disinfectants and antibioticsThe different bacteria were grown in 50mL conical tubes, as described above, until reaching an OD600 of approximately 1.0. Five millilitres of each were then transferred to a new 50mL conical tube, 10 sterile glass beads were added, and the tube was vortexed for 20s to reduce clumping. The tubes were left to stand at room temperature for 15min then 100μL was added to 9.9mL of 7H9OAD-Tw. One millilitre of this suspension was then added to 9mL of the same media, which was then mixed and used in the 96 well plate assay.
Assay of disinfectant activity using fluorescent mycobacteriaThe assay was performed using black 96 well plates, one disinfectant per plate. 200μL of the highest concentration of the disinfectant to be tested was placed into the wells in the first column, and 100μL 7H9 OAD-Tw added to the wells in the other columns. 100μL from the wells with the highest disinfectant concentration were diluted into the 100μL 7H9 OAD-Tw in the next column, and the dilution process serially repeated to obtain final concentrations of 4%, 2%, 1%, 0.5%, 0.25%, 0.125%, 0.06% until 0.00003%. 100μL of the mycobacterial suspension were then added into each well. Positive controls without disinfectants and negative controls with media alone were included on each plate. The plates were incubated with agitation at 37°C and the fluorescence read periodically over 4 days in a fluorimeter.
MIC determination with Alamar BlueThe Alamar Blue tests25–27 were performed in transparent 96 well plates with inocula, controls and detergent concentrations prepared as described above. Detergent concentrations were tested in duplicate. The plates were incubated at 37°C with agitation for different times depending upon the mycobacterial species being tested (M. smegmatis 3–4 days and M. chelonae and M. abscessus 5–6 days), and then 30μL of Alamar Blue was added to the positive control wells. If, after a further 24h of incubation, the initial blue in the positive controls had turned a fuchsia color, Alamar Blue was added to the other wells and incubated for another 24h. In reading the results, the positive and negative controls were used as references. The MIC corresponded to the lowest concentration in which the intensity of the color change was equal to or less than the negative controls.
MIC determination by plate dilutionFor the plate dilution method,28 60mm Petri dishes were prepared using 7H10-OAD media containing serial twofold decreasing concentrations of the disinfectants from 0.50% through 0.0005%. Solidified plates were spread in duplicate with 100μL of an inoculum containing ∼104 bacteria per mL and incubated at 37°C for 3 days for M. smegmatis, 5 days for M. abscessus and 8 days for M. chelonae. Control plates had no disinfectant.
Evaluation of antibiotic activity against M. abscessusM. abscessus was grown and the inocula prepared and diluted, as described above, to contain 104–5 bacteria per mL. The antibiotics clarithromycin, rifampicin, mefloxine and amikacin were purchased from Sigma–Aldrich. Moxifloxacin was obtained from the NIH AIDS repository program. The five drugs were tested in triplicate in each 96 well plate assay, at concentrations of 128, 64, 32, 16, 8, 4, 2, 1 and 0.5μg/mL, obtained by serial dilutions as described above. 100μL of the bacterial suspension was added to the appropriate wells, the plates were covered and sealed with tape to prevent evaporation, and then incubated at 37°C. with agitation. Fluorescence was measured daily for 3 days, and each assay was performed at least 3 times.
ResultsConstruction of GFP labeled mycobacterial strainsIn this work we employed a GFP-based expression system to assess the growth inhibitory properties of distinct QACs disinfectants and antibiotics against different mycobacterial species. The gfp gene was fused to M. tuberculosis dnaA promoter, as expression of this promoter is induced during the growth cycle of mycobacteria, and thus expression of gfp and its innate fluorescence should reflect bacterial growth. The multicopy (pdnaA-gfp) and integrating single copy (pintdnaA-gfp) plasmid versions were electroporated separately into M. smegmatis. Transformants with either plasmid were seen to fluoresce under a fluorescent microscope, but the fluorescence was much stronger in the strains carrying the multicopy version of the fusion (data not shown). Preliminary studies with strains carrying the integrated version of the pintdnaA-gfp fusion indicated that to obtain an adequate signal to noise ratio it was necessary to begin with of least a million bacteria per well, but at this density the cultures were rapidly saturated and the effects of the drugs and disinfectants on growth inhibition were difficult to detect (Fig. 2A and B).
