Transcatheter aortic valve implantation (TAVI) is a less invasive technique compared to surgical aortic valve replacement (SAVR), used in patients with severe aortic stenosis, especially in those with a high surgical risk and frailty.1 However, a potentially fatal complication of this procedure has been acknowledged as “suicide left ventricle” (SLV). Although its pathophysiology is still poorly understood, after the relief of the aortic valve stenosis with a subsequent reduction in left ventricular pressures, a dynamic intraventricular obstruction occurs, manifested as severe hemodynamic instability. This phenomenon has been described in both SAVR and TAVI procedures.1–3
Based on the literature, dynamic intraventricular obstruction has been reported in approximately 15% of patients undergoing SAVR.4 However, its exact incidence in TAVI is unknown. A retrospective observational study published by Kaewkes et al. (n=1729) found that 1.8% of patients who underwent TAVI had significant left ventricular obstruction features present in the transthoracic echocardiogram (TTE), nevertheless, only one patient developed hemodynamic compromise.5
Some risk factors that can contribute to the development of an SLV are female sex, a sigmoid-shaped septum, asymmetric septal hypertrophy, a small ventricular cavity, a normal left ventricular ejection fraction (LVEF), high peri-procedural transvalvular gradients, and a narrow left ventricle outflow tract (LVOT).3,4 In most cases, dynamic intraventricular gradients occur in mid-ventricular locations, especially when there is concentric hypertrophy of the interventricular septum.4,6