(A) Comparison of the fluorescence of cultures (RFU-Relative Fluorescent Units) with their Optical Density (OD600) for M. smegmatis strains with no gfp fusion (WT) or M. smegmatis with either the multicopy (pdnaA-gfp) or single copy (pintdnaA-gfp) integrated version of the GFP fusion. (B) Comparison of the fluorescence (RFU) with colony counts (CFU – Colony Forming Units) of the same strains shown in (A). All results are from representative experiments that were repeated at least three times.
In contrast, strains carrying the multicopy plasmid version of the dnaA-gfp fusion provided sufficient fluorescence to monitor bacterial growth starting with 104–5 bacteria per well, and were used for all subsequent studies. For these strains the increase in fluorescence produced by bacterial growth in 96 well plates correlated closely with determinations of bacterial growth both by optical density (OD600) (Fig. 2A), and by colony counts of serial dilutions plated on solid media in Petri dishes (Fig. 2B).
Use of GFP fluorescence to determine relative activity of four QACs against M. smegmatis, M. chelonae, M. abscessus. M. terrae and M. bovis BCGStrains of M. smegmatis, M. chelonae, M. abscessus, M. terrae and M. bovis BCG were transformed with the pdnaA-gfp and inoculated into serially decreasing concentrations of CTAB, DTAB, HPC and DBLAB. Fluorescence was monitored daily, as shown for M. abscessus upon exposure to disinfectant DBLAB (Fig. 3).
Activity of DBLAB against M. abscessus carrying the pdnaA-gfp fusion on a multi-copy plasmid was determined in a 96 well format. Bacterial growth was measured daily over 4 days with a fluorimeter and expressed as Relative Fluorescent Units (RFU) shown on log scale. The experiment was performed in triplicate.
After four days of growth, DTAB appeared to be the most active compound, suppressing growth of all five strains at concentrations as low as 0.062%. With HPC and CTAB, growth was suppressed at concentrations as low as 0.25%. In CTAB, there was essentially no growth at concentrations of 0.25% or higher, slight growth of M. chelonae and M. abscessus at concentrations from 0.125% through 0.016%, and abundant growth at lower concentrations. A similar pattern was seen for M. abscessus in HPC. M. smegmatis was markedly more susceptible to CTAB and DTAB than the other two strains, while M. abscessus was more resistant to DBLAB and HPC than both M. chelonae and M. smegmatis. M. bovis BCG was even more sensitive than M. smegmatis, and M. terrae slightly more resistant (Fig. 4).
Activity of four QACs disinfectants tested in decreasing concentrations against M. smegmatis, M. abscessus, M. chelonae, M. terrae and M. bovis BCG, all carrying the dnaA-gfp fusion on a multi-copy plasmid. The assays were performed in a 96 well format and bacterial growth after 4 days of incubation was measured as Relative Fluorescent Units (RFU) and shown on a log scale. The QACs tested were DBLAB, HPC, CTAB and DTAB. All results are averaged from experiments performed at least three times.
In parallel studies, the capacity of the QACs disinfectants to inhibit the mycobacterial growth of M. smegmatis, M. chelonae and M. abscessus was measured in a microwell Alamar Blue assay and also by the plate dilution method. The MICs obtained with the three methods are compared in Fig. 5. The plate assay showed the least variation of inhibitory concentrations with the three strains and tended to produce lower MICs. M. abscessus showed the least differences with the three methods and M. chelonae the greatest discrepancies. M. chelonae and M. abscessus were generally more resistant than M. smegmatis. DBLAB, the ingredient of the commercial product had much higher MICs than the other three QAC disinfectants tested. The results of the Alamar Blue and fluorescent assays differed from those of the plate assay, with a roughly equivalent degree of variance. In most cases the differences in the MICs amongst the different methods were within a range of 2–3-fold.
Comparison of the results of three different methods for determining the MICs of four QACs disinfectants (A) DBLAB, (B) HPC, (C) CTAB and (D) DTAB against M. smegmatis, M. abscessus, and M. chelonae. The methods tested were: GFP, monitoring the fluorescence of strains carrying the multicopy dnaA-gfp fusion; AB, Alamar Blue, 96 well plate format; P, plate assays. All results are from representative experiments that were repeated at least three times.
To evaluate the utility of the fluorescent assay for determining the sensibility of mycobacteria to therapeutic agents, we tested various antibiotics against gfp labeled M. abscessus in an assay similar to that used for the QACs above (Fig. 6). Of the antibiotics tested against M. abscessus, clarithromycin clearly had the best activity, followed by moxifloxacin>amikacin>rifampin>mefloquine, in that order.