SLV treatment should focus on increasing preload and afterload to improve left ventricle filling pressures and reduce contractility.1 Conventional treatment includes volume loading and beta-blockers as the mainstays for this pathology.7 Other options include strategies such as right ventricular pacing, surgical myomectomy, and alcohol septal ablation.1,3,4,6 Furthermore, for severe and refractory cases, extracorporeal membrane oxygenation (ECMO) therapy has been described.2
A 75-year-old male was referred to our institution in consideration for an aortic valve replacement. At the time of admission, he was hemodynamically stable but presented a 12-month history of progressive decline in functional class (New York Heart Association III), syncope, and chest pain. Vital signs showed blood pressure of 150/100mmHg, heart rate of 80beats per minute, a respiratory rate of 14 breaths per minute with oxygen saturation of 92%, and he was afebrile. Physical examination revealed a body mass index of 26.8kg/m2 (height 1.77m and weight 84kg), jugular venous distension, an ejection systolic murmur in the aortic region radiating to the neck vessels, and lower extremity edema. His past medical history included severe aortic valve stenosis diagnosed 2 years before, dyslipidemia, hypertension, and paroxysmal atrial fibrillation with poor treatment adherence.
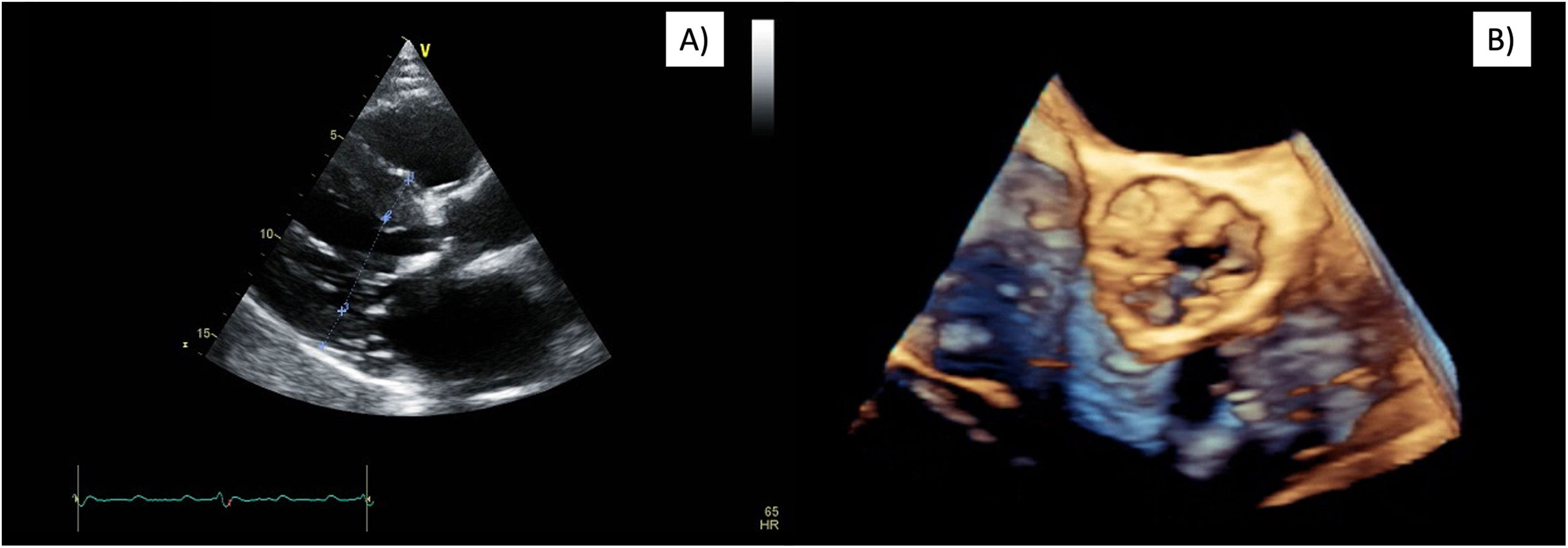
The electrocardiogram showed sinus rhythm and left ventricular hypertrophy. Preoperative TTE revealed severe aortic valve stenosis with significant thickening and reduced mobility of the valve leaflets (valve area of 0.62cm2, mean gradient of 58mmHg, maximum 103mmHg, and a peak velocity of 5m/s). In addition, moderate mitral regurgitation and mild tricuspid insufficiency were reported. The left ventricle exhibited a mildly reduced LVEF of 49%, a relatively small cavity (42mm at end-diastole), concentric hypertrophy with a septal thickness of 18mm and a posterior wall thickness of 16mm (Fig. 1). LVOT diameter was 1.59cm, and a peak gradient of 2mmHg was measured. The right ventricle was enlarged and presented reduced ventricular function (TAPSE of 12mm). A pulmonary artery systolic pressure of 72mmHg and a mean of 42mmHg were quantified. CT scan measured an aortic annulus at 23mm, severe calcification (5054.5AU), and revealed a sigmoid septum. A coronary angiogram showed no significant coronary artery disease.
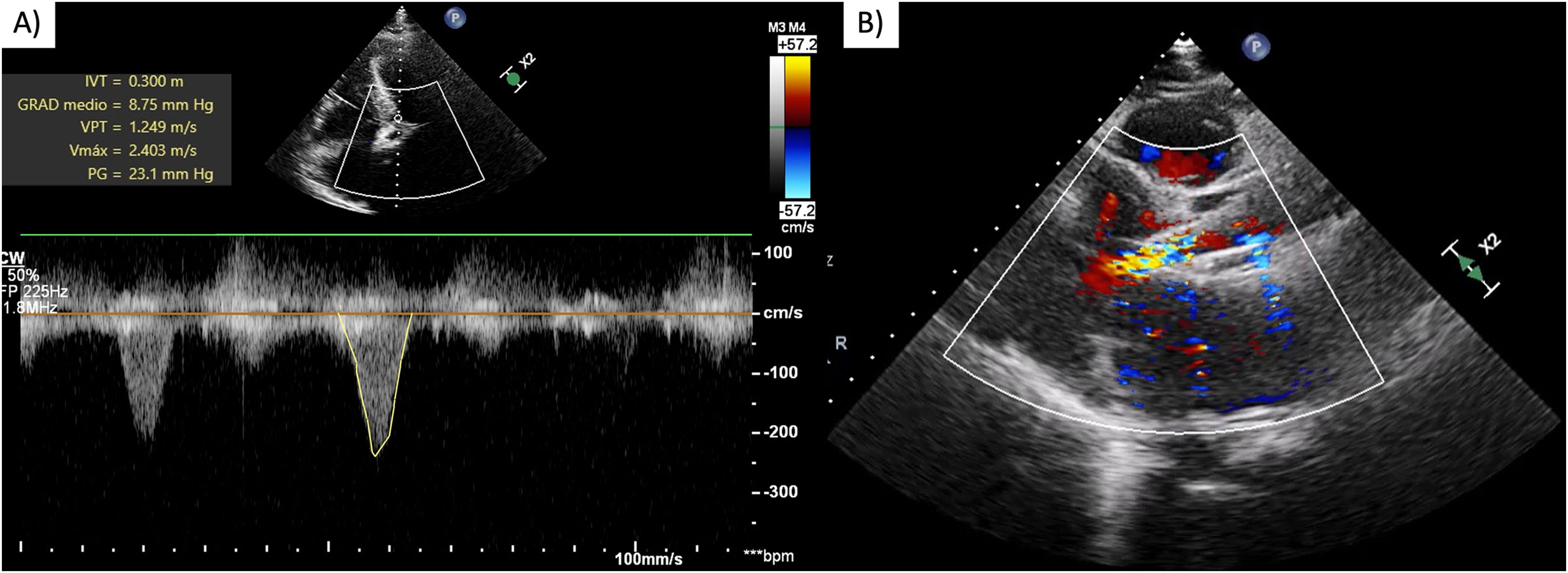
After evaluating the patient's characteristics, surgical risk, and comorbidities, it was determined that he met the appropriate criteria for a TAVI. An active pacemaker lead was fixed in the right ventricle via jugular access and a right transfemoral TAVI was performed. However, during the balloon aortic valvuloplasty, the procedure was complicated by severe arterial hypotension with a mean arterial pressure (MAP) of 33mmHg for which norepinephrine (0.56mcg/kg/min), vasopressin (0.1U/kg/min) and dobutamine (5mcg/kg/min) were started with a slightly increased MAP at 51mmHg. Subsequently, the patient suffered cardiac arrest with ventricular fibrillation and pulseless electrical activity, therefore advanced cardiac life support was initiated. During the resuscitation, a self-expanding 29mm biologic aortic valve prosthesis was advanced and successfully implanted, resulting in the return of spontaneous circulation with pacemaker dependency. An emergent coronary angiography was performed and ruled out coronary artery occlusion and no bleeding sites were detected. TTE showed good prosthesis function with no paravalvular leaks, mildly reduced systolic function, an LVOT peak gradient of 23mmHg (previously 2mmHg) and a peak velocity of 2.4m/s. No SAM of the mitral valve was documented (Fig. 2). As an SLV was suspected a 2L of fluid resuscitation with Hartman solution was started and dobutamine was suspended.