DiscussionWe have evaluated the activity of four QACs against different species of NTM and five antibiotics against M. abscessus using GFP labeled bacteria, and have shown that measurement of GFP fluorescence, when expressed from the growth-dependant dnaA promoter, is a convenient way to determine the sensitivity of mycobacteria to both QAC disinfectants and antibiotics. Further, we have confirmed that M. abscessus and M. chelonae are significantly more resistant to QACs than M. smegmatis.29,30 Although the activities of different QACs vary somewhat depending upon the mycobacterial species, DBLAB was the least effective of those tested. However, using M. smegmatis to assess the activity of disinfectants against all NTM could lead to disastrous over-estimations of the capacity of QACs and other disinfectants to eliminate the species that are most commonly associated with infections, such as M. abscessus or M. chelonae.
In other studies, not shown, we confirmed that M. terrae, proposed as a proxy for the susceptibility of M. tuberculosis,31 is more resistant to the QACs than M. bovis BCG. Therefore, while BCG is a closer model for determining the resistance profile of M. tuberculosis, testing with M. terrae should provide of margin of safety. In studies similar to those in Fig. 6, not shown, we also found that fluorescent M. bovis BCG can be employed to assess the activity of antibiotics against M. tuberculosis. The differences in the relative sensitivity of different mycobacteria to disinfectants and other anti-bacterial agents is likely determined by the composition of the complex, lipid rich, relatively impermeable cell envelope, but the possible involvement of efflux pumps32 or differences in porin-like structures33 cannot be excluded. The GFP method produces results that, although often slightly different from the MICs obtained with the plate dilution method, are similar to those obtained with the Alamar Blue 96 well plate method, which also uses liquid media. It is less labor intensive than the other methods, but the format requires a fluorimeter that can read multi-well plates. The fluorescent mycobacteria can provide a facile and rapid system for evaluating the growth inhibiting effects of detergents and antibiotics and could be used for high throughput screening of new compounds against NTM as well as M. tuberculosis, with either M. bovis BCG or M. terrae as surrogates that do not require P3 biosafety facilities. However, the method may not work if the chemical or extract to be tested emits fluorescence with a wavelength close to that of GFP, although the bacteria could be labeled with alternative molecules that fluoresce at different wavelengths.
There are several limitations to the results of this study. Many situations requiring disinfection involve organic material, but in our GFP assay only routine growth media is used, which could lead to an overestimation of the effectiveness of the QACs tested. We performed the experiments using inocula from cultures late in the exponential phase (OD600∼1.0), and grew the bacteria at 37°C, but it is possible that the results could vary if early log or late stationary phase cultures were used, or if the bacteria were grown at a different temperature. M. abscessus would likely have grown better at 30°C, and therefore the MICs for this bacteria could be higher at this lower temperature. To test the possibility that the Tween 80 added to the media was affecting the activity of the QAC compounds, we eliminated Tween from the culture media and found that the MIC values were unchanged. Our studies using the GFP method to determine the relative MICs of antibiotics against mycobacteria (Fig. 6) were not validated with parallel colony counts, however, because of the close correlation of colony counts with fluorescence in our preliminary studies (Fig. 1B), we believe that the relative activity of the antibiotics can be trusted, although perhaps not the absolute MIC values. We considered that after four days in culture the QACs might begin to break down and lose activity, so we did not examine growth after this time point. It is possible that the QACs selected for few resistant organisms whose growth would eventually result in higher fluorescence at later time points. However, the four days should have been sufficient for at least 25 rounds of replication, which would have been reflected by an increase in the fluorescence. We previously published studies34 showing that exposure to QACs at concentrations as high as 9% can induce a non-genetic resistance state in M. abscessus and M. chelonae, but this resistance is only to QAC exposures up to 1h, while in the studies described here the QACs are present in the media during the entire four days during which fluorescence was monitored.
Finally, although the QAC contained in the commercial disinfectant product is much less active than the three other QACs tested, by itself this may not be a cause for alarm, as the MICs for all the bacteria tested were still well below the 9% concentration of the compound in the commercial presentation, and the lower activity may be advantageous in terms of less toxicity to humans. However, it is clear from this work and previous studies that M. smegmatis is generally much more sensitive to QACs disinfectants than M. abscessus and M. chelonae, and therefore the innocuous M. smegmatis should not be used as a surrogate to assess activity against strains with greater intrinsic resistance and also greater pathogenic potential.
Conflicts of interestThe authors declare no conflicts of interest.
This work was funded by FONACIT Proyecto G-2005000393 and LOCTI project “Las Cepas de Tuberculosis Mas Virulentas de Venezuela.”