Immediate post-TAVI transthoracic echocardiogram. Continuous wave Doppler showing (A) intraventricular gradient of 23mmHg and maximum velocity of 2.4m/s, no SAM of the mitral valve was documented, (B) LV mid-cavitary gradient. LV=left ventricle, TAVI=transcatheter aortic valve implantation, SAM=systolic anterior motion.
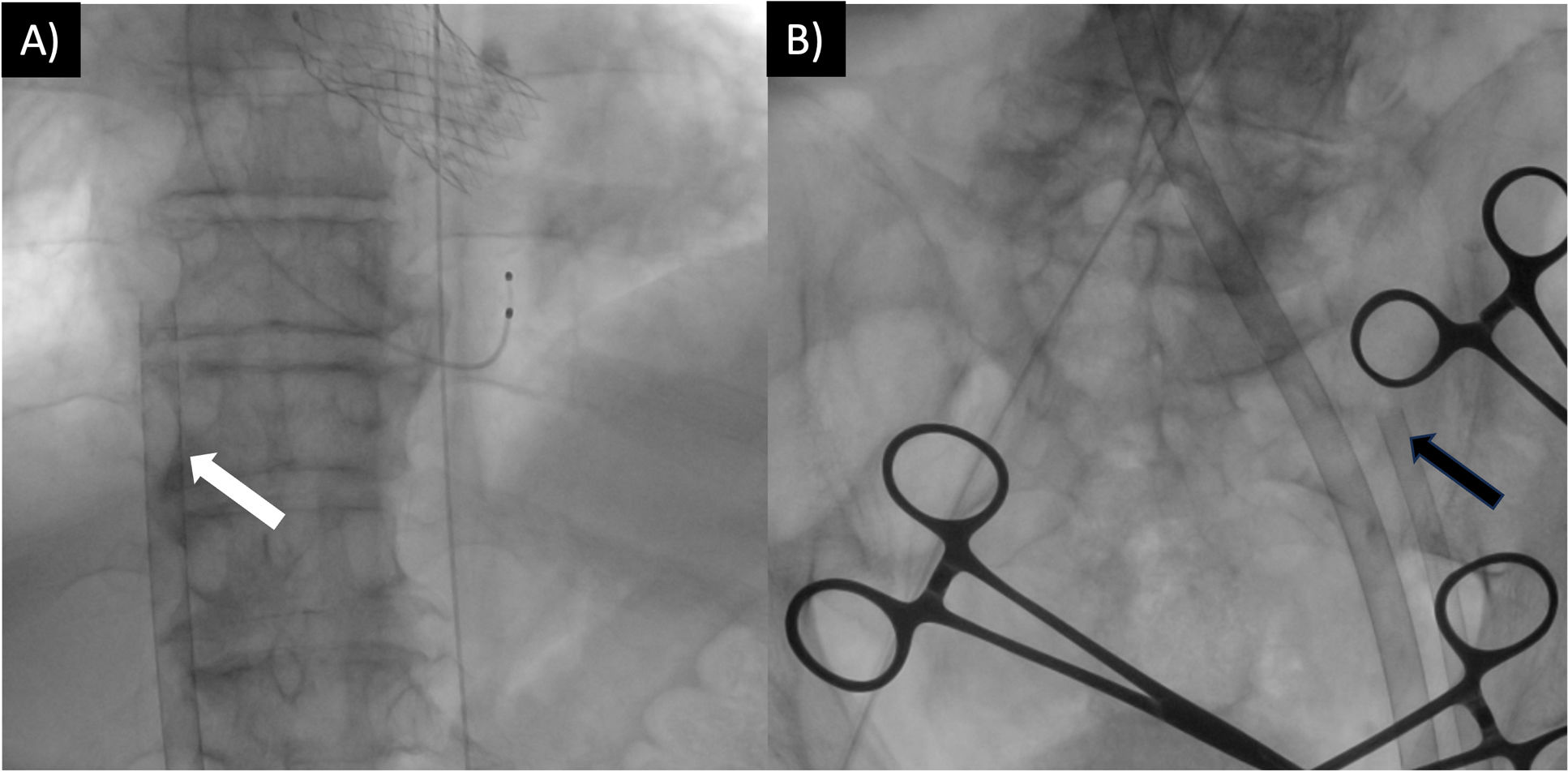
In the next 30min, the patient presented with intermittent episodes of ventricular fibrillation, pulseless electrical activity, and persistent hemodynamic deterioration (MAP 53mmHg, lactate 3.3mmol/L) despite escalating vasopressor support. As there was no response to medical treatment, therapy was escalated to mechanical circulatory support using peripheral veno-arterial (VA) ECMO (Fig. 3). A 23Fr left femoral extraction venous multi-fenestrated cannula (Medtronic, Minneapolis, MN, USA) was placed at the Cavo atrial junction, a 17Fr left arterial femoral return cannula (Medtronic) was placed, and a 6Fr distal perfusion line was placed in the left superficial femoral artery. Assistance was started at 3000rpm, with a flow of 3.4L/min.
Percutaneous ECMO cannulation with ultrasound-guided puncture and fluoroscopic guidance, following trans aortic valve implantation. (A) White arrow indicates the left femoral extraction venous cannula placed at the Cavo atrial junction. (B) Black arrow indicates the return cannula placed in the left superficial femoral artery. The right femoral artery was used for valve implantation and balloon valvuloplasty. ECMO=extracorporeal membrane oxygenation.
The patient was transferred to the cardiovascular intensive care unit, where he was found to have an adequate post-resuscitation clinical neurological status (Full Outline of UnResponsiveness score 13) and CT ruled out anoxic-ischemic lesions. In the first 48h, the patient demonstrated clinical improvement and was successfully extubated. At 72h, vital signs stabilized (MAP 96mmHg, heart rate 85beats per minute, oxygen saturation 95%), bedside echocardiogram showed left ventricular contractility improvement, and vasoactive support was reduced (norepinephrine 0.1mcg/kg/min). Laboratory results showed a lactate level of 1.0 and creatinine elevation peaked at 1.7mg/dL with gradual decrease in the following days (baseline 1.1mg/dL). ECMO flow was reduced to 1.0L/min with good tolerance. Due to improved hemodynamic status and meeting prognostic success criteria, the patient was transferred to the hemodynamics department, where he was decannulated after 3 days of assistance under fluoroscopic vision. The femoral artery was closed with a vascular closure device and direct pressure was applied to the femoral vein. The vasopressor support was discontinued 24h later.
Six days after the procedure, his condition continued to improve, TTE revealed a residual intraventricular gradient of 15mmHg, adequate aortic prosthesis position, leaflet excursion, and no paravalvular leaks. The patient was discharged after 11 days of hospitalization in compensated status.
TAVI is a minimally invasive procedure used in patients diagnosed with severe aortic stenosis. Among its complications, a phenomenon known as SLV has been described. This is characterized by the development of dynamic intraventricular gradients, left ventricle hypercontractility, and/or systolic anterior motion of the mitral valve, after a sudden reduction in afterload, leading to dynamic obstruction of the LVOT and hemodynamic collapse.2,4
We present the case of a 75-year-old male who experienced severe hypotension and multiple episodes of cardiac arrest while undergoing TAVI. After ruling out the most common acute complications, SLV was considered. Predictive factors in our patient for developing SLV were a sigmoid-shaped septum, increased basal septal thickness (18mm), a narrow LVOT (15mm), a small hypertrophied ventricular cavity, and high pre-procedural valve gradients.
Regardless of the described conservative treatment,7 our patient experienced refractory cardiogenic shock and cardiac arrest during the TAVI procedure. As a result, early support with VA-ECMO was initiated, leading to the optimization of hemodynamic parameters, improvement of cardiac function, and stabilization of the patient preventing further end-organ damage.
While this complication is rare, it is crucial to understand its characteristics, pathophysiology, preventive and therapeutic measures due to its increased mortality risk.1 Whereas SLV is suspected, the conventional management of cardiogenic shock should be modified to encourage increasing filling pressures, raising diastolic filling time, and reducing inotropy; inotropes, vasodilators, and intra-aortic balloon pumps can aggravate the LVOT obstruction, promoting further instability.1,7
Other strategies such as intended desynchronization of the left ventricle via right ventricular pacing, and more invasive and permanent procedures such as surgical myomectomy, and alcohol septal ablation have also been described as bail-out measures for SLV refractory to medical treatment. Nonetheless, these can represent a high risk in patients undergoing TAVI due to their comorbidities and frailty.1,3,4,6 So it is questionable whether these measures are strictly necessary, especially in this group of patients, as ECMO assistance can help stabilize the patient while the hemodynamics are optimized and the SLV is reversed.
SLV scenarios in which ECMO therapy becomes more relevant and could be considered as a rescue strategy include (1) No adequate response to initial medical treatment where the obstruction further deteriorates hemodynamic status to a cardiogenic shock state. (2) Patient presents cardiorespiratory arrest refractory to advanced cardiac life support measures.
Sudden hemodynamic collapse during or following TAVI must include SLV as a differential diagnosis for prompt treatment and stabilization. This condition should be suspected when severe hypotension, high intraventricular gradients, and/or the presence of systolic anterior motion of the mitral valve are present. If the patient is stable, it is reasonable to start conservative treatment with intravenous fluids and beta-blockers, however, if the patient is unstable, the use of pure vasoconstrictors and early hemodynamic support with VA-ECMO can improve the patient's outcomes. To prevent LVOT obstruction from worsening, any measure that reduces the afterload or increases the left ventricle contractility must be avoided when SLV is suspected.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Ethical considerationsThe patient's anonymity has been maintained, written informed consent was obtained from the patient.
FundingNone funding or other forms of financial support were received.
Conflict of interestThe authors have nothing to disclose